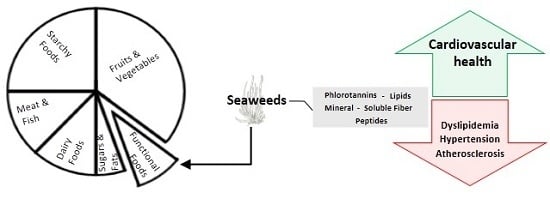
Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods
Abstract
:1. Introduction
1.1. Cardiovascular Diseases (CVDs)
1.2. Relevant Macroalgae Components in the Area of Functional Food

2. Overview of Relevant Biological Pathways Underlying CVDs
3. Evidences of Protective Effects of Seaweeds with Impact on CVDs
3.1. Dyslipidemia
3.2. Hypertension
| Seaweed Species | Extraction | Inhibition | Ref |
|---|---|---|---|
| ACE-I inhibition of extracts | |||
| Twenty-six red algae | MeOH and Aq Ext 20 °C and 70 °C | Aq Ext 20 °C IC50 (µg/mL): Lomentaria catenata = 13.78; Lithophyllum okamurae = 12.21; MeOH Ext 20 °C IC50 (µg/mL): Ahnfeltiopsis flabelliformis = 13.84; Laurencia okamurae = 106.15; MeOH Ext70 °C: Bonnemaisonia hamifera, Grateloupia filicina, Sinkoraena lancifolia, Grateloupia lanceolata, Gracilaria vermiculophylla and L. okamurae ranging from 25.82 to 124.69 | [85] |
| Ten Korean seaweeds | EtOH Ext | Ecklonia stolonifera, E. cava, Pelvetia siliquosa, Undaria Pinnatifida and Gigartina tenella: above 50% inhibition of ACE at 163.93 µg/mL | [86] |
| Ecklonia cava | EtOH, EtAc, CHCl3, Hex, DE | Best inhibition for EtOH Ext, IC50 = 0.96 mg/mL | [87] |
| ACE-I inhibition associated with antioxidants | |||
| Ecklonia stolonifera | Purified Phlorotannins | Best inhibition recorded for eckol, dieckol and phlorofucofuroeckol. IC50 (μM): eckol = 70.82; phlorofucofuroeckol A = 12.74; dieckol = 34.25 | [86] |
| Ecklonia cava | Purified Phlorotannins | IC50 (mM): phloroglucinol = 2.57 ± 0.09; eckol = 2.27 ± 0.08; triphlorethol-A = 2.01 ± 0.36; dieckol = 1.47 ± 0.04; eckstolonol = 2.95 ± 0.28 | [87] |
| Saccharina japonica (SJ) Sargassum horneri (SH) | Supercritic CO2 vs. Acet: MeOH | IC50 (µg/mL): SJ CO2 Ext = 0.89 ± 0.07; SJ Acet:MeOH Ext = 1.05 ± 0.14; SH CO2 Ext = 0.97 ± 0.11; SH Acet:MeOH Ext = 1.28 ± 0.50; | [89] |
| ACE-I or Renin inhibition associated with peptides | |||
| Porphyra columbina | Enzymatic in thermostatic reactor (A/AF) | ACE-I IC50 (g/L): A = 1.2 ± 0.1; AF = 1.7 ± 0.0 | [90] |
| Porphyra yezoensis | pH and enzymatic | ACE-I IC50 (g/L): 1.6 | [91] |
| Palmaria palmata | Papain | Ile-Arg-Leu-Ile-Ile-Val-Leu-Met-Pro-Ile-Leu-Met-Ala Renin inhibitory bioassay: ↓ renin activities by 58.97% (±1.26) at 1 mg/mL. | [92] |
| Solieria chordalis (SC) Palmaria palmata (PP) | Chymotrypsin (ChTr) or trysin (Tr) | <10 kDa fractions of SC: hydrolyzed with ChTr (IC50 ACE 3.50 mg/mL) or Tr (IC50 ACE 20.34 mg/mL); <10 kDa fractions of PP: hydrolyzed with ChTr (ACE IC50 460.05 mg/mL) | [93] |
| Caulerpa microphysa | Pepsin, alcalase, flavourzyme | ACE-I IC50 (mg/L): pepsin = 0.20; flavourzyme = 29.74; alcalase = 31.71 | [94] |
| Undaria pinnafida | Pepsin | ACE-I IC50 (µM): Ala-Ile-Tyr-Lys = 213; Tyr-Lys-Tyr-Tyr = 64.2; Lys-Phe-Tyr-Gly = 90.5; Tyr-Asn-Lys-Leu = 21 | [95] |
| Undaria pinnatifida | Aq hot Ext dyalisis, chromatography | ACE-I IC50 (µM): Tyr-His = 5.1; Lys-Trp = 10.8; Lys-Tyr = 7.7; Lys-Phe = 28.3; Phe-Tyr = 3.7; Val-Trp = 10.8; Val-Phe = 43.7; Ile-Tyr = 2.7; Ile-Trp = 12.4; Val-Tyr = 11.3 | [88] |
| Seaweed Species (Extract) | Model Dose | Effects | Ref. |
|---|---|---|---|
