Author Contributions
Conceptualization, T.S. and M.R.H.; Methodology, T.S. and M.R.H.; Software, T.S. and M.R.H.; Validation, T.S. and M.R.H.; Formal Analysis, T.S. and M.R.H.; Investigation, T.S. and M.R.H.; Resources, T.S. and M.R.H.; Data Curation, T.S. and M.R.H.; Writing—Original Draft Preparation, T.S. and M.R.H.; Writing—Review and Editing, T.S. and M.R.H.; Visualization, T.S. and M.R.H.; Supervision, T.S. and M.R.H.; Project Administration, T.S. and M.R.H.; Funding Acquisition, T.S. and M.R.H. All authors have read and agreed to the published version of the manuscript.
Abbreviations
AC, astrocyte; ACef, astrocyte end-feet; AGE/RAGE, advanced glycation end products/receptor for advanced glycation end products; AQP4, aquaporin-4; BBB, blood–brain barrier; BEC(s), brain endothelial cell(s); BECact/dys, brain endothelial cell activation/dysfunction; BG, basal ganglia; CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CBF = cerebral blood flow; CCVD, cerebrocardiovascular disease; CSF, cerebrospinal fluid; CSO, centrum semiovale; E-selectin, endothelial–leukocyte adhesion molecule; ecGCx = endothelial cell glycocalyx; EPVS, enlarged perivascular spaces; HTN, hypertension; ISF, interstitial fluid; ICAM-1, intercellular adhesion molecule 1; LDL, low-density lipoprotein (LDL); LOAD, late-onset Alzheimer’s disease; MCI, mild cognitive impairment; MetS, metabolic syndrome; MRI, magnetic resonance imaging; MS, multiple sclerosis; NO, nitric oxide; NVU, neurovascular unit; Pc, pericyte; PD, Parkinson’s disease; PVS, perivascular spaces; PVU, perivascular unit; SAS, subarachnoid space; SVD, small vessel disease; TEM, transmission electron microscopy; TIA, transient ischemic attack; TJ/AJ, tight and adherens junctions; VaD, vascular dementia; VCAM-1, vascular cell adhesion molecule 1; VRS, Virchow–Robin spaces; WMH, white matter hyperintensities.
Figure 1.
Relationship of perivascular spaces PVS to the whole brain. (A) illustrates the structurally labeled whole brain with demarcations of specific regions (dashed lines). (B) depicts the pia artery within the subarachnoid space (SAS) that penetrates the deeper brain structures in a perpendicular manner with adjacent PVS with blue coloration (B1) and horizontally–diagonally (B2), wherein the perivascular spaces (PVS) allow for the influx (black arrow) of the cerebrospinal fluid (CSF) to the parenchymal interstitial fluid space (ISF) via the arteriolar PVS. Panel (B3) depicts the efflux (blue arrow) of the interstitial fluid metabolic waste material (WM) of the pial venular PVS to the pial vein PVS that enter the subarachnoid space to eventually drain into the dural venous sinus space.
Figure 1.
Relationship of perivascular spaces PVS to the whole brain. (A) illustrates the structurally labeled whole brain with demarcations of specific regions (dashed lines). (B) depicts the pia artery within the subarachnoid space (SAS) that penetrates the deeper brain structures in a perpendicular manner with adjacent PVS with blue coloration (B1) and horizontally–diagonally (B2), wherein the perivascular spaces (PVS) allow for the influx (black arrow) of the cerebrospinal fluid (CSF) to the parenchymal interstitial fluid space (ISF) via the arteriolar PVS. Panel (B3) depicts the efflux (blue arrow) of the interstitial fluid metabolic waste material (WM) of the pial venular PVS to the pial vein PVS that enter the subarachnoid space to eventually drain into the dural venous sinus space.
Figure 2.
Illustration of the transition from a true capillary to a post-capillary venule with accompanying transition electron microscopy (TEM) images. (A) illustrates a true capillary without perivascular spaces (PVS). (B) illustrates a post-capillary venule with PVS, which contains a resident macrophage (#) and lymphocyte (@). Note the direction of blood flow within the capillary lumen (CL) (open red arrows from left to right in these images (A,B)). Note the EC tight and adherens junction (*). Additionally, note that the closed red arrows in (A,B) point to the corresponding TEMs in (a’,b’). (a’) demonstrates a cross-section electron micrograph of a true capillary and how the astrocyte end-feet (ACef) directly abut the basement membrane of the mural endothelial cell(s) (ECs) and pericyte end-feet process (Pcef PcP). (b’) depicts a longitudinal section of the post-capillary venule with prominent PVS. RBC = red blood cell.
Figure 2.
