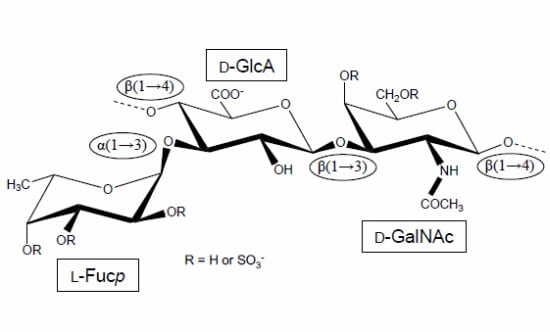
Holothurian Fucosylated Chondroitin Sulfate
Abstract
:1. The First Reports Were Mostly Concerned with the Structure and Physicochemical Properties

2. Medical Effects
2.1. Anticoagulation and Antithrombosis: The First and Predominantly Studied Clinical Actions
| Species | Fuc0S | Fuc3S | Fuc4S | Fuc2S4S | Fuc3S4S | aPTT | References |
|---|---|---|---|---|---|---|---|
| Ludwigothurea griseaa | 0 | − | ~49 | ~20 | ~17 | 55 b | [5,14] |
| Pearsonothuria graeffei | − | − | 81.6 | 18.4 | − | 35 c | [20] |
| Holothuria vagabunda | 25.6 | − | 50.2 | 15.8 | 8.4 | 42 c | [20] |
| Stichopus tremulus | − | − | 24.8 | 22.4 | 52.8 | 135 c | [20] |
| Isostichopus badionotus | − | − | 4.1 | 95.9 | − | 183 c | [20] |
| Thelenata ananas | 0 | ~25 | ~22 | ~53 | 0 | 348 d | [21,22] |
| Stichopus japonicuse | 0 | Nd f | 11.1 | 55.6 | 33.3 | Ns g | [23] |
| Holothuria edulish | − | − | Nd | 18 | Nd | 89 i | [24] |
| Apostichopus japonicash | − | − | Nd | 45 | Nd | 116 i | [24] |
| Holothuria nobilisj | − | Nd | Nd | − | Nd | 59 i | [24] |
| Acaudina molpadioideak | − | − | − | − | − | Nc l | [25] |
| Athyonidium chilensisk | − | − | − | − | − | Nc l | [26] |
2.2. Hemodialysis
2.3. Atherosclerosis
2.4. Cellular Growth, Angiogenesis and Fibrosis
2.5. Tumor Metastasis and Inflammation
2.6. Viral Infection
2.7. Hyperglycemia
3. Chemical Modifications and Synthesis
4. Major Conclusions and Perspectives
| Biological Systems | Mechanisms of Action | Structural Requirement | Method | Reference |
|---|---|---|---|---|
| Coagulation/Thrombosis | Serpin-dependent action: FucCS potentiated the inhibition activity of blood cofactor antithrombin and heparin cofactor II over thrombin and factor Xa | Branch 2,4-O-di-sulfated Fucp unit | In vitro TCT, aPTT, and tests using purified blood cofactors through chromogenic substrates. | [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] |
| In vivo arterial and venous thrombotic models using mice and rats | ||||
| Serpin-independent activity: FucCS inhibits formation of the intrinsic tenase complex besides interfering in the activity of factors VIII and V | Fucosyl branch units. The best sulfation pattern is still unknown | In vitro inhibitory assays using blood cofactors | ||
| Hemodialysis | Anticoagulant activity | Fucosyl branch units since mammalian unfucosylated CS has no action in hemodialysis. The best sulfation pattern of FucCS branch units is still unknown | In vivo anticoagulant experimental beagle-dog method using a hollow-fiber dialyzer | [39] |
| In vivo dog model of renal failure | [40] | |||
| Atherosclerosis | Interaction with lipoproteins | Fucosyl branch units. The best sulfation pattern is still unknown | Affinity liquid-chromatography | [41] |
| Inhibitory activity over neointimal formation | In vivo balloon-injured rat carotid artery experimental model | [42] | ||
| Cellular growth | FucCS exhibits stimulating effects on vascular SMC proliferation and endothelial cell proliferation, migration | Fucosyl branch units. The best sulfation pattern is still unknown | In vivo assays using SMC from rat thoracic aorta and HUVEC in culture with or without added fibroblast growth factors (FGF-1 and FGF-2) | [43] |