| Ulva ohnoi (UO) Derbesia tenuissima (DT) | High-carbohydrate, HF diet-fed rats 5% for 8 weeks | UO: ↓ total final body fat mass by 24% and sBP by 29 mmHg; ↑ Glc utilization and insulin sensitivity; DT: ↓ TG by 38% and TC by 17% | [59] |
| Ulva linza (UL) Lessonia trabeculata (LT) | High-sucrose, HF diet-fed rats|400 mg·kg−1 for 8 weeks | UL, LT: ↓ levels of intra-abdominal fat, arterial BP, insulin resistance, TC, TG, SOD; ↓ liver expression levels SOD and GPx and ↑ CAT in control groups and ↓ in algae-fed rats; LT: ↓ GPx activity | [96] |
| Gracilaria changii | HF, HC diet-induced rats|5% and 10% for 8 weeks | 5%: ↓ TC (−39.19%), LDL-C (−36.36%), and TG (−25.45%); 10%: ↓ TC, LDL-C and TG content by 40.34%, 35.95% and 30.91%, respectively; lowest AI; 5% and 10%: in plasma = ↓ LipPerox; ↓ AST and ALT levels; in erythrocyte = ↑ SOD, CAT and GSH-Px | [60] |
| Not detailed | Healthy children from 3 to 6 years diet including seaweed intake using 3-day dietary records | Cross-section study in healthy preschoolers: Girls with higher seaweed intake had significantly lower systolic BP (102.4, 99.2 and 96.9 mmHg for girls with the lowest, middle and highest tertiles of seaweed intake, respectively); seaweed intake was negatively related to dBP in boys and to sBP in girls. | [97] |
| Undaria pinnatifida (UP) | Men/Women with MS|Gr1:1 month (m) 4 g/day UP; Gr2: 1 m 4 g plus 1 m g/day UP (pills) | Randomized double-blinded placebo-controlled trial. Gr2: ↓ systolic BP 10.5 mmHg after a month of 6 g/day seaweed (primarily in subjects with high-normal baseline BP); ↓ waist circumference for women participants (↓ 2.1 cm after 4 g/day and further 1.8 cm after 1 m 6 g/day seaweed). No changes in lipid profile. | [98] |
| Undaria pinnatifida (UP) | 19 patients MS|3.3 g in capsules | sBP: ↓ 13 mmHg below the baseline after 4 weeks and 8 mmHg after 8 weeks. dBP: ↓ 9 mmHg after 4 weeks and 8 mmHg after 8 weeks; hypercholesterolemia ↓ 8% by week 4 | [99] |
| Extracts | |||
| Sargassum subrepandum (MeOH Ext) | Rats with atherogenic diet|100 mg/kg b.wt | ↓ TC, TG, LDL-C and ↑ HDL-C; ↓ MDA, NO, leptin, TNF-alpha levels; ↑ adiponectin level; | [100] |
| Ulva fasciata (Ulvans/Aq Ext at 4 °C or 100 °C plus EtOH pp) | HC rats|175 mg/kg for 4 weeks | Both Ext: No side effects; ↓ TC, TG, TG, LDL-C and VLDL-C; ↓ liver NO•; ↓ ICAM-1 and VCAM-1; ↑ IL-10; ↓ atherogenic plaques in the aorta more than fluvastatin; | [65] |
| Ulva lactuca (Ulvans/Aq Ext at 100 °C plus EtOH pp) | HC rats|175 mg/kg for 4 weeks | ↓TL, TG, TC, LDL-C and VLDL-C; ↑ HDL-C; ↓ AI, creatine kinase and LDH; ↓ liver ALT, AST and ALP activities and serum urea, creatinine and urea/creatinine ratio; ↑ hepatic CAT, GSH-Px; ↑ GSH, Total thiol levels | [67] |
| Not detailed (Low-MW Commercial alginates) | DOCA salt-induced hypertensive rats|250 or 500 mg/kg for 30 days | ↓ sBP; dose-dependent normalization of changes induced by DOCA salt, with the exception of further increasing sodium excretion | [101] |
| Gloiopeltis complanata (Funoran/Aq hot Ext plus various purification steps) | HC, high-sal fed rats|1000 mg/kg for 20 days | ↓ sBP; ↓ TC, TG, LDL-C, AI; ↑ urine excretion of sodium, chloride; ↑ urine Na/K ratio | [102] |
| Not detailed (Seaweed fiber (SF)) | Hypertensive Patients|Pills with 0.33 g; 25 min before meals for 4 weeks | ↓ mean and sBP; ↑ plasma renin activity; ↓ urinary secretion of Na, K and Na/K ratio | [103] |