Illustration of the transition from a true capillary to a post-capillary venule with accompanying transition electron microscopy (TEM) images. (A) illustrates a true capillary without perivascular spaces (PVS). (B) illustrates a post-capillary venule with PVS, which contains a resident macrophage (#) and lymphocyte (@). Note the direction of blood flow within the capillary lumen (CL) (open red arrows from left to right in these images (A,B)). Note the EC tight and adherens junction (*). Additionally, note that the closed red arrows in (A,B) point to the corresponding TEMs in (a’,b’). (a’) demonstrates a cross-section electron micrograph of a true capillary and how the astrocyte end-feet (ACef) directly abut the basement membrane of the mural endothelial cell(s) (ECs) and pericyte end-feet process (Pcef PcP). (b’) depicts a longitudinal section of the post-capillary venule with prominent PVS. RBC = red blood cell.
Figure 3.
Pial pre-capillary influx arteriole, post-capillary efflux venules, and perivascular spaces (PVS). (
A) illustrates the PVS bounded by the arteriole and venous endothelial/pericyte basement membranes and the pia matter/astrocyte end-feet basal lamina (glia limitans). The arteriole and precapillary PVS are responsible for the influx of cerebrospinal fluid (CSF) from the cerebrospinal fluid (CSF) and subarachnoid space (SAS) to the parenchymal interstitial fluid (ISF) space; some studies have demonstrated a retrograde efflux of ISF and CSF against the flow to the SAS, while the post-capillary venule and veins are responsible for the efflux of the ISF and the admixed CSF, and metabolic waste to the SAS and eventually to the dural venous sinus (DVS) via arachnoid granulation(s) (AG). Importantly, note how the pia matter membrane covering abruptly disappears at the level of the true capillary that is now covered by only the astrocyte end-feet (ACef) (glia limitans) on the outer capillary neurovascular unit. Additionally, note that the pia matter layer is thought to be not present in the post-capillary venules and veins [
2]. (
B) illustrates an arachnoid villus and its AG for the exchange of ISF, CSF, and metabolic waste with the DVS blood and the dural lymphatics (cyan). The metabolic waste is also known to drain along cranial nerve sheaths or through the nasal lymphatic system. (
C) depicts the astrocyte end-feet (ACef) barrier with only a few 20 nm gaps and thus creates the rate-limiting barrier for water and solute exchange. Importantly, the ACef contain the polarized aquaporin-4 (AQP4) water channel, which has also been shown to be important in fluid and solute exchange.
Figure 3.
Pial pre-capillary influx arteriole, post-capillary efflux venules, and perivascular spaces (PVS). (
A) illustrates the PVS bounded by the arteriole and venous endothelial/pericyte basement membranes and the pia matter/astrocyte end-feet basal lamina (glia limitans). The arteriole and precapillary PVS are responsible for the influx of cerebrospinal fluid (CSF) from the cerebrospinal fluid (CSF) and subarachnoid space (SAS) to the parenchymal interstitial fluid (ISF) space; some studies have demonstrated a retrograde efflux of ISF and CSF against the flow to the SAS, while the post-capillary venule and veins are responsible for the efflux of the ISF and the admixed CSF, and metabolic waste to the SAS and eventually to the dural venous sinus (DVS) via arachnoid granulation(s) (AG). Importantly, note how the pia matter membrane covering abruptly disappears at the level of the true capillary that is now covered by only the astrocyte end-feet (ACef) (glia limitans) on the outer capillary neurovascular unit. Additionally, note that the pia matter layer is thought to be not present in the post-capillary venules and veins [
2]. (
B) illustrates an arachnoid villus and its AG for the exchange of ISF, CSF, and metabolic waste with the DVS blood and the dural lymphatics (cyan). The metabolic waste is also known to drain along cranial nerve sheaths or through the nasal lymphatic system. (
C) depicts the astrocyte end-feet (ACef) barrier with only a few 20 nm gaps and thus creates the rate-limiting barrier for water and solute exchange. Importantly, the ACef contain the polarized aquaporin-4 (AQP4) water channel, which has also been shown to be important in fluid and solute exchange.
![Medicina 59 00917 g003]()
Figure 4.
