Localization and Characterization of Ferritin in Demospongiae: A Possible Role on Spiculogenesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Intracellular Structures

2.2. One Dimensional Gel Electrophoresis and Western Blotting Analysis of Ferritin in S. domuncula and Primmorph Tissues

2.3. Sequence Alignment

2.4. Immunohistology of S. domuncula Filaments/Spicules and Tissue

2.5. Elemental Analysis (ICP-MS) of S. domuncula Filaments
2.6. Magnetic Resonance Imaging (MRI) of S. domuncula Filaments and Spicules

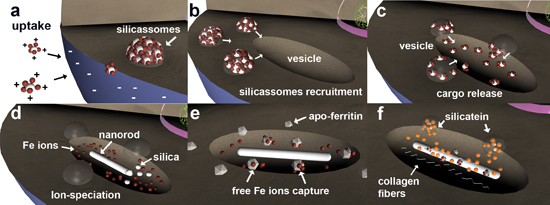
3. Discussion

4. Experimental Section
4.1. Primmorphs Cell Culture
4.2. Sample Preparation and Microscopy Analysis
4.3. Protein Extraction
4.4. One-Dimensional Gel Electrophoresis and Western Immunoblotting
4.5. Sequence Analysis of Ferritin Deduced Protein
4.6. Preparation of Suberites Domuncula Spicules and Axial Filaments
4.7. Histological Preparation of Suberites Domuncula Tissue
4.8. Immunohistology of Suberites Domuncula Filaments, Spicules and Tissue
4.9. Induced Couple Plasma—Mass Spectrometry (ICP-MS) Analysis of S. domuncula Filaments
4.10. MRI Measurements, Imaging and Computer Tomography (CT)
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiao, S.; Yuan, X.; Knoll, A.H. Eumetazoan fossils in terminal Proterozoic phosphorites? Proc. Natl. Acad. Sci. USA 2000, 97, 13684–13689. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Li, J.; Schröder, H.C.; Qiao, L.; Wang, X.H. The unique skeleton of siliceous sponges (Porifera; Hexactinellida and Demospongiae) that evolved first from the Urmetazoa during the Proterozoic: A review. Biogeosciences 2007, 4, 219–232. [Google Scholar]
- Knoll, A.H.; Carroll, S.B. Early animal evolution: Emerging views from comparative biology and geology. Science 1999, 284, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Walker, G. Snowball Earth: The Story of the Great Global Catastrophe that Spawned Life as We Know It; Crown Publishers: New York, NY, USA, 2003. [Google Scholar]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Cha, J.N.; Shimizu, K.; Zhou, Y.; Christianssen, S.C.; Chmelka, B.F.; Stucky, G.D.; Morse, D.E. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Rothenberger, M.; Boreiko, A.; Tremel, W.; Reiber, A.; Schröder, H.C. Formation of siliceous spicules in the marine demosponge Suberites domuncula. Cell Tissue Res. 2005, 321, 285–297. [Google Scholar]
- Müller, W.E.G.; Wang, X.H.; Belikov, S.I.; Tremel, W.; Schloßmacher, U.; Natoli, A.; Brandt, D.; Boreiko, A.; Tahir, M.N.; Müller, I.M.; et al. Formation of siliceous spicules in demosponges: Example Suberites domuncula. In Handbook of Biomineralization Volume 1, The Biology of Biominerals Structure Formation; Bäuerlein, E., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 59–82. [Google Scholar]
- Müller, W.E.G.; Jochum, K.; Stoll, B.; Wang, X.H. Formation of giant spicule from quartz glass by the deep sea sponge Monorhaphis. Chem. Mater. 2008, 20, 4703–4711. [Google Scholar]
- Wang, X.H.; Boreiko, A.; Schloßmacher, U.; Brandt, D.; Schröder, H.C.; Li, J.; Kaandorp, J.A.; Götz, H.; Duschner, H.; Müller, W.E.G. Axial growth of hexactinellid spicules: Formation of cone-like structural units in the giant basal spicules of the hexactinellid Monorhaphis. J. Struct. Biol. 2008, 164, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Belikov, S.; Tremel, W.; Perry, C.C.; Gieskes, W.W.C.; Boreiko, A.; Schröder, H.C. Siliceous spicules in marine demosponges (example Suberites domuncula). Micron 2006, 37, 107–120. [Google Scholar]