| Palmaria palmata (protein hydrolysate and tridecapeptide IRLIIVLMPILMA) | Spontaneously Hypertensive rats|50 mg/kg b.wt | After 24 h ingestion: ↓ 34 mm Hg in sBP; IRLIIVLMPLIMA: ↓ 33 mm Hg | [104] |
3.3. Biological Pathways Underlying Atherosclerotic-Related Events
4. Functional Food with Macroalgae for Promoting Cardiovascular Health
| Product | Seaweed Species | Relevant Results | Ref |
|---|---|---|---|
| Meat-based products | |||
| Gel/emulsion meat systems | Himanthalia elongate (HE), Undaria pinnatifida (UP), Porphyra umbilicalis (PU) at 2.5% or 5% | ↑ water- and fat-binding properties except in the case of PU at 2.5%. | [121] |
| Gel/emulsion meat systems | Himanthalia elongate (HE), Undaria pinnatifida (UP), Porphyra umbilicalis (PU) at 5.6% | All: ↑ n-3 PUFA and ↓ n-6/n-3 PUFA ratio; ↓ Na and ↑ K, Ca, Mg, Mn, antioxidants ↓ TI by for PU and UP | [122] |
| Low-fat frankfurters | Himanthalia elongata (HE) at 5.5% (algae plus 50% substitution of animal fat by olive oil) | Effect of HE add: little effect on lipid and amino acid profiles but ↑ dietary fiber content and Ca and ↓ Na/K ratios | [123] |
| Restructured meats | Himanthalia elongata at 5% | Effects in hypercholesterolemic rats: ↓ TC; ↑ expression CYP7A1 and Cu, Zn-SOD; ↓ expression CAT, Mn-SOD and GPx; | [127] |
| Restructured meats | Undaria pinnatifida (UP), Porphyra umbilicalis (PU) at 5% | Effects in hypercholesterolemic rats: PU = ↓ TC; ↑ expression Mn-SOD and CAT and AE activity; UP meat mainly had benefits as antioxidant | [131] |
| Restructured meats | Undaria pinnatifida (UP), Porphyra umbilicalis (PU) at 5% | UP moderately ameliorated the lipid profile in hypercholesterolemic rats: ↓ TC and VLDL-C | [132] |
| Restructured meats | Himanthalia elongata at 5% | Effects on hypercholesterolemic rats: ↑ AE activity; ↓ VLDL-C, ILDL-C + LDL-C | [130] |
| Pork/chicken patties | Laminaria japonica (LJ) (replacement of 2.25 g pork/chichen by 2.25 g LJ) | ↓ increased in postprandial glucose blood levels; ↓ TC and LDL-C | [133] |
| Others | |||
| Bread | Tridecapeptide IRLIIVLMPILMA from Palmaria palmata at 4% | Activity against renin IRLIIVLMPILMA maintained after baking process | [134] |
| Bread | Ascophyllum nodosum at 4% | Single blind cross over trial: ↓ in energy intake at a test meal 4 h later; no significant changes in Glc and cholesterol | [135] |
| Tea | Ecklonia cava (EC), Undaria pinnatifida (UP), Hizikia fusiforme (HF), Ulva pertusa (UP) | ACE inhibition IC50 (mg DW/mL): EC = 5.33 ± 0.24, UP = 26.4 ± 1.05, HF = 7.79 ± 0.46; UP = ND | [136] |
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). Fact Sheet N°317. Updated January 2015. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 15 July 2015).
- World Health Organization (WHO). Cardiovascular Diseases. Available online: http://www.who.int/cardiovascular_diseases/about_cvd/en/ (accessed on 15 July 2015).
- World Hearth Federation. Cardiovascular Disease Risk Factors. Available online: http://www.world-heart-federation.org/cardiovascular-health/cardiovascular-disease-risk-factors/ (accessed on 15 July 2015).