| Angiogenesis | FucCS accelerates angiogenesis by interactions with FGF-2 | Fucosyl branch units. The best sulfation pattern is still unknown | In vitro experiments for tubulogenesis using endothelial cells in Matrigel | [44] |
| Fibrosis | Inhibition of fibrosis via P-selectin-mediated mechanism | Fucosyl branch units. The best sulfation pattern is still unknown | In vivo renal fibrosis models of animals submitted to unilateral ureteral obstruction. Biochemical and histological analyses | [45] |
| Inflammation | Inhibitory activities over P- and L-selectins | Fucosyl branch units. The best sulfation pattern is still unknown | In vitro experiments using P- and L-selectin binding to immobilized sialyl Lewis(×) | [46] |
| Cancer metastasis | Inhibitory effects on selectin-mediated cancer metastasis | Fucosyl branch units. The best sulfation pattern is still unknown | LS180 carcinoma cell attachment to immobilized P- and L-selectins | [46] |
| Virus infection | FucCS binds to gp120 protein of HIV particles | Not assigned | In vitro cytopathic effect assay and a HIV-1 p24 detection assay (biolayer interferometry technology) | [47] |
| In vitro inhibitory assays to verify blocking potential of FucCS on entry and replication of HIV strains | [48] | |||
| Hyperglycemia | FucCS enhances insulin-stimulated GLUT4 translocation and phosphorylation of Tyr-IR-β, Tyr612-IRS-1, p85-PI3K, Ser473-PKB, and Thr308-PKB | Fucosyl branch units. The best sulfation pattern is still unknown | In vivo experiments using skeletal muscle from insulin-resistant mice | [49] |
Acknowledgements
Conflicts of Interest
References
- Vieira, R.P.; Mourão, P.A. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. J. Biol. Chem. 1988, 263, 18176–18183. [Google Scholar]
- Vieira, R.P.; Mulloy, B.; Mourão, P.A. Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-beta-d-glucuronosyl residues. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar]
- Vieira, R.P.; Pedrosa, C.; Mourão, P.A. Extensive heterogeneity of proteoglycans bearing fucose-branched chondroitin sulfate extracted from the connective tissue of sea cucumber. Biochemistry 1993, 32, 2254–2262. [Google Scholar] [CrossRef]
- Ruggiero, J.; Vieira, R.P.; Mourão, P.A. Increased calcium affinity of a fucosylated chondroitin sulfate from sea cucumber. Carbohydr. Res. 1994, 256, 275–287. [Google Scholar] [CrossRef]
- Mourão, P.A.; Pereira, M.S.; Pavão, M.S.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.C.; Abildgaard, U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef]
- Mourão, P.A.; Guimarães, B.; Mulloy, B.; Thomas, S.; Gray, E. Antithrombotic activity of a fucosylated chondroitin sulphate from echinoderm: Sulphated fucose branches on the polysaccharide account for its antithrombotic action. Br. J. Haematol. 1998, 101, 647–652. [Google Scholar] [CrossRef]
- Pacheco, R.G.; Vicente, C.P.; Zancan, P.; Mourão, P.A. Different antithrombotic mechanisms among glycosaminoglycans revealed with a new fucosylated chondroitin sulfate from an echinoderm. Blood Coagul. Fibrinol. 2000, 11, 563–573. [Google Scholar] [CrossRef]
