Preparation and Characterization of Antioxidant Nanoparticles Composed of Chitosan and Fucoidan for Antibiotics Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CS/F NPs

| Compound | Characteristic Peaks |
|---|---|
| Chitosan (CS) | -NH3+ (1560 cm−1); -C=O (1650 cm−1); C-O-C (1150 cm−1); C-O (1026 cm−1) |
| Fucoidan (F) | S=O (1160–1260 cm−1); C-O-S (845 cm−1) |
| CS/F NPs | Both the characteristic peaks of CS and fucoidan were present, but a red shift of the C=O group of CS appeared. |
| Group | Chitosan (mg/mL, pH 6.0) | Fucoidan (mg/mL, pH 6.0) | Weight Ratio (CS:F) | Charge Ratio (CS:F) |
|---|---|---|---|---|
| C1F1 | 5 | 5 | 1:1 | 1.05:1 |
| C2F1 | 10 | 5 | 2:1 | 2.11:1 |
| C3F1 | 15 | 5 | 3:1 | 3.16:1 |
| C4F1 | 20 | 5 | 4:1 | 4.21:1 |
| C5F1 | 25 | 5 | 5:1 | 5.26:1 |
| Particles Size (nm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| C1F1 | C2F1 | C3F1 | C4F1 | C5F1 | ||||
| pH 6.0 | 372 ± 38 | 340 ± 5 | 331 ± 6 | 344 ± 2 | 326 ± 21 | |||
| pH 6.6 | 865 ± 129 | 331 ± 6 | 312 ± 2 | 327 ± 5 | 316 ± 3 | |||
| pH 7.0 | 1050 ± 149 | 334 ± 11 | 298 ± 8 | 330 ± 4 | 293 ± 10 | |||
| pH 7.2 | 1051 ± 124 | 429 ± 25 | 309 ± 4 | 324 ± 4 | 274 ± 6 | |||
| pH 7.4 | 1252 ± 114 | 747 ± 52 | 497 ± 54 | 348 ± 4 | 271 ± 6 | |||
| Zeta potential (mV) | ||||||||
| C1F1 | C2F1 | C3F1 | C4F1 | C5F1 | ||||
| pH 6.0 | 8.0 ± 0.2 | 11.7 ± 0.8 | 13.8 ± 0.3 | 14.5 ± 0.6 | 13.2 ± 0.9 | |||
| pH 6.6 | 0.9 ± 2.8 | 7.8 ± 0.5 | 9.0 ± 0.7 | 10.4 ± 1.6 | 9.6 ± 0.4 | |||
| pH 7.0 | −3.1 ± 2.7 | 4.1 ± 0.5 | 5.4 ± 0.8 | 6.1 ± 0.6 | 5.9 ± 0.5 | |||
| pH 7.2 | −3.7 ± 1.5 | 3.3 ± 0.6 | 3.4 ± 0.1 | 3.9 ± 0.6 | 4.4 ± 0.7 | |||
| pH 7.4 | −4.7 ± 1.3 | 0.7 ± 0.6 | −1.1 ± 1.4 | 3.6 ± 3.1 | 1.8 ± 0.5 | |||
| PDIs | ||||||||
| C1F1 | C2F1 | C3F1 | C4F1 | C5F1 | ||||
| pH 6.0 | 0.24 ± 0.02 | 0.28 ± 0.01 | 0.31 ± 0.01 | 0.25 ± 0.02 | 0.35 ± 0.03 | |||
| pH 6.6 | 0.29 ± 0.04 | 0.29 ± 0.01 | 0.27 ± 0.03 | 0.25 ± 0.01 | 0.37 ± 0.02 | |||
| pH 7.0 | 0.37 ± 0.07 | 0.29 ± 0.03 | 0.27 ± 0.02 | 0.26 ± 0.01 | 0.32 ± 0.03 | |||
| pH 7.2 | 0.33 ± 0.04 | 0.38 ± 0.03 | 0.30 ± 0.04 | 0.26 ± 0.01 | 0.29 ± 0.02 | |||
| pH 7.4 | 0.41 ± 0.04 | 0.28 ± 0.03 | 0.28 ± 0.01 | 0.26 ± 0.02 | 0.24 ± 0.01 | |||


2.2. DPPH Scavenging Activity of CS/F NPs

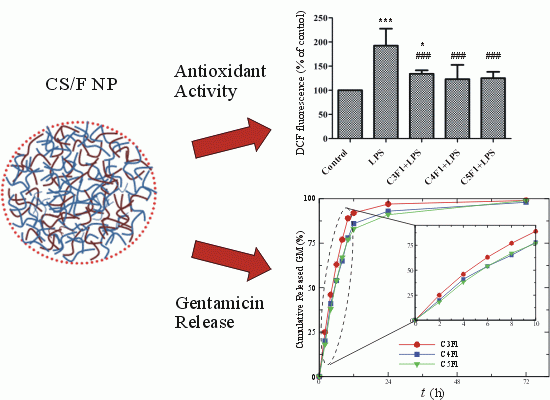
2.3. Effects of CS/F NPs on ROS Production