- Siti, H.N.; Yusof, K.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Castro Cabezas, M. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Stoner, L.; Lucero, A.A.; Palmer, B.R.; Jones, L.M.; Young, J.M.; Faulkner, J. Inflammatory biomarkers for predicting cardiovascular disease. Clin. Biochem. 2013, 46, 1353–1371. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Word Health Organization (WHO). Diet, Nutrition and the Prevention of Cardiovascular Diseases; WHO Tecnhical Report Series 916; Word Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Winterman, D. Future Foods: What Will We Be Eating in 20 Years’ Time? Available online: http://www.bbc.com/news/magazine-18813075 (accessed on 13 July 2015).
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Gallagher, E.; Tasdemir, D.; Hayes, M. Heart health peptides from macroalgae and their potential use in functional foods. J. Agric. Food Chem. 2011, 59, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.M.; Carvalho, L.G.; Silva, P.J.; Rodrigues, M.S.; Pereira, O.R.; Pereira, L. Bioproducts from seaweeds: A review with special focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Brownlee, I.; Fairclough, A.; Hall, A.; Paxman, J. The potential health benefits of seaweed and seaweed extract. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses. Marine Biology: Earth Sciences in the 21st Century; Pomin, V.H., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 119–136. [Google Scholar]
- Shimazu, T.; Kuriyama, S.; Hozawa, A.; Ohmori, K.; Sato, Y.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Dietary patterns and cardiovascular disease mortality in Japan: A prospective cohort study. Int. J. Epidemiol. 2007, 36, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Miura, A.; Taira, K. Implications from and for food cultures for cardiovascular diseases: Japanese food, particularly Okinawan diets. Asia Pac. J. Clin. Nutr. 2001, 10, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets. Functional Food Market: GCC Industry Analysis and Opportunity Assessment 2014–2020; Research and Markets: Dublin, UK, 2014. [Google Scholar]
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Health benefits of algal polysaccharides in human nutrition. Adv. Food Nutr. Res. 2012, 66, 75–145. [Google Scholar] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.; de Morais, A.; de Morais, R. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Freile-Pelerguín, Y.; Robledo, D. Bioactive Compounds from Algae. In Bioactive Compounds from Marine Foods: Plant and Animal Sources; Blanca, H.-L., Miguel, H., Eds.; John Wiley & Sons: West Sussex, UK, 2013; pp. 113–130. [Google Scholar]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Yeh, T.S.; Hung, N.H.; Lin, T.C. Analysis of iodine content in seaweed by GC-ECD and estimation of iodine intake. J. Food Drug Anal. 2014, 22, 189–196. [Google Scholar] [CrossRef]
- Zalesin, K.C.; Franklin, B.A.; Miller, W.M.; Peterson, E.D.; McCullough, P.A. Impact of obesity on cardiovascular disease. Med. Clin. North Am. 2011, 95, 919–937. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, Hypertension, and Cardiovascular Disease An Update. Hypertension 2001, 37, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, P.S. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr. Pract. 2012, 18, 1–78. [Google Scholar] [PubMed]