Ultrastructure comparison of perivascular spaces (PVS) and enlarged perivascular spaces (EPVS) utilizing transmission electron microscopy (TEM). (A) demonstrates a true capillary in a control C57B6-7 model. Note how the pseudo-colored golden astrocyte end-feet (ACef) tightly abut the basement membrane (BM) of the neurovascular unit (NVU) brain endothelial cell (BEC or EC) that does not have a PVS. (B) demonstrates a very small nanometer PVS (pseudo-colored green). Note how the pseudo-colored golden ACef do not tightly abut the combined BEC and pericyte (Pc) BM as in the true capillary in (A). However, this nanometer-sized PVS is bounded by the abluminal ACef and pia matter (glia limitans) of this terminal arteriole before it transitions to a true capillary without a PVS. (C) depicts a post-capillary venule with an EPVS varying from 2 to 6.5 μm in diameter in the obese diabetic black and tan brachyuric ob/ob (BTBR ob/ob) transgenic mouse model. (D) depicts EPVS with a 3-micrometer diameter space in the BTBR ob/ob model. Note how the surrounding ACef now abut the EPVS on its most abluminal boundary in (C,D) that are bounded by its innermost BEC and Pc basement membrane. Magnifications and scale bars vary and are present in (A–D). AQP4 = aquaporin-4 water channel; CL = capillary lumen; EC = brain endothelial cell; Pcfp = pericyte foot process or end-feet; Pc N = pericyte nucleus; RBC = red blood cell.
Figure 4.
Ultrastructure comparison of perivascular spaces (PVS) and enlarged perivascular spaces (EPVS) utilizing transmission electron microscopy (TEM). (A) demonstrates a true capillary in a control C57B6-7 model. Note how the pseudo-colored golden astrocyte end-feet (ACef) tightly abut the basement membrane (BM) of the neurovascular unit (NVU) brain endothelial cell (BEC or EC) that does not have a PVS. (B) demonstrates a very small nanometer PVS (pseudo-colored green). Note how the pseudo-colored golden ACef do not tightly abut the combined BEC and pericyte (Pc) BM as in the true capillary in (A). However, this nanometer-sized PVS is bounded by the abluminal ACef and pia matter (glia limitans) of this terminal arteriole before it transitions to a true capillary without a PVS. (C) depicts a post-capillary venule with an EPVS varying from 2 to 6.5 μm in diameter in the obese diabetic black and tan brachyuric ob/ob (BTBR ob/ob) transgenic mouse model. (D) depicts EPVS with a 3-micrometer diameter space in the BTBR ob/ob model. Note how the surrounding ACef now abut the EPVS on its most abluminal boundary in (C,D) that are bounded by its innermost BEC and Pc basement membrane. Magnifications and scale bars vary and are present in (A–D). AQP4 = aquaporin-4 water channel; CL = capillary lumen; EC = brain endothelial cell; Pcfp = pericyte foot process or end-feet; Pc N = pericyte nucleus; RBC = red blood cell.
![Medicina 59 00917 g004]()
Figure 5.
Comparisons between lacunes, enlarged perivascular spaces, and white matter hyperintensities. mm = micrometer.
Figure 5.
Comparisons between lacunes, enlarged perivascular spaces, and white matter hyperintensities. mm = micrometer.
Figure 6.
Magnetic resonance imaging (MRI) comparison of enlarged perivascular spaces (EPVS), white matter hyperintensities (WMH), and lacunes. (A) depicts EPVS localized to the basal ganglia (symmetrical) with yellow color masking of EPVS (EPVS localized to centrum semiovale not shown). (B) depicts WMH localized to the periventricular regions (deep white matter WMH not shown). (C) depicts a T-2-weighted lacune (arrow). (D) depicts a FLAIR image with cyan color masking. Note the encircling white line to suggest hyperintensity FLAIR. V = ventricle.
Figure 6.
Magnetic resonance imaging (MRI) comparison of enlarged perivascular spaces (EPVS), white matter hyperintensities (WMH), and lacunes. (A) depicts EPVS localized to the basal ganglia (symmetrical) with yellow color masking of EPVS (EPVS localized to centrum semiovale not shown). (B) depicts WMH localized to the periventricular regions (deep white matter WMH not shown). (C) depicts a T-2-weighted lacune (arrow). (D) depicts a FLAIR image with cyan color masking. Note the encircling white line to suggest hyperintensity FLAIR. V = ventricle.
Figure 7.
Possible sequence of events that lead to cerebral small vessel disease (SVD) and the formation of enlarged perivascular spaces (EPVS), white matter hyperintensities (WMH), and lacunes. BEC = brain endothelial cell; BEC act/dys = brain endothelial cell activation and dysfunction; BG = basal ganglia; BM = basement membrane; CBF = cerebral blood flow; CSO = centrum semiovale; MGC = microglia cell; MRI = magnetic resonance imaging; NO = nitric oxide; NVU = neurovascular unit; TIA = transient ischemic attack.
Figure 7.
Possible sequence of events that lead to cerebral small vessel disease (SVD) and the formation of enlarged perivascular spaces (EPVS), white matter hyperintensities (WMH), and lacunes. BEC = brain endothelial cell; BEC act/dys = brain endothelial cell activation and dysfunction; BG = basal ganglia; BM = basement membrane; CBF = cerebral blood flow; CSO = centrum semiovale; MGC = microglia cell; MRI = magnetic resonance imaging; NO = nitric oxide; NVU = neurovascular unit; TIA = transient ischemic attack.