- Custodio, R.M.; Prokic, I.; Steffen, R.; Koziol, C.; Borojevic, R.; Brümmer, F.; Nickel, M.; Müller, W.E.G. Primmorphs generated from dissociated cells of the sponge Suberites domuncula: A model system for studies of cell proliferation and cell death. Mech. Ageing Dev. 1998, 105, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wiens, M.; Batel, R.; Steffen, R.; Borojevic, R.; Custodio, R.M. Establishment of a primary cell culture from a sponge: Primmorphs from Suberites domuncula. Mar. Ecol. Progr. Ser. 1999, 178, 205–219. [Google Scholar]
- Schröder, H.C.; Perovic-Ottstadt, S.; Rothenberger, M.; Wiens, M.; Schwertner, H.; Batel, R.; Korzhev, M.; Müller, I.M.; Müller, W.E.G. Silica transport in the demosponge Suberites domuncula: Fluorescence emission analysis using the PDMPO probe and cloning of a potential transporter. Biochem. J. 2004, 381, 665–673. [Google Scholar]
- Schröder, H.C.; Natalio, F.; Shukoor, M.I.; Tremel, W.; Schloßmacher, U.; Wang, X.; Müller, W.E.G. Apposition of silica lamellae during growth of spicules in the demosponge Suberites domuncula: Biological/biochemical studies and chemical/biomimetical confirmation. J. Struct. Biol. 2007, 159, 325–334. [Google Scholar]
- Mugnaioli, E.; Natalio, F.; Schloβmacher, U.; Wang, X.; Müller, W.E.G.; Kolb, U. Crystalline nanorods as possible templates for the synthesis of amorphous biosilica during spicule formation in Demospongiae. ChemBioChem 2009, 10, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Krasko, A.; Batel, R.; Schröder, H.C.; Müller, I.M.; Müller, W.E.G. Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur. J. Biochem. 2000, 267, 4878–4887. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Worwood, M. Editors: Iron in Biochemistry and Medicine II; Academic Press: London, UK, 1980. [Google Scholar]
- Blakemore, R. Magnetotactic Bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, J.L.; Jones, D.S.; MacFadden, B.J. Magnetite Biomineralization and Magnetoreception in Organisms: A New Biomagnetism (Topics in Geobiology); Kluwer Academic/Plenum Publishers: New York, NY, USA, 1985. [Google Scholar]
- Towe, K.M.; Rützler, K. Lepidocrocite Iron Mineralization in Keratose Sponge Granules. Science 1968, 162, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Towe, K.M.; Bradley, W.P. Mineralogical constitution of colloidal hydrous ferric oxides. J. Colloid Interface Sci. 1967, 24, 384–392. [Google Scholar] [CrossRef]
- Mann, S.; Bannister, J.V.; Williams, R.J.P. Structure and composition of ferritin cores isolated from human spleen, limpet (Patella vulgata) hemolymph and bacterial (Pseudomonas aeruginosa) cells. J. Mol. Biol. 1986, 188, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Massover, W.H.; Cowley, J.M. The ultrastructure of ferritin macromolecules. The lattice structure of the core crystallites. Proc. Nat. Acad. Sci. USA 1973, 70, 3847–3851. [Google Scholar] [CrossRef]
- Mayer, D.E.; Rohrer, J.S.; Schoeller, D.A.; Harris, D.C. Fate of oxygen during ferritin iron incorporation. Biochemistry 1983, 22, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.A.; Prissel, M.M.; Young, M.J.; Douglas, T. Constrained metal oxide mineralization: Lessons from ferritin applied to other protein cage architectures. In Handbook of Biomineralization Volume 1, Biomimetic and Bioinspired Chemistry; Bäuerlein, E., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 313–333. [Google Scholar]