2.4. Anti-Inflammatory Effect of CS/F NPs

2.5. Cytotoxicity of CS/F NPs

2.6. In Vitro Release of Gentamicin (GM)

3. Experimental Section
3.1. Preparation of CS/F NPs
3.2. Characterization of CS/F NPs
3.3. Cell Culture
3.4. DPPH Scavenging Test

3.5. Reactive Oxygen Species Determination
3.6. Nitric Oxide Assay
3.7. Interleukin-6 Assay
3.8. MTT Assay
3.9. Preparation of GM-Loaded CS/F NPs and in Vitro Release
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Westerman, E.M.; de Boer, A.H.; le Brun, P.P.; Touw, D.J.; Roldaan, A.C.; Frijlink, H.W.; Heijerman, H.G. Dry powder inhalation of colistin in cystic fibrosis patients: A single dose pilot study. J. Cyst. Fibros. 2007, 6, 284–292. [Google Scholar] [CrossRef]
- Richardson, C.H.; de Matas, M.; Hosker, H.; Mukherjee, R.; Wong, I.; Chrystyn, H. Determination of the relative bioavailability of salbutamol to the lungs following inhalation from dry powder inhaler formulations containing drug substance manufactured by supercritical fluids and micronization. Pharm. Res. 2007, 24, 2008–2017. [Google Scholar] [CrossRef]
- Beaulac, C.; Sachetelli, S.; Lagace, J. Aerosolization of low phase transition temperature liposomal tobramycin as a dry powder in an animal model of chronic pulmonary infection caused by Pseudomonas aeruginosa. J. Drug Target 1999, 7, 33–41. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Kohane, D.S.; Bloch, K.D.; Stasch, J.P.; Volpato, G.P.; Bellas, E.; Evgenov, N.V.; Buys, E.S.; Gnoth, M.J.; Graveline, A.R.; et al. Inhaled agonists of soluble guanylate cyclase induce selective pulmonary vasodilation. Am. J. Respir. Crit. Care Med. 2007, 176, 1138–1145. [Google Scholar] [CrossRef]
- Azarmi, S.; Tao, X.; Chen, H.; Wang, Z.; Finlay, W.H.; Lobenberg, R.; Roa, W.H. Formulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particles. Int. J. Pharm. 2006, 319, 155–161. [Google Scholar] [CrossRef]
- Shoyele, S.A.; Cawthorne, S. Particle engineering techniques for inhaled biopharmaceuticals. Adv. Drug Deliv. Rev. 2006, 58, 1009–1029. [Google Scholar] [CrossRef]
- Yoo, D.; Guk, K.; Kim, H.; Khang, G.; Wu, D.; Lee, D. Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. Int. J. Pharm. 2013, 450, 87–94. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 600–612. [Google Scholar] [CrossRef]
- Makino, K.; Yamamoto, N.; Higuchi, K.; Harada, N.; Ohshima, H.; Terada, H. Phagocytic uptake of polystyrene microspheres by alveolar macrophages: Effects of the size and surface properties of the microspheres. Colloid Surf. B Biointerfaces 2003, 27, 33–39. [Google Scholar] [CrossRef]
- Schurch, S.; Gehr, P.; Im Hof, V.; Geiser, M.; Green, F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respir. Physiol. 1990, 80, 17–32. [Google Scholar] [CrossRef]
- Yang, L.; Luo, J.; Shi, S.; Zhang, Q.; Sun, X.; Zhang, Z.; Gong, T. Development of a pulmonary peptide delivery system using porous nanoparticle-aggregate particles for systemic application. Int. J. Pharm. 2013, 451, 104–111. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Liu, C.; Zhang, N. Biodegradable solid lipid nanoparticle flocculates for pulmonary delivery of insulin. J. Biomed. Nanotechnol. 2012, 8, 834–842. [Google Scholar] [CrossRef]
- Chattopadhyay, S. Aerosol generation using nanometer liposome suspensions for pulmonary drug delivery applications. J. Liposome Res. 2013, 23, 255–267. [Google Scholar] [CrossRef]
- Dokka, S.; Toledo, D.; Shi, X.; Castranova, V.; Rojanasakul, Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm. Res. 2000, 17, 521–525. [Google Scholar] [CrossRef]
- Dailey, L.A.; Schmehl, T.; Gessler, T.; Wittmar, M.; Grimminger, F.; Seeger, W.; Kissel, T. Nebulization of biodegradable nanoparticles: Impact of nebulizer technology and nanoparticle characteristics on aerosol features. J. Control. Release 2003, 86, 131–144. [Google Scholar] [CrossRef]
- Dailey, L.A.; Kleemann, E.; Wittmar, M.; Gessler, T.; Schmehl, T.; Roberts, C.; Seeger, W.; Kissel, T. Surfactant-free, biodegradable nanoparticles for aerosol therapy based on the branched polyesters, DEAPA-PVAL-g-PLGA. Pharm. Res. 2003, 20, 2011–2020. [Google Scholar]