- Halperin, R.O.; Sesso, H.D.; Ma, J.; Buring, J.E.; Stampfer, M.J.; Gaziano, J.M. Dyslipidemia and the risk of incident hypertension in men. Hypertension 2006, 47, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.R.; Carr, C.S.; al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Lichstein, P.R. The Medical Interview. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, CA, USA, 1990. [Google Scholar]
- Hall, J.E.; Granger, J.P.; do Carmo, J.M.; da Silva, A.A.; Dubinion, J.; George, E.; Hamza, S.; Speed, J.; Hall, M.E. Hypertension: Physiology and pathophysiology. Compr. Physiol. 2012, 2, 2393–2442. [Google Scholar] [PubMed]
- Oparil, S.; Zaman, M.A.; Calhoun, D.A. Review Pathogenesis of Hypertension. Ann. Intern. Med. 2003, 139, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Leenen, F.H.H.; Chen, L.; Golovina, V.A.; Hamlyn, J.M.; Pallone, T.L.; van Huysse, J.W.; Zhang, J.; Wier, W.G. How NaCl raises blood pressure: A new paradigm for the pathogenesis of salt-dependent hypertension. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1031–H1049. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guideline: Potassium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- World Health Organization (WHO). Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Geiger, H.; Wanner, C. Magnesium in disease. CKJ Clin. Kidney J. 2012, 5, i25–i38. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- McAreavey, D.; RoMcAreavbertson, J.I. Angiotensin converting enzyme inhibitors and moderate hypertension. Drugs 1990, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Uk, J.M.; Uk, H.D.; Poland, M.T.; Kjekshus, J.; France, P.L.; Denmark, C.T.; Committee, E.S.C.; Cpg, G.; Priori, S.G.; Angeles, M.; et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease: The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur. Heart J. 2004, 25, 1454–1470. [Google Scholar] [CrossRef] [PubMed]
- Barreras, A.; Gurk-Turner, C. Angiotensin II receptor blockers. Proc. (Bayl. Univ. Med. Cent.) 2003, 16, 123–126. [Google Scholar] [PubMed]
- Sanoski, C.A. Aliskiren: An oral direct renin inhibitor for the treatment of hypertension. Pharmacotherapy 2009, 29, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, L.G.; Bonanno, E.; Sangiorgi, G.; Mauriello, A. Role of inflammation in atherosclerosis. J. Nucl. Med. 2007, 48, 1800–1815. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L.; Uzick, M. Cardiovascular Disease: C-Reactive Protein and the Inflammatory Disease Paradigm: HMG-CoA Reductase Inhibitors, alpha-Tocopherol, Red Yeast Rice, and Olive Oil Polyphenols. A Review of the Literature. Altern. Med. Rev. 2001, 6, 248–271. [Google Scholar] [PubMed]
- Cardoso, S.M.; Catarino, M.D.; Semião, M.S.; Pereira, O.R. Virgin Olive Oil As a Source of Anti-Inflammatory Agents. In Virgin Olive Oil: Production, Composition, Uses and Benefits for Man; de Leonardis, A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014; pp. 187–209. [Google Scholar]
- Badimon, L.; Padró, T.; Vilahur, G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Hear. J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar]