- Theil, E.C.; Hase, T. Plant and microbial ferritins. In Iron Chelation in Plants and Soil Microorganisms; Acadernic Press: San Diego, CA, USA, 1993; Volume 5, pp. 133–156. [Google Scholar]
- Theil, E.C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 1987, 56, 289–315. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, 597–603. [Google Scholar] [CrossRef]
- Krasko, A.; Schroder, H.C.; Batel, R.; Grebenjuk, V.A.; Steffen, R.; Muller, I.M.; Muller, W.E.G. Iron induces proliferation and morphogenesis in primmorphs from the marine sponge Suberites domuncula. DNA Cell Biol. 2002, 21, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Kaluzhnaya, O.V.; Belikov, S.I.; Schröder, H.C.; Rothenberger, M.; Zapf, S.; Kaandorp, J.A.; Borejko, A.; Müller, I.M.; Müller, W.E.G. Dynamics of skeletal formation in the Lake Baikal sponge Lubomirskia baicalensis. Part I: Biological and biochemical studies. Naturwissenschaften 2005, 92, 128–133. [Google Scholar]
- Brooks, R.A.; Vymazal, J.; Goldfarb, R.B.; Bulte, J.W.; Aisen, P. Relaxometry and magnetometry of ferritin. Magn. Reson. Med. 1998, 40, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, F.C.; Heywood, B.R.; Mann, S. Magneto-ferritin: In vitro synthesis of a novel magnetic protein. Science 1992, 257, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Sandford, F. Physical and chemical analysis of the siliceous skeletons in six sponges of two groups (demospongiae and hexactinellida). Microsc. Res. Tech. 2003, 62, 336–355. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Cowley, J.M.; Janney, D.; Gerkin, R.C.; Buseck, P.R. The structure of ferritin cores determined by electron nanodiffraction. J. Struct. Biol. 2000, 131, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Quintana, C.; Bonnet, N.; Jeantet, A.Y.; Chemel, P. Crystallographic study of the ferritin molecule: New results obtained from natural cystals in situ (mollusc oocyte) and from isolated molecules horse spleen. Biol. Cell 1987, 59, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Krasko, A.; Le Pennec, G.; Schröder, H.C. Biochemistry and Cell Biology of Silica Formation in Sponges. Microsc. Res. Tech. 2003, 62, 368–377. [Google Scholar]
- Jambor, J.L.; Dutrizac, J.E. Occurrence and Constitution of Natural and Synthetic Ferrihydrite, a widespread Iron Oxyhydroxide. Chem. Rev. 1998, 98, 2549–2585. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, R.L.; Vandergaast, S.J.; Childs, C.W. A structural model for natural siliceous ferrihydrite. Clays Clay Miner. 1992, 6, 675–681. [Google Scholar] [CrossRef]
- Davis, C.C.; Chen, H.-W.; Edwards, M. Modeling silica sorption to iron hydroxide. Environ. Sci. Technol. 2002, 36, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Hydes, D.J. Aluminum in Seawater: Control by Inorganic Processes. Science 1979, 205, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Le Pennec, G.; Perovic, S.; Ammar, S.M.A.; Grebenjuk, V.A.; Steffen, R.; Brümmer, F.; Müller, W.E.G. Cultivation of primmorphs from the marine sponge Suberites domuncula: Morphogenetic potential of silicon and iron. A review. J. Biotechnol. 2003, 100, 93–108. [Google Scholar]