Figure 8.
Enlarged perivascular spaces (EPVS) may exist in an evolutionary spectrum over time to result in cerebral small vessel disease (SVD) with neuroinflammation, impaired cognition, and neurodegeneration. ACfp = astrocyte foot processes or end-feet; BBB = blood–brain barrier; CSF = cerebrospinal fluid; ISF = interstitial fluid; LOAD = late-onset Alzheimer’s disease; mm = micrometer; MRI = magnetic resonance imaging; NVU = neurovascular unit; PVS = perivascular spaces; SAS = subarachnoid space; WMH = white matter hyperintensities.
Figure 8.
Enlarged perivascular spaces (EPVS) may exist in an evolutionary spectrum over time to result in cerebral small vessel disease (SVD) with neuroinflammation, impaired cognition, and neurodegeneration. ACfp = astrocyte foot processes or end-feet; BBB = blood–brain barrier; CSF = cerebrospinal fluid; ISF = interstitial fluid; LOAD = late-onset Alzheimer’s disease; mm = micrometer; MRI = magnetic resonance imaging; NVU = neurovascular unit; PVS = perivascular spaces; SAS = subarachnoid space; WMH = white matter hyperintensities.
Figure 9.
Examples of transmission electron microscopic (TEM) images for endothelial cell activation (EC
act). (
A) demonstrates the normal TEM appearance of the brain endothelial cell (BEC). Note the thinness and electron density of this BEC. (
B) depicts the abrupt appearance of thickened regions of electron-lucent areas (red arrows) of EC
act compared to the control model in (
A). (
C) depicts basement membrane (BM) thickening with increased vacuoles (V) and vesicles (v). (
D,
E) depict monocyte (
D) and lymphocyte (
E) (white arrows depict cellular adhesion to activated endothelium), platelet (outlined by yellow dashed lines and yellow arrows), and red blood cell (RBC) adhesion (white arrows) (
F,
G) to the activated ECs. Original magnification = ×2000; scale bar = 1 μm. Modified with permission CC by 4.0 [
6]. Images in (
C–
G) were reproduced and modified with permission by CC 4.0 [
19].
ACfp =
astrocyte foot processes;
Cl,
capillary lumen;
EC =
brain endothelial cells;
ECact =
endothelial cell activation;
MP =
microparticle of the platelet.
Figure 9.
Examples of transmission electron microscopic (TEM) images for endothelial cell activation (EC
act). (
A) demonstrates the normal TEM appearance of the brain endothelial cell (BEC). Note the thinness and electron density of this BEC. (
B) depicts the abrupt appearance of thickened regions of electron-lucent areas (red arrows) of EC
act compared to the control model in (
A). (
C) depicts basement membrane (BM) thickening with increased vacuoles (V) and vesicles (v). (
D,
E) depict monocyte (
D) and lymphocyte (
E) (white arrows depict cellular adhesion to activated endothelium), platelet (outlined by yellow dashed lines and yellow arrows), and red blood cell (RBC) adhesion (white arrows) (
F,
G) to the activated ECs. Original magnification = ×2000; scale bar = 1 μm. Modified with permission CC by 4.0 [
6]. Images in (
C–
G) were reproduced and modified with permission by CC 4.0 [
19].
ACfp =
astrocyte foot processes;
Cl,
capillary lumen;
EC =
brain endothelial cells;
ECact =
endothelial cell activation;
MP =
microparticle of the platelet.
![Medicina 59 00917 g009]()
Figure 10.
Summary of the observational transmission electron microscopic (TEM) remodeling changes in activated brain endothelial cells in obesity, metabolic syndrome, type 2 diabetes mellitus, and hypertensive rodent models. MtROS = mitochondria reactive oxygen species.
Figure 10.
Summary of the observational transmission electron microscopic (TEM) remodeling changes in activated brain endothelial cells in obesity, metabolic syndrome, type 2 diabetes mellitus, and hypertensive rodent models. MtROS = mitochondria reactive oxygen species.
Figure 11.
Brain endothelial cell activation, attenuation and/or loss of paracellular tight and adherens junctions, and increased transcytosis may each contribute to enlarged perivascular spaces. (
A,
C,
E) demonstrate the appearance of normal BECs found in control preclinical models. Note the yellow dashed line that parallels the elongated tight and adherens junctions (TJ/AJ) in the control model (
C). (
B,
D,
F) images depict activation of brain endothelial cells (BECs) as compared to their respective control model images in (
A,
C,
E). (
B) depicts abrupt swelling and hyperluceny of BECs; (
D) depicts the disruption, attenuation, and loss of TJ/AJs; and (
F) depicts increased transcytotic micro- and macropinocytotic vesicles. Each of these aberrant BECs contributes to increased permeability through different mechanisms, i.e., increased permeability via paracellular and transcytotic routes. (
A,
B) were provided with permission by CC 4.0 [
6]. (
C,
D) original images from streptozotocin-induced diabetes in CD-1 male mice at 16 weeks of age. (
E,
F) were provided with permission by CC 4.0 [
36].