- Mourão, P.A.; Pereira, M.S. Searching for alternatives to heparin: sulfated fucans from marine invertebrates. Trends Cardiovasc. Med. 1999, 9, 225–232. [Google Scholar] [CrossRef]
- Mourão, P.A.; Boisson-Vidal, C.; Tapon-Bretaudière, J.; Drouet, B.; Bros, A.; Fischer, A. Inactivation of thrombin by a fucosylated chondroitin sulfate from echinoderm. Thromb. Res. 2001, 102, 167–176. [Google Scholar] [CrossRef]
- Mourão, P.A. Use of sulfated fucans as anticoagulant and antithrombotic agents: Future perspectives. Curr. Pharm. Des. 2004, 10, 967–981. [Google Scholar] [CrossRef]
- Zancan, P.; Mourão, P.A. Venous and arterial thrombosis in rat models: dissociation of the antithrombotic effects of glycosaminoglycans. Blood Coagul. Fibrinol. 2004, 15, 45–54. [Google Scholar] [CrossRef]
- Fonseca, R.J.; Mourão, P.A. Fucosylated chondroitin sulfate as a new oral antithrombotic agent. Thromb. Haemost. 2006, 96, 822–829. [Google Scholar]
- Buyue, Y.; Sheehan, J.P. Fucosylated chondroitin sulfate inhibits plasma thrombin generation via targeting of the factor IXa heparin-binding exosite. Blood 2009, 114, 3092–3100. [Google Scholar]
- Fonseca, R.J.; Santos, G.R.; Mourão, P.A. Effects of polysaccharides enriched in 2,4-disulfated fucose units on coagulation, thrombosis and bleeding. Practical and conceptual implications. Thromb. Haemost. 2009, 102, 829–836. [Google Scholar]
- Fonseca, R.J.; Oliveira, S.N.; Pomin, V.H.; Mecawi, A.S.; Araujo, I.G.; Mourão, P.A. Effects of oversulfated and fucosylated chondroitin sulfates on coagulation. Challenges for the study of anticoagulant polysaccharides. Thromb. Haemost. 2010, 103, 994–1004. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Wu, N.; Guo, X.; Liao, N.; Ye, X.; Liu, D.; Xue, C.; Chai, W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. Biochim. Biophys. Acta 2013, 1830, 3054–3066. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, D.; Wang, S.; Tao, L.; Wang, A.; Chen, W.; Zhu, Z.; Zheng, S.; Gao, X.; Lu, Y. Holothurian glycosaminoglycan inhibits metastasis and thrombosis via targeting of nuclear factor-κB/tissue factor/Factor Xa pathway in melanoma B16F10 cells. PLoS One 2013, 8, e56557. [Google Scholar]
- Imanari, T.; Washio, Y.; Huang, Y.; Toyoda, H.; Suzuki, A.; Toida, T. Oral absorption and clearance of partially depolymerized fucosyl chondroitin sulfate from sea cucumber. Thromb. Res. 1999, 93, 129–135. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Ushio, T.; Haginaka, J. Chiral resolution of basic drugs by capillary electrophoresis with new glycosaminoglycans. J. Chromatogr. A 1999, 864, 163–171. [Google Scholar] [CrossRef]
- Chen, S.; Xue, C.; Yin, L.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Wu, M.; Huang, R.; Wen, D.; Gao, N.; He, J.; Li, Z.; Zhao, J. Structure and effect of sulfated fucose branches on anticoagulant activity of the fucosylated chondroitin sulfate from sea cucumber Thelenata ananas. Carbohydr. Polym. 2012, 87, 862–868. [Google Scholar] [CrossRef]
