Isolation and Assessment of the in Vitro Anti-Tumor Activity of Smenothiazole A and B, Chlorinated Thiazole-Containing Peptide/Polyketides from the Caribbean Sponge, Smenospongia aurea
Abstract
:1. Introduction

2. Results
2.1. Isolation and Stereostructural Determination

| Pos. | δH [mult., J (Hz)] | δC [mult.] | COSY | HMBC | |
|---|---|---|---|---|---|
| 1 | 7.71 (d, 3.3) | 142.9 (CH) | 2 | ||
| 2 | 7.48 (d, 3.3) | 120.1 (CH) | 1 | 1, 3 | |
| 3 | - | 174.4 (C) | |||
| 4 | 5.47 (dd, 8.2, 2.9) | 60.7 (CH) | 5a, 5b | 3, 5, 6, 7, 8 | |
| 5 | a | 2.37 (m) | 33.2 (CH2) | 4, 5b, 6a, 6b | 3, 4, 6, 7 |
| b | 2.21 (m) | 4, 5a, 6a, 6b | 3, 6 | ||
| 6 | a | 2.17 (m) | 25.4 (CH2) | 4a, 5b, 6b, 7a, 7b | 3, 7 |
| b | 2.11 (m) | 4a, 5b, 6a, 7a, 7b | 4, 5, 7 | ||
| 7 | a | 4.01 (ddd, 10.1, 8.4, 7.1) | 48.7 (CH2) | 6a, 6b, 7b | 5, 6 |
| b | 3.88 (ddd, 10.1, 7.8, 4.0) | 6a, 6b, 7a | 5, 6 | ||
| 8 | - | 173.5 (C) | |||
| 9 | 4.54 (d, 7.9) | 58.0 (CH) | 10 | 8, 10, 11, 12, 13 | |
| 10 | 2.14 (m) | 31.9 (CH) | 9, 11, 12 | 8, 9, 11, 12 | |
| 11 | 1.01 (d, 6.7) | 19.9 (CH3) | 10 | 9, 10, 12 | |
| 12 | 0.97 (d, 6.7) | 18.9 (CH3) | 10 | 9, 10, 11 | |
| 13 | - | 172.2 (C) | |||
| 14 | - | 133.4 (C) | |||
| 15 | 1.79 (br. t, 1.5) | 13.2 (CH3) | 16, 17 | 13, 14, 16 | |
| 16 | 6.16 (tq, 7.5, 1.5) | 133.4 (CH) | 15, 17 | 14, 15, 17 | |
| 17 | 3.03 (br. d, 7.5) | 30.2 (CH2) | 15, 16, 19 | 14, 16, 18, 19, 20 | |
| 18 | - | 141.3 (C) | |||
| 19 | 6.12 (br. s) | 116.0 (CH) | 17, 20 | 17, 18, 20 | |
| 20 | 3.44 (br. s) | 41.9 (CH2) | 19, 22/26 | 17, 18, 19, 21, 22/26 | |
| 21 | - | 139.6 (C) | |||
| 22/26 | 7.19 (br. d, 8.2) | 129.9 (CH) | 19, 23/25, 24 | 20, 24, 26/22 | |
| 23/25 | 7.29 (br. t, 8.2) | 129.4 (CH) | 22/26, 24 | 21, 25/23 | |
| 24 | 7.21 (br. t, 8.2) | 128.1 (CH) | 22/26, 23/25 | 22/26 |


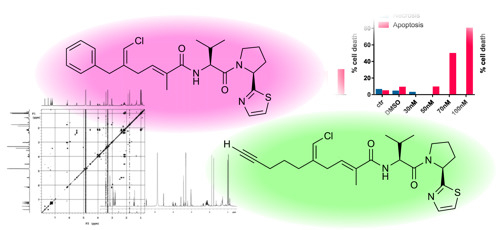
2.2. In Vitro Evaluation of Cytotoxic Activity of Smenothiazoles
2.2.1. Morphological Changes Induced by Treatment with Smenothiazoles Lead to Apoptosis
2.2.2. Smenothiazoles Reduce Cell Viability