ACfp or ACef =
astrocyte foot process or end-feet;
BM =
basement membrane;
CL =
capillary lumen;
EC =
brain endothelial cell(s);
LPS =
lipopolysaccharide;
Pcfp =
pericyte foot processes;
RBC =
red blood cell.
Figure 11.
Brain endothelial cell activation, attenuation and/or loss of paracellular tight and adherens junctions, and increased transcytosis may each contribute to enlarged perivascular spaces. (
A,
C,
E) demonstrate the appearance of normal BECs found in control preclinical models. Note the yellow dashed line that parallels the elongated tight and adherens junctions (TJ/AJ) in the control model (
C). (
B,
D,
F) images depict activation of brain endothelial cells (BECs) as compared to their respective control model images in (
A,
C,
E). (
B) depicts abrupt swelling and hyperluceny of BECs; (
D) depicts the disruption, attenuation, and loss of TJ/AJs; and (
F) depicts increased transcytotic micro- and macropinocytotic vesicles. Each of these aberrant BECs contributes to increased permeability through different mechanisms, i.e., increased permeability via paracellular and transcytotic routes. (
A,
B) were provided with permission by CC 4.0 [
6]. (
C,
D) original images from streptozotocin-induced diabetes in CD-1 male mice at 16 weeks of age. (
E,
F) were provided with permission by CC 4.0 [
36].
ACfp or ACef =
astrocyte foot process or end-feet;
BM =
basement membrane;
CL =
capillary lumen;
EC =
brain endothelial cell(s);
LPS =
lipopolysaccharide;
Pcfp =
pericyte foot processes;
RBC =
red blood cell.
![Medicina 59 00917 g011]()
Figure 12.
Transmission electron microscopic images of lanthanum nitrate staining of the endothelial glycocalyx (ecGCx) and an illustration of the (ecGCx) as it occurs in the brain neurovascular unit. (
A) demonstrates the normal lanthanum nitrate (LAN) perfusion fixation staining of the endothelial glycocalyx (ecGCx) (white arrows) in a post-capillary higher-order venule with a single lining of vascular smooth muscle cells (VSMC). This magnified image demonstrates the normal LAN positive staining of the ecGCx in a healthy control male CD-1 mouse brain from the frontal cortical gray matter layer III with the original intact scale bar = 2 μm and yellow scale bar = 1 μm with a 500 nm to 1.5 μm thickness of the ecGCx. Note the boxed-in insert in the lower right-hand corner with a white outline, which is the original image from which the magnified image in (
A) is derived. (
B) illustrates another representative image in a true capillary without a PVS that is positive for LAN staining of the ecGCx from cortical layer III frontal gray matter. Note the intense electron-dense staining of lanthanum nitrate of the apical brain endothelial cell(s) (BEC) of the ecGCx such that the structural content of the ecGCx cannot be visualized in either (
A) or (
B); however, the components of the ecGCx will be illustrated in (
C). (
C) illustrates the various proteoglycans (PGNs) (purple), glycoproteins (GPs) (green), hyaluronan (HA) (blue), glycosaminoglycans (GAGs) (purple and green triangles), and their sulfation sites (red circles). Note the red boxed-in region on the upper-right-hand side of this image that lists the numerous toxicities capable of causing ecGCx dysfunction, attenuation, and/or shedding. This modified and adapted image was provided with permission by CC 4.0 [
6,
42].
A =
albumin;
AGE/RAGE =
advanced glycation end; BM =
basement membrane;
CAD =
cadherin;
CAM =
cellular adhesion molecule;
CD44 =
cluster of differentiation 44;
EC =
endothelial cell(s);
ecSOD =
extracellular superoxide dismutase;
F =
fibrinogen;
FGF2 =
fibroblast Growth Factor 2;
FOCM =
folate-mediated one-carbon metabolism;
GCx =
glycocalyx;
ICAM-1 =
intercellular adhesion molecule;
Ox LDL =
oxidized low-density lipoprotein;
LPL =
lipoprotein lipase;
MMPs =
matrix metalloproteinases;
N =
nucleus;
Na+ =
sodium;
O =
orosomucoids;
Pc =
vascular mural cell pericyte(s);
PECAM-1 =
platelet endothelial cell adhesion molecule-1;
RONS =
reactive oxygen species;
VEC =
vascular endothelial cell(s);
TFPI =
tissue factor pathway inhibitor;
TJ/AJ =
tight and adherens junctions;
VCAM =
vascular cell adhesion protein;
VE CAD =
vascular endothelial cadherins;
VEGF =
vascular endothelial growth factor;
XOR =
xanthine oxioreductase.