- Dailey, L.A.; Jekel, N.; Fink, L.; Gessler, T.; Schmehl, T.; Wittmar, M.; Kissel, T.; Seeger, W. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol. Appl. Pharmacol. 2006, 215, 100–108. [Google Scholar] [CrossRef]
- Fiore, V.F.; Lofton, M.C.; Roser-Page, S.; Yang, S.C.; Roman, J.; Murthy, N.; Barker, T.H. Polyketal microparticles for therapeutic delivery to the lung. Biomaterials 2010, 31, 810–817. [Google Scholar] [CrossRef]
- Huang, Y.C.; Liu, T.J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012, 8, 1048–1056. [Google Scholar]
- Huang, Y.C.; Yang, Y.T. Effect of basic fibroblast growth factor released from chitosan-fucoidan nanoparticles on neurite extension. J. Tissue Eng. Regen Med. 2013. [Google Scholar] [CrossRef]
- Nishino, T.; Yokoyama, G.; Dobashi, K.; Fujihara, M.; Nagumo, T. Isolation, purification, and characterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia kurome and their blood-anticoagulant activities. Carbohydr. Res. 1989, 186, 119–129. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifant’ev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Luo, D.Z.; Wang, X.M. Fucoidan: Advances in the study of its anti-inflammatory and anti-oxidative effects. Yao Xue Xue Bao 2008, 43, 1186–1189. [Google Scholar]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005, 137, 289–294. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R.D. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A.; Ng, K.Y. Preparation and evaluation of zinc-pectin-chitosan composite particles for drug delivery to the colon: Role of chitosan in modifying in vitro and in vivo drug release. Int. J. Pharm. 2011, 406, 11–20. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Sajadi Tabassi, S.A.; Jaafari, M.R. Preparation, characterization, and mucoadhesive properties of chitosan-coated microspheres encapsulated with cyclosporine A. Drug Dev. Ind. Pharm. 2008, 34, 492–498. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sanchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Portero, A.; Remunan-Lopez, C.; Nielsen, H.M. The potential of chitosan in enhancing peptide and protein absorption across the TR146 cell culture model-an in vitro model of the buccal epithelium. Pharm. Res. 2002, 19, 169–174. [Google Scholar] [CrossRef]
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm. Res. 1994, 11, 1358–1361. [Google Scholar] [CrossRef]
- Fernandez-Urrusuno, R.; Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef]
- Bandi, N.; Wei, W.; Roberts, C.B.; Kotra, L.P.; Kompella, U.B. Preparation of budesonide- and indomethacin-hydroxypropyl-beta-cyclodextrin (HPBCD) complexes using a single-step, organic-solvent-free supercritical fluid process. Eur. J. Pharm. Sci. 2004, 23, 159–168. [Google Scholar] [CrossRef]
- Tang, D.W.; Yu, S.H.; Ho, Y.C.; Mi, F.L.; Kuo, P.L.; Sung, H.W. Heparinized chitosan/poly(gamma-glutamic acid) nanoparticles for multi-functional delivery of fibroblast growth factor and heparin. Biomaterials 2010, 31, 9320–9332. [Google Scholar] [CrossRef]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; la Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Ozbas-Turan, S.; Akbuga, J.; Sezer, A.D. Topical application of antisense oligonucleotide-loaded chitosan nanoparticles to rats. Oligonucleotides 2010, 20, 147–153. [Google Scholar] [CrossRef]
- Sezer, A.D.; Akbuga, J. Comparison on in vitro characterization of fucospheres and chitosan microspheres encapsulated plasmid DNA (pGM-CSF): Formulation design and release characteristics. AAPS PharmSciTech 2009, 10, 1193–1199. [Google Scholar] [CrossRef]
- Yu, D.W.; Yang, T.; Sonoda, T.; Gong, Y.; Cao, Q.; Gaffney, K.; Jensen, P.J.; Freedberg, I.M.; Lavker, R.M.; Sun, T.T. Osteopontin gene is expressed in the dermal papilla of pelage follicles in a hair-cycle-dependent manner. J. Investig. Dermatol. 2001, 117, 1554–1558. [Google Scholar] [CrossRef]