- Wu, M.; Xu, S.; Zhao, J.; Kang, J.; Ding, H. Physicochemical characteristics and anticoagulant activities of low molecular weight fractions by free-radical depolymerization of a fucosylated chondroitin sulphate from sea cucumber Thelenata ananas. Food Chem. 2010, 122, 716–723. [Google Scholar] [CrossRef]
- Yoshida, H.-I.; Minami, Y. Structure of DHG, a depolymerized holothurian glycosaminoglycan from sea cucumber, Stichopus japonicus. Tetrahedron lett. 1992, 33, 4959–4962. [Google Scholar] [CrossRef]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef]
- Ye, L.; Xu, L.; Li, J. Preparation and anticoagulant activity of a fucosylated polysaccharide sulfate from a sea cucumber Acaudina molpadioidea. Carbohydr. Polym. 2012, 87, 2052–2057. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Osorio-Román, I.O.; Torres, R. Vibrational spectrsocopy characterization and anticoagulant activity of a sulfated polysaccharide from sea cucumber Athyonidium chilensis. Carbohydr. Polym. 2012, 88, 959–965. [Google Scholar] [CrossRef]
- Nagase, H.; Enjyoji, K.-I.; Minamiguchi, K.; Kitazato, K.T.; Kitazato, K.; Saito, H.; Kato, H. Depolymerized holothurian glycosaminoglycan with novel anticoagulant actions: antithrombin III- and heparin cofactor II-independent inhibition of factor X activation by factor IXa-factor VIIIa complex and heparin cofactor II-dependent inhibition of thrombin. Blood 1995, 85, 1527–1534. [Google Scholar]
- Glauser, B.F.; Pereira, M.S.; Monteiro, R.Q.; Mourão, P.A. Serpin-independent anticoagulant activity of a fucosylated chondroitin sulfate. Thromb. Haemost. 2008, 100, 420–428. [Google Scholar]
- Nagase, H.; Kitazato, K.T.; Sasaki, E.; Hattori, M.; Kitazato, K.; Saito, H. Antithrombin III-independent effect of depolymerized holothurian glycosaminoglycan (DHG) on acute thromboembolism in mice. Thromb. Haemost. 1997, 77, 399–402. [Google Scholar]
- Sheehan, J.P.; Walke, E.N. Depolymerized holothurian glycosaminoglycan and heparin inhibit the intrinsic tenase complex by a common antithrombin-independent mechanism. Blood 2006, 107, 3876–3882. [Google Scholar] [CrossRef]
- Nagase, H.; Enjyoji, K.; Shima, M.; Kitazato, K.; Yoshioka, A.; Saito, H.; Kato, H. Effect of depolymerized holothurian glycosaminoglycan (DHG) on the activation of factor VIII and factor V by thrombin. J. Biochem. 1996, 119, 63–69. [Google Scholar] [CrossRef]
- Kitazato, K.; Kitazato, K.T.; Nagase, H.; Minamiguchi, K. DHG, a new depolymerized holothurian glycosaminoglycan, exerts an antithrombotic effect with less bleeding than unfractionated or low molecular weight heparin, in rats. Thromb. Res. 1996, 84, 111–120. [Google Scholar] [CrossRef]
- Minamiguchi, K.; Nagase, H.; Kitazato, K.T.; Kitazato, K. Interaction of a new depolymerized holothurian glycosaminoglycan with proteins in human plasma. Thromb. Res. 1996, 83, 253–264. [Google Scholar] [CrossRef]
- Kitazato, K.; Kitazato, K.T.; Sasaki, E.; Minamiguchi, K.; Nagase, H. Prolonged bleeding time induced by anticoagulant glycosaminoglycans in dogs is associated with the inhibition of thrombin-induced platelet aggregation. Thromb. Res. 2003, 112, 83–91. [Google Scholar] [CrossRef]