Figure 12.
Transmission electron microscopic images of lanthanum nitrate staining of the endothelial glycocalyx (ecGCx) and an illustration of the (ecGCx) as it occurs in the brain neurovascular unit. (
A) demonstrates the normal lanthanum nitrate (LAN) perfusion fixation staining of the endothelial glycocalyx (ecGCx) (white arrows) in a post-capillary higher-order venule with a single lining of vascular smooth muscle cells (VSMC). This magnified image demonstrates the normal LAN positive staining of the ecGCx in a healthy control male CD-1 mouse brain from the frontal cortical gray matter layer III with the original intact scale bar = 2 μm and yellow scale bar = 1 μm with a 500 nm to 1.5 μm thickness of the ecGCx. Note the boxed-in insert in the lower right-hand corner with a white outline, which is the original image from which the magnified image in (
A) is derived. (
B) illustrates another representative image in a true capillary without a PVS that is positive for LAN staining of the ecGCx from cortical layer III frontal gray matter. Note the intense electron-dense staining of lanthanum nitrate of the apical brain endothelial cell(s) (BEC) of the ecGCx such that the structural content of the ecGCx cannot be visualized in either (
A) or (
B); however, the components of the ecGCx will be illustrated in (
C). (
C) illustrates the various proteoglycans (PGNs) (purple), glycoproteins (GPs) (green), hyaluronan (HA) (blue), glycosaminoglycans (GAGs) (purple and green triangles), and their sulfation sites (red circles). Note the red boxed-in region on the upper-right-hand side of this image that lists the numerous toxicities capable of causing ecGCx dysfunction, attenuation, and/or shedding. This modified and adapted image was provided with permission by CC 4.0 [
6,
42].
A =
albumin;
AGE/RAGE =
advanced glycation end; BM =
basement membrane;
CAD =
cadherin;
CAM =
cellular adhesion molecule;
CD44 =
cluster of differentiation 44;
EC =
endothelial cell(s);
ecSOD =
extracellular superoxide dismutase;
F =
fibrinogen;
FGF2 =
fibroblast Growth Factor 2;
FOCM =
folate-mediated one-carbon metabolism;
GCx =
glycocalyx;
ICAM-1 =
intercellular adhesion molecule;
Ox LDL =
oxidized low-density lipoprotein;
LPL =
lipoprotein lipase;
MMPs =
matrix metalloproteinases;
N =
nucleus;
Na+ =
sodium;
O =
orosomucoids;
Pc =
vascular mural cell pericyte(s);
PECAM-1 =
platelet endothelial cell adhesion molecule-1;
RONS =
reactive oxygen species;
VEC =
vascular endothelial cell(s);
TFPI =
tissue factor pathway inhibitor;
TJ/AJ =
tight and adherens junctions;
VCAM =
vascular cell adhesion protein;
VE CAD =
vascular endothelial cadherins;
VEGF =
vascular endothelial growth factor;
XOR =
xanthine oxioreductase.
![Medicina 59 00917 g012]()
Figure 13.
This image illustrates a bidirectional relationship between brain endothelial cell activation/dysfunction (BECact/dys), aberrant mitochondria (aMt) and dysfunction, attenuation, and/or shedding of the brain endothelial glycocalyx (BEC ecGCx). BECact/dys further illustrates the role of BEC oxidative stress and reactive oxygen species (ROS) and specifically mitochondrial ROS (mtROS) (left-hand box). This schematic also shows that obesity, insulin resistance (IR), leptin resistance (LR), impaired fasting glucose (IFG), impaired glucose tolerance (IGT), lipotoxicity (including modified low-density lipoprotein-cholesterol (mod-LDL-C)), and overt type 2 diabetes mellitus (T2DM) are related to increased Mt fission, decreased mitophagy, and the subsequent accumulation of leaky aMt that leak mtROS (right-hand box). Leaky aMt may be responsible for the attenuation and/or loss of the ecGCx via ROS-activated matrix metalloproteinases (red-dashed arrows) and in turn, may result in the loss of the ecGCx that may contribute to an increase in aMt (black-dashed arrows). Moreover, mtROS (superoxide or hydrogen peroxide (H2O2)) could oxidize the essential fully reduced and essential tetrahydrobiopterin (BH4) cofactor to oxidized biopterin (BH3 and BH2) that will not enable eNOS to synthesize nitric oxide (NO) and results in eNOS uncoupling. eNOS uncoupling results in decreased bioavailable NO, as occurs in BECact/dys. Importantly, the depicted bidirectional interaction could result in a vicious cycle. This vicious cycle results in blood–brain barrier (BBB) disruption with increased neurovascular unit (NVU) BEC permeability that would support the entry of excess fluid into the PVS, which could result in an EPVS. This vicious cycle could be interrupted by either preventing the accumulation of aMt (improved mitophagy) or preventing the dysfunction, attenuation, and/or loss (shedding) of the ecGCx. BH3 and BH2 = oxidized biopterin; NADPH Ox = nicotinamide adenine dinucleotide phosphate reduced oxidase.