2.2.3. Smenothiazoles Induce Apoptosis with Different Percentage


3. Discussion

4. Experimental Section
4.1. General Experimental Procedures
4.2. Collection, Extraction and Isolation
4.3. Absolute Configuration of Amino Acids
4.3.1. Ozonolysis and Hydrolysis
4.3.2. Marfey’s Derivatization with d- and l-FDAA
4.3.3. LC-MS Analysis
4.4. Smenothiazole A (3)
4.5. Smenothiazole B (4)
4.6. Cell Culture
4.7. MTT Assay
4.8. Cell Apoptosis Assay
4.9. Cell Cycle Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tirino, V.; Desiderio, V.; Paino, F.; de Rosa, A.; Papaccio, F.; La Noce, M.; Laino, L.; de Francesco, F.; Papaccio, G. Cancer stem cells in solid tumors: An overview and new approaches for their isolation and characterization. FASEB J. 2013, 27, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Garvalov, B.K.; Acker, T. Cancer stem cells: A new framework for the design of tumor therapies. J. Mol. Med. (Berl.) 2011, 89, 95–107. [Google Scholar] [CrossRef]
- Von Schwarzenberg, K.; Vollmar, A.M. Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 2013, 332, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Giddings, L.A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2013, 12, 293–304. [Google Scholar] [CrossRef]
- Lamoral-Theys, D.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Kiss, R.; Costantino, V. Evaluation of the antiproliferative activity of diterpene isonitriles from the sponge Pseudoaxinella flava in apoptosis-sensitive and apoptosis-resistant cancer cell lines. J. Nat. Prod. 2011, 74, 2299–2303. [Google Scholar] [CrossRef]
- Teta, R.; Irollo, E.; della Sala, G.; Pirozzi, G.; Mangoni, A.; Costantino, V. Smenamides A and B, chlorinated peptide/polyketide hybrids containing a dolapyrrolidinone unit from the caribbean sponge Smenospongia aurea. Evaluation of their role as leads in antitumor drug research. Mar. Drugs 2013, 11, 4451–4463. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Cirino, G.; de Gruttola, L.; Roviezzo, F. Tedanol: A potent anti-inflammatory ent-pimarane diterpene from the Caribbean Sponge Tedania ignis. Bioorg. Med. Chem. 2009, 17, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Büelhmann, P.; Affolter, M. Structure determination of organic compounds. In Tables of Spectral Data, 3rd ed.; Springer-Verlag Berlin: Heidelberg, Germany, 2000; p. 186. [Google Scholar]
- Marfey, P. Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg. Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Rogers, E.W.; Edison, A.S.; Molinski, T.F. Trisoxazole macrolides and thiazole-containing cyclic peptides from the nudibranch Hexabranchus sanguineus. J. Nat. Prod. 2009, 72, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Apramides A–G, novel lipopeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Marquez, B.L.; Nogle, L.M.; McPhail, K.; Goeger, D.E.; Roberts, M.A.; Gerwick, W.H. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem Biol. 2004, 11, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Rottacker, E.C.; Kastovsky, J.; Byrum, T.; Choi, H.; Ellismanm, M.H.; Komàrek, J.; Gerwick, W.H. Moorea producens gen.nov., sp. nov and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62, 1171. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wang, B.; Kulkarni, A.; Geders, T.W.; Grindberg, R.V.; Gerwick, L.; Hakansson, K.; Wipf, P.; Smith, J.L.; Gerwick, W.H.; et al. Metamorphic enzyme assembly in polyketide diversification. Nature 2009, 459, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, J.H.; Cjoi, H.; Pereira, A.R.; Ban, Y.H.; Yoo, Y.J.; Won Park, J.; Sherman, D.H.; Gerwick, W.H.; Yoon, Y.J. Heterologous production of 4-O-demethylbarbamide, a marine cyanobacterial natural product. Org. Lett. 2012, 14, 5824–5827. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, G.; Hochmuth, T.; Costantino, V.; Teta, R.; Gerwick, W.; Gerwick, L.; Piel, J.; Mangoni, A. Polyketide genes in the marine sponge plakortis simplex: A new group of mono-modular type-I polyketide synthases from sponge symbionts. Environ. Microbiol. Rep. 2013, 5, 809–818. [Google Scholar] [CrossRef] [PubMed]
- The Sponge Guide. Available online: http://www.spongeguide.org (accessed on 7 July 2013).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Teta, R.; Miceli, R.; Ceccarelli, L.S.; Della Sala, G.; Camerlingo, R.; Irollo, E.; Mangoni, A.; Pirozzi, G.; Costantino, V. Isolation and Assessment of the in Vitro Anti-Tumor Activity of Smenothiazole A and B, Chlorinated Thiazole-Containing Peptide/Polyketides from the Caribbean Sponge, Smenospongia aurea. Mar. Drugs 2015, 13, 444-459. https://doi.org/10.3390/md13010444
Esposito G, Teta R, Miceli R, Ceccarelli LS, Della Sala G, Camerlingo R, Irollo E, Mangoni A, Pirozzi G, Costantino V. Isolation and Assessment of the in Vitro Anti-Tumor Activity of Smenothiazole A and B, Chlorinated Thiazole-Containing Peptide/Polyketides from the Caribbean Sponge, Smenospongia aurea. Marine Drugs. 2015; 13(1):444-459. https://doi.org/10.3390/md13010444
Chicago/Turabian StyleEsposito, Germana, Roberta Teta, Roberta Miceli, Luca S. Ceccarelli, Gerardo Della Sala, Rosa Camerlingo, Elena Irollo, Alfonso Mangoni, Giuseppe Pirozzi, and Valeria Costantino. 2015. "Isolation and Assessment of the in Vitro Anti-Tumor Activity of Smenothiazole A and B, Chlorinated Thiazole-Containing Peptide/Polyketides from the Caribbean Sponge, Smenospongia aurea" Marine Drugs 13, no. 1: 444-459. https://doi.org/10.3390/md13010444
APA StyleEsposito, G., Teta, R., Miceli, R., Ceccarelli, L. S., Della Sala, G., Camerlingo, R., Irollo, E., Mangoni, A., Pirozzi, G., & Costantino, V. (2015). Isolation and Assessment of the in Vitro Anti-Tumor Activity of Smenothiazole A and B, Chlorinated Thiazole-Containing Peptide/Polyketides from the Caribbean Sponge, Smenospongia aurea. Marine Drugs, 13(1), 444-459. https://doi.org/10.3390/md13010444