- Chasteen, N.D.; Harrison, P.M. Mineralization in ferritin: An efficient means of iron storage. J. Struct. Biol. 1999, 126, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Loader, C.E.; Timmons, C.J. Studies in photochemistry. Part V. The photocyclodehydrogenation of 2-furyl- and 2-thienyl-ethylenes: The mass spectra of the products. J. Chem. Soc. C 1967, 1677–1681. [Google Scholar] [CrossRef]
- Antelo, B.; Castedo, L.; Delamano, J.; Gómez, A.; López, C.; Toto, G. Photochemical Ring Closure of 1-Tosyl-1,2-diarylethenes. J. Org. Chem. 1996, 61, 1188–1189. [Google Scholar] [CrossRef]
- Karmininski-Zamola, G.; Fiser-Jakic, L.; Jakopcic, K. Photochemistry of furans. Photochemical transformations of some substituted 2-phenyl-3-furylacrylic acids. Tetrahedron 1982, 38, 1392–1335. [Google Scholar]
- Klint, D.; Karlson, G.; Bovin, J.-O. Cryo-TEM Snapshots of ferritin adsorbed on small zeolite crystals. Angew. Chem. Int. Ed. 1990, 38, 2560–2562. [Google Scholar] [CrossRef]
- Imsiecke, G.; Müller, W.E.G. Unusual presence and intranuclear storage of silica crystals in the freshwater sponges Ephydatia muelleri and Spongilla lacustris (Porifera: Spongillidae). Cell. Mol. Biol. 1995, 41, 827–832. [Google Scholar] [PubMed]
- Croce, G.; Frache, A.; Milanesio, M.; Viterbo, D.; Bavestrello, G.; Benatti, U.; Giovine, M.; Amenitsch, H. Fiber diffraction study of spicules from marine sponges. Microsc. Res. Tech. 2003, 62, 378–381. [Google Scholar]
- Garrone, R. Collagène, spongine et squelette minéral chez l'éponge Haliclona rosea (O.S.). J. Micros. 1969, 8, 581–598. [Google Scholar]
- Donadey, C.; Paris, J.; Vacelet, J. Occurrence and ultrastructure of microraphides in Axinella polypoides. In New Perspectives in Sponge Biology (International Conference on the Biology of Sponges, 1985); Rützler, K., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1990; pp. 259–263. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.R.; Lipman, D.J. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 1988, 85, 2444–2448. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. ClustalW and ClustalX version 2. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, M.B.; Naik, R.R.; Sarosi, P.M.; Agarwal, G.; Stone, M.O.; Sandhage, K.H. Ceramic Nanoparticle Assemblies with Tailored Shapes and Tailored Chemistries via Biosculpting and Shape-Preserving Inorganic Conversion. J. Nanosci. Nanotech. 2005, 5, 63–67. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Natalio, F.; Wiese, S.; Friedrich, N.; Werner, P.; Tahir, M.N. Localization and Characterization of Ferritin in Demospongiae: A Possible Role on Spiculogenesis. Mar. Drugs 2014, 12, 4659-4676. https://doi.org/10.3390/md12084659
Natalio F, Wiese S, Friedrich N, Werner P, Tahir MN. Localization and Characterization of Ferritin in Demospongiae: A Possible Role on Spiculogenesis. Marine Drugs. 2014; 12(8):4659-4676. https://doi.org/10.3390/md12084659
Chicago/Turabian StyleNatalio, Filipe, Stefanie Wiese, Norman Friedrich, Peter Werner, and Muhammad Nawaz Tahir. 2014. "Localization and Characterization of Ferritin in Demospongiae: A Possible Role on Spiculogenesis" Marine Drugs 12, no. 8: 4659-4676. https://doi.org/10.3390/md12084659
APA StyleNatalio, F., Wiese, S., Friedrich, N., Werner, P., & Tahir, M. N. (2014). Localization and Characterization of Ferritin in Demospongiae: A Possible Role on Spiculogenesis. Marine Drugs, 12(8), 4659-4676. https://doi.org/10.3390/md12084659