- Sasaki, E.; Minamiguchi, K.; Kitazato, K.T.; Nagase, H.; Kitazato, K. Neutralization of DHG, a new depolymerized holothurian glycosaminoglycan, by protamine sulfate and platelet factor 4. Haemostasis 1997, 27, 174–183. [Google Scholar]
- Huang, Y.; Toyoda, H.; Koshiishi, I.; Toida, T.; Imanari, T. Determination of a depolymerized holothurian glycosaminoglycan in plasma after intravenous administration by postcolumn HPLC. Chem. Pharm. Bull. 1995, 43, 2182–2186. [Google Scholar] [CrossRef]
- Nagase, H.; Enjyoji, K.; Kamikubo, Y.; Kitazato, K.T.; Kitazato, K.; Saito, H.; Kato, H. Effect of depolymerized holothurian glycosaminoglycan (DHG) on tissue factor pathway inhibitor: In vitro and in vivo studies. Thromb. Haemost. 1997, 78, 864–870. [Google Scholar]
- Zhao, L.; Lai, S.; Huang, R.; Wu, M.; Gao, N.; Xu, L.; Qin, H.; Peng, W.; Zhao, J. Structure and anticoagulant activity of fucosylated glycosaminoglycan degraded by deaminative cleavage. Carbohydr. Polym. 2013, 98, 1514–1523. [Google Scholar] [CrossRef]
- Minamiguchi, K.; Kitazato, K.T.; Sasaki, E.; Nagase, H.; Kitazato, K. The anticoagulant and hemorrhagic effects of DHG, a new depolymerized holothurian glycosaminoglycan, on experimental hemodialysis in dogs. Thromb. Haemost. 1997, 77, 1148–1153. [Google Scholar]
- Minamiguchi, K.; Kitazato, K.T.; Nagase, H.; Sasaki, E.; Ohwada, K.; Kitazato, K. Depolymerized holothurian glycosaminoglycan (DHG), a novel alternative anticoagulant for hemodialysis, is safe and effective in a dog renal failure model. Kidney Intern. 2003, 63, 1548–1555. [Google Scholar] [CrossRef]
- Tovar, A.M.; Mourão, P.A. High affinity of a fucosylated chondroitin sulfate for plasma low density lipoprotein. Atherosclerosis 1996, 26, 185–195. [Google Scholar] [CrossRef]
- Igarashi, M.; Takeda, Y.; Mori, S.; Takahashi, K.; Fuse, T.; Yamamura, M.; Saito, Y. Depolymerized holothurian glycosaminoglycan (DHG) prevents neointimal formation in balloon-injured rat carotid artery. Atherosclerosis 1997, 129, 27–31. [Google Scholar] [CrossRef]
- Tapon-Bretaudière, J.; Drouet, B.; Matou, S.; Mourão, P.A.; Bros, A.; Letourneur, D.; Fischer, A.M. Modulation of vascular human endothelial and rat smooth muscle cell growth by a fucosylated chondroitin sulfate from echinoderm. Thromb. Haemost. 2000, 84, 332–337. [Google Scholar]
- Tapon-Bretaudière, J.; Chabut, D.; Zierer, M.; Matou, S.; Helley, D.; Bros, A.; Mourão, P.A.; Fischer, A.M. A fucosylated chondroitin sulfate from echinoderm modulates in vitro fibroblast growth factor 2-dependent angiogenesis. Mol. Cancer Res. 2002, 1, 96–102. [Google Scholar]
- Melo-Filho, N.M.; Belmiro, C.L.; Gonçalves, R.G.; Takiya, C.M.; Leite, M., Jr.; Pavão, M.S.; Mourão, P.A. Fucosylated chondroitin sulfate attenuates renal fibrosis in animals submitted to unilateral ureteral obstruction: A P-selectin-mediated event? Am. J. Physiol. Renal. Physiol. 2010, 299, F1299–F1307. [Google Scholar] [CrossRef]
- Borsig, L.; Wang, L.; Cavalcante, M.C.; Cardilo-Reis, L.; Ferreira, P.L.; Mourão, P.A.; Esko, J.D.; Pavão, M.S. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber. Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef]