Figure 13.
This image illustrates a bidirectional relationship between brain endothelial cell activation/dysfunction (BECact/dys), aberrant mitochondria (aMt) and dysfunction, attenuation, and/or shedding of the brain endothelial glycocalyx (BEC ecGCx). BECact/dys further illustrates the role of BEC oxidative stress and reactive oxygen species (ROS) and specifically mitochondrial ROS (mtROS) (left-hand box). This schematic also shows that obesity, insulin resistance (IR), leptin resistance (LR), impaired fasting glucose (IFG), impaired glucose tolerance (IGT), lipotoxicity (including modified low-density lipoprotein-cholesterol (mod-LDL-C)), and overt type 2 diabetes mellitus (T2DM) are related to increased Mt fission, decreased mitophagy, and the subsequent accumulation of leaky aMt that leak mtROS (right-hand box). Leaky aMt may be responsible for the attenuation and/or loss of the ecGCx via ROS-activated matrix metalloproteinases (red-dashed arrows) and in turn, may result in the loss of the ecGCx that may contribute to an increase in aMt (black-dashed arrows). Moreover, mtROS (superoxide or hydrogen peroxide (H2O2)) could oxidize the essential fully reduced and essential tetrahydrobiopterin (BH4) cofactor to oxidized biopterin (BH3 and BH2) that will not enable eNOS to synthesize nitric oxide (NO) and results in eNOS uncoupling. eNOS uncoupling results in decreased bioavailable NO, as occurs in BECact/dys. Importantly, the depicted bidirectional interaction could result in a vicious cycle. This vicious cycle results in blood–brain barrier (BBB) disruption with increased neurovascular unit (NVU) BEC permeability that would support the entry of excess fluid into the PVS, which could result in an EPVS. This vicious cycle could be interrupted by either preventing the accumulation of aMt (improved mitophagy) or preventing the dysfunction, attenuation, and/or loss (shedding) of the ecGCx. BH3 and BH2 = oxidized biopterin; NADPH Ox = nicotinamide adenine dinucleotide phosphate reduced oxidase.
![Medicina 59 00917 g013]()
Figure 14.
Metabolic syndrome (MetS), enlarged perivascular spaces (EPVS), and cerebral small vessel disease (SVD). The central X has four arms consisting of hyperlipidemia (lower left), hyperinsulinemia of insulin resistance (IR) (lower right), essential hypertension (upper right), and hyperglycemia (upper left). It is currently known that enlarged PVS (EPVS) are a biomarker of SVD. Visceral adipose tissue (VAT), increased triglyceride/glucose index (TG index), and hypertension are known to associate with SVD. Each of these four arms is either directly or indirectly associated with EPVS and SVD. Importantly, note that the triad of obesity, MetS, and decreased bioavailable nitric oxide (NO) are associated with capillary rarefaction and EPVS are known to be biomarkers of SVD. AGE = advanced glycation end-products; RAGE = receptor for AGE; AGE/RAGE = advanced glycation end-products and its receptor interaction; BEC act/dys = brain endothelial cell activation and dysfunction; eNOS = endothelial nitric oxide synthase; FFA = free fatty acids–unsaturated long chain fatty acids; IGT = impaired glucose tolerance; LOAD = late-onset Alzheimer’s disease; O2 •− = superoxide; ROS = reactive oxygen species; RSI = reactive species interactome; Sk = skeletal; T2DM = type 2 diabetes mellitus; TG Index = triglyceride/glucose index; TIA = transient ischemia attack; VAT = visceral adipose tissue.
Figure 14.