- Sanders, N.N.; de Smedt, S.C.; Demeester, J. The physical properties of biogels and their permeability for macromolecular drugs and colloidal drug carriers. J. Pharm. Sci. 2000, 89, 835–849. [Google Scholar] [CrossRef]
- Lieu, D.K.; Degraffenried, L.A.; Isseroff, R.R.; Kurzrock, E.A. Beta1 integrin expression pattern in transitional urothelium does not allow for efficient stem cell enrichment as in other epithelia. Tissue Eng. 2007, 13, 263–270. [Google Scholar] [CrossRef]
- Jarmila, V.; Vavrikova, E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—A review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [CrossRef]
- Ruperez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.Y. Inhibitory effects of honokiol on LPS and PMA-induced cellular responses of macrophages and monocytes. BMB Rep. 2009, 42, 574–579. [Google Scholar] [CrossRef]
- Ji, J.G.; Hao, S.L.; Wu, D.J.; Huang, R.; Xu, Y. Preparation, characterization and in vitro release of chitosan nanoparticles loaded with gentamicin and salicylic acid. Carbohydr. Polym. 2011, 85, 803–808. [Google Scholar] [CrossRef]
- Achour, O.; Bridiau, N.; Godhbani, A.; le Joubioux, F.; Bordenave Juchereau, S.; Sannier, F.; Piot, J.M.; Fruitier Arnaudin, I.; Maugard, T. Ultrasonic-assisted preparation of a low molecular weight heparin (LMWH) with anticoagulant activity. Carbohydr. Polym. 2013, 97, 684–689. [Google Scholar] [CrossRef]
- Page, C. Heparin and related drugs: Beyond anticoagulant activity. ISRN Pharmacol. 2013, 2013, 910743. [Google Scholar] [CrossRef]
- Fermas, S.; Gonnet, F.; Sutton, A.; Charnaux, N.; Mulloy, B.; Du, Y.; Baleux, F.; Daniel, R. Sulfated oligosaccharides (heparin and fucoidan) binding and dimerization of stromal cell-derived factor-1 (SDF-1/CXCL 12) are coupled as evidenced by affinity CE-MS analysis. Glycobiology 2008, 18, 1054–1064. [Google Scholar] [CrossRef]
- Chabut, D.; Fischer, A.M.; Colliec-Jouault, S.; Laurendeau, I.; Matou, S.; le Bonniec, B.; Helley, D. Low molecular weight fucoidan and heparin enhance the basic fibroblast growth factor-induced tube formation of endothelial cells through heparan sulfate-dependent alpha6 overexpression. Mol. Pharmacol. 2003, 64, 696–702. [Google Scholar] [CrossRef]
- Kuno, T.; Naito, S.; Ito, H.; Ohta, M.; Kido, N.; Kato, N. Staining of the O-specific polysaccharide chains of lipopolysaccharides with alkaline bismuth. Microbiol. Immunol. 1986, 30, 1207–1211. [Google Scholar] [CrossRef]
- Nishio, K.; Horie, M.; Akazawa, Y.; Shichiri, M.; Iwahashi, H.; Hagihara, Y.; Yoshida, Y.; Niki, E. Attenuation of lipopolysaccharide (LPS)-induced cytotoxicity by tocopherols and tocotrienols. Redox Biol. 2013, 1, 97–103. [Google Scholar] [CrossRef]
- Kohroki, J.; Muto, N.; Tanaka, T.; Itoh, N.; Inada, A.; Tanaka, K. Induction of differentiation and apoptosis by dithizone in human myeloid leukemia cell lines. Leuk Res. 1998, 22, 405–412. [Google Scholar] [CrossRef]
- Frutos Cabanillas, P.; Diez Pena, E.; Barrales-Rienda, J.M.; Frutos, G. Validation and in vitro characterization of antibiotic-loaded bone cement release. Int. J. Pharm. 2000, 209, 15–26. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, Y.-C.; Li, R.-Y. Preparation and Characterization of Antioxidant Nanoparticles Composed of Chitosan and Fucoidan for Antibiotics Delivery. Mar. Drugs 2014, 12, 4379-4398. https://doi.org/10.3390/md12084379
Huang Y-C, Li R-Y. Preparation and Characterization of Antioxidant Nanoparticles Composed of Chitosan and Fucoidan for Antibiotics Delivery. Marine Drugs. 2014; 12(8):4379-4398. https://doi.org/10.3390/md12084379
Chicago/Turabian StyleHuang, Yi-Cheng, and Rou-Ying Li. 2014. "Preparation and Characterization of Antioxidant Nanoparticles Composed of Chitosan and Fucoidan for Antibiotics Delivery" Marine Drugs 12, no. 8: 4379-4398. https://doi.org/10.3390/md12084379
APA StyleHuang, Y. -C., & Li, R. -Y. (2014). Preparation and Characterization of Antioxidant Nanoparticles Composed of Chitosan and Fucoidan for Antibiotics Delivery. Marine Drugs, 12(8), 4379-4398. https://doi.org/10.3390/md12084379