- Lian, W.; Wu, M.; Huang, N.; Gao, N.; Xiao, C.; Li, Z.; Zhang, Z.; Zheng, Y.; Peng, W.; Zhao, J. Anti-HIV-1 activity and structure-activity-relationship study of a fucosylated glycosaminoglycan from an echinoderm by targeting the conserved CD4 induced epitope. Biochim. Biophys. Acta 2013, 1830, 4681–4691. [Google Scholar] [CrossRef]
- Huang, N.; Wu, M.Y.; Zheng, C.B.; Zhu, L.; Zhao, J.H.; Zheng, Y.T. The depolymerized fucosylated chondroitin sulfate from sea cucumber potently inhibits HIV replication via interfering with virus entry. Carbohydr. Res. 2013, 380, 64–69. [Google Scholar] [CrossRef]
- Hu, S.; Chang, Y.; Wang, J.; Xue, C.; Shi, D.; Xu, H.; Wang, Y. Fucosylated chondroitin sulfate from Acaudina molpadioides improves hyperglycemia via activation of PKB/GLUT4 signaling in skeletal muscle of insulin resistant mice. Food Funct. 2013, 4, 1639–1646. [Google Scholar] [CrossRef]
- Gao, N.; Wu, M.; Liu, S.; Lian, W.; Li, Z.; Zhao, J. Preparation and characterization of O-acylated fucosylated chondroitin sulfate from sea cucumber. Mar. Drugs 2012, 10, 1647–1661. [Google Scholar] [CrossRef]
- Wu, N.; Ye, X.; Guo, X.; Liao, N.; Yin, X.; Hu, Y.; Sun, Y.; Liu, D.; Chen, S. Depolymerization of fucosylated chondroitin sulfate from sea cucumber, Pearsonothuria graeffei, via 60Co irradiation. Carbohydr. Polym. 2013, 93, 604–614. [Google Scholar] [CrossRef]
- Wu, M.; Xu, S.; Zhao, J.; Kang, H.; Ding, H. Free-radical depolymerization of glycosaminoglycan from sea cucumber Thelenata ananas by hydrogen peroxide and copper ions. Carbohydr. Polym. 2013, 93, 1116–1124. [Google Scholar]
- Tamura, J.-I.; Tanaka, H.; Nakamura, A.; Takeda, N. Synthesis of β-d-GalNAc(4,6-diS)(1–4)[α-l-Fuc(2,4-diS)(1–3)]-β-d-GlcA, a novel trisaccharide unit of chondroitin sulfate with a fucose branch. Tetrahedron lett. 2013, 54, 3940–3943. [Google Scholar] [CrossRef]
- Pomin, V.H. Fucanomics and galactanomics: Marine distribution, medicinal impact, conceptions, and challenges. Mar. Drugs 2010, 80, 793–811. [Google Scholar]
- Pomin, V.H. Fucanomics and galactanomics: current status in drug discovery, mechanism of action and role of the well-defined chemical structure. Biochim. Biophys. Acta 2012, 1820, 1971–1979. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mourão, P.A. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1019–1027. [Google Scholar]
- Pomin, V.H. Review: An overview about the structure-function relationship of marine sulfated homopolysaccharides with regular chemical structures. Biopolymers 2009, 91, 601–609. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar. Drugs 2014, 12, 232-254. https://doi.org/10.3390/md12010232
Pomin VH. Holothurian Fucosylated Chondroitin Sulfate. Marine Drugs. 2014; 12(1):232-254. https://doi.org/10.3390/md12010232
Chicago/Turabian StylePomin, Vitor H. 2014. "Holothurian Fucosylated Chondroitin Sulfate" Marine Drugs 12, no. 1: 232-254. https://doi.org/10.3390/md12010232
APA StylePomin, V. H. (2014). Holothurian Fucosylated Chondroitin Sulfate. Marine Drugs, 12(1), 232-254. https://doi.org/10.3390/md12010232