Metabolic syndrome (MetS), enlarged perivascular spaces (EPVS), and cerebral small vessel disease (SVD). The central X has four arms consisting of hyperlipidemia (lower left), hyperinsulinemia of insulin resistance (IR) (lower right), essential hypertension (upper right), and hyperglycemia (upper left). It is currently known that enlarged PVS (EPVS) are a biomarker of SVD. Visceral adipose tissue (VAT), increased triglyceride/glucose index (TG index), and hypertension are known to associate with SVD. Each of these four arms is either directly or indirectly associated with EPVS and SVD. Importantly, note that the triad of obesity, MetS, and decreased bioavailable nitric oxide (NO) are associated with capillary rarefaction and EPVS are known to be biomarkers of SVD. AGE = advanced glycation end-products; RAGE = receptor for AGE; AGE/RAGE = advanced glycation end-products and its receptor interaction; BEC act/dys = brain endothelial cell activation and dysfunction; eNOS = endothelial nitric oxide synthase; FFA = free fatty acids–unsaturated long chain fatty acids; IGT = impaired glucose tolerance; LOAD = late-onset Alzheimer’s disease; O2 •− = superoxide; ROS = reactive oxygen species; RSI = reactive species interactome; Sk = skeletal; T2DM = type 2 diabetes mellitus; TG Index = triglyceride/glucose index; TIA = transient ischemia attack; VAT = visceral adipose tissue.
![Medicina 59 00917 g014]()
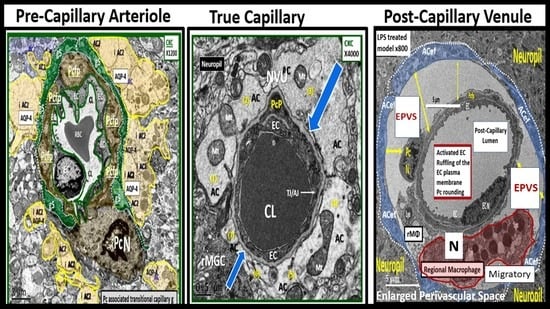
Figure 15.
Obesity, metabolic syndrome (MetS), and enlarged perivascular spaces (EPVS) may be associated with brain capillary rarefaction. (A) demonstrates the normal precapillary arteriole (left), a true capillary (center), and a post-capillary venule (right) with their associated normal perivascular spaces (PVS). It is important to note that the pia matter lining abruptly stops at the true capillary and that the astrocyte end-feet directly abut the basement membrane (BM) of the neurovascular unit (NVU) mural cells (endothelial cell(s) (EC) and pericyte end-feet (Pcef)). (B) depicts the associated aberrant remodeling changes associated with the increased capillary rarefaction that is associated with obesity and MetS by utilizing a semi-transparent masking process. Note that as capillary rarefaction (capillary loss) is completed, there is a loss of the central capillary that runs through the PVS and how the PVS undergoes volume expansion due to the PVS now becoming only a fluid-filled space (light blue) similar to the PVS in (A), but now without a capillary running through the PVS. Additionally, note that capillary rarefaction also includes the NVU true capillary and how this also assists in the formation of EPVS when there is blood–brain barrier disruption. This depiction of how capillary rarefaction may be one of the multiple causes of EPVS fits nicely with the other causes that are thought to be important for the development of EPV, which include PVS obstruction, decreased pial artery, and arteriole pulsatility due to vascular stiffening and cerebral atrophy. Interestingly, the capillary rarefaction would contribute to regional ischemia and subsequent cerebral atrophy. ACef = astrocyte end-feet; CL = capillary lumen; EC = endothelial cell; Pcef = pericyte end-feet; RBC = red blood cell.
Figure 15.
Obesity, metabolic syndrome (MetS), and enlarged perivascular spaces (EPVS) may be associated with brain capillary rarefaction. (A) demonstrates the normal precapillary arteriole (left), a true capillary (center), and a post-capillary venule (right) with their associated normal perivascular spaces (PVS). It is important to note that the pia matter lining abruptly stops at the true capillary and that the astrocyte end-feet directly abut the basement membrane (BM) of the neurovascular unit (NVU) mural cells (endothelial cell(s) (EC) and pericyte end-feet (Pcef)). (B) depicts the associated aberrant remodeling changes associated with the increased capillary rarefaction that is associated with obesity and MetS by utilizing a semi-transparent masking process. Note that as capillary rarefaction (capillary loss) is completed, there is a loss of the central capillary that runs through the PVS and how the PVS undergoes volume expansion due to the PVS now becoming only a fluid-filled space (light blue) similar to the PVS in (A), but now without a capillary running through the PVS. Additionally, note that capillary rarefaction also includes the NVU true capillary and how this also assists in the formation of EPVS when there is blood–brain barrier disruption. This depiction of how capillary rarefaction may be one of the multiple causes of EPVS fits nicely with the other causes that are thought to be important for the development of EPV, which include PVS obstruction, decreased pial artery, and arteriole pulsatility due to vascular stiffening and cerebral atrophy. Interestingly, the capillary rarefaction would contribute to regional ischemia and subsequent cerebral atrophy. ACef = astrocyte end-feet; CL = capillary lumen; EC = endothelial cell; Pcef = pericyte end-feet; RBC = red blood cell.
![Medicina 59 00917 g015]()
Figure 16.
Eight core reasons why perivascular spaces are important.
Figure 16.
Eight core reasons why perivascular spaces are important.