- Pahan, K. Lipid-lowering drugs. Cell Mol. Life Sci. 2006, 63, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-M.; Shih, W.-T.; Yang, Y.-H.; Chen, P.-C.; Chu, Y.-H. Use of traditional Chinese medicine in patients with hyperlipidemia: A population-based study in Taiwan. J. Ethnopharmacol. 2015, 168, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Mirhoseini, M.; Shirzad, H.; Sedighi, M.; Shahinfard, N.; Rafieian-Kopaei, M. A Review on Promising Natural Agents Effective on Hyperlipidemia. J. Evid. Based Complement. Altern. Med. 2015, 20, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.J.; Morcillo, M.; Tenorio, M.D.; Mateos-Aparicio, I.; Andrés, V.; Redondo-Cuenca, A. Health-promoting effects in the gut and influence on lipid metabolism of Himanthalia elongata and Gigartina pistillata in hypercholesterolaemic Wistar rats. Eur. Food Res. Technol. 2014, 238, 409–416. [Google Scholar] [CrossRef]
- Kumar, S.; Magnusson, M.; Ward, L.; Paul, N.; Brown, L. Seaweed Supplements Normalise Metabolic, Cardiovascular and Liver Responses in High-Carbohydrate, High-Fat Fed Rats. Mar. Drugs 2015, 13, 788–805. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant and hypolipidaemic properties of red seaweed, Gracilaria changii. J. Appl. Phycol. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.K. Insulinotrophic and hypolipidemic effects of Ecklonia cava in streptozotocin-induced diabetic mice. Asian Pac. J. Trop. Med. 2012, 5, 374–379. [Google Scholar] [CrossRef]
- Ruqqia, K.; Sultana, V.; Ara, J.; Ehteshamul-Haque, S.; Athar, M. Hypolipidaemic potential of seaweeds in normal, triton-induced and high-fat diet-induced hyperlipidaemic rats. J. Appl. Phycol. 2015, 27, 571–579. [Google Scholar] [CrossRef]
- Riaz, B.; Najam, R.; Anser, H.; Ali, M.S. Evaluation of Iyengariastellata for its hypolipidemic and hepatoprotective activity. Pak. J. Pharm. Sci. 2014, 27, 1775–1779. [Google Scholar] [PubMed]
- Dousip, A.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B.H.; Lim, T.P. Effect of seaweed mixture intake on plasma lipid and antioxidant profile of hyperholesterolaemic rats. J. Appl. Phycol. 2014, 26, 999–1008. [Google Scholar] [CrossRef]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Matloub, A.A.; Aly, H.F.; El, A.; Farrag, R.; Fouad, G.I. Hypolipidemic and Anti-atherogenic Effect of Sulphated Polysaccharides from the Green Alga Ulva fasciata. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 1–12. [Google Scholar]
- Hoang, M.H.; Kim, J.-Y.; Lee, J.H.; You, S.G.; Lee, S.-J. Antioxidative, hypolipidemic, and anti-inflammatory activities of sulfated polysaccharides from Monostroma nitidum. Food Sci. Biotechnol. 2015, 24, 199–205. [Google Scholar] [CrossRef]
- Hassan, S.; El-Twab, S.A.; Hetta, M.; Mahmoud, B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi J. Biol. Sci. 2011, 18, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Cuong, H.D.; Thuy, T.T.T.; Huong, T.T.; Ly, B.M.; Van, T.T.T. Structure and hypolipidaemic activity of fucoidan extracted from brown seaweed Sargassum henslowianum. Nat. Prod. Res. 2015, 29, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jeon, J.; Lee, J.S. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phyther. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.V.; Bogdanovich, L.N.; Ivanova, T.B.; Byankina, A.O.; Kryzhanovskiy, S.P.; Yermak, I.M. Effect of carrageenan food supplement on patients with cardiovascular disease results in normalization of lipid profile and moderate modulation of immunity system markers. PharmaNutrition 2014, 2, 33–37. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Konno, K.; Hosokawa, M.; Maeda, H.; Sashima, T.; Miyashita, K. Fucoxanthin and fucoxanthinol enhance the amount of docosahexaenoic acid in the liver of KKAy obese/diabetic mice. J. Agric. Food Chem. 2007, 55, 5025–5029. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Baba, N.; Hosokawa, M.; Sashima, T.; Miyashita, K. Enhancement of hepatic docosahexaenoic acid and arachidonic acid contents in C57BL/6J mice by dietary fucoxanthin. Fish. Sci. 2009, 75, 261–263. [Google Scholar] [CrossRef]
- Miyashita, K.; Narayan, B.; Tsukui, T.; Kamogawa, H.; Abe, M.; Hosokawa, M. Brown seaweed lipids as potential source of omega-3 PUFA in biological systems. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: West Sussex, UK, 2011; pp. 329–339. [Google Scholar]
- Aki, T.; Yamamoto, M.; Takahashi, T.; Tomita, K.; Toyoura, R.; Iwashita, K.; Kawamoto, S.; Hosokawa, M.; Miyashita, K.; Ono, K. Regulation of polyunsaturated fatty acid biosynthesis by seaweed fucoxanthin and its metabolite in cultured hepatocytes. Lipids 2014, 49, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; D’Orazio, N.; Franceschelli, S.; Speranza, L. Marine carotenoids and cardiovascular risk markers. Mar. Drugs 2011, 9, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L. Stearidonic acid (18:4n-3): Metabolism, nutritional importance, medical uses and natural sources. Eur. J. Lipid Sci. Technol. 2007, 109, 1226–1236. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Aschwin, E.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J.D. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; Mcsorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Airanthi, M.K.W.-A.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Hosokawa, M.; Niwano, Y.; Miyashita, K. Effects of dietary fucoxanthin on cholesterol metabolism in diabetic/obese KK-Ay mice. Lipids Health Dis. 2012, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Lee, K.-W.; Jeon, Y.-J. Screening of Extracts from Red Algae in Jeju for Potentials Marine Angiotensin-I Converting Enzyme (ACE) Inhibitory Activity. Algae 2006, 21, 343–348. [Google Scholar] [CrossRef]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish. Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.; Ko, S.C.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on angiotensin I-converting enzyme (ACE) inhibitory activity. Nutr. Res. Pract. 2011, 5, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Maekawa, K.; Chen, J.R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sivagnanam, S.; Yin, S.; Choi, J.; Park, Y.; Woo, H.; Chun, B. Biological Properties of Fucoxanthin in Oil Recovered from Two Brown Seaweeds Using Supercritical CO2 Extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Caballero, M.S.; Sabbag, N.; González, R.J.; Drago, S.R. Bio-accessibility of bioactive compounds (ACE inhibitors and antioxidants) from extruded maize products added with a red seaweed Porphyra columbina. LWT—Food Sci. Technol. 2014, 55, 51–58. [Google Scholar] [CrossRef]
- Qu, W.; Ma, H.; Pan, Z.; Luo, L.; Wang, Z.; He, R. Preparation and antihypertensive activity of peptides from Porphyra yezoensis. Food Chem. 2010, 123, 14–20. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Bondu, S.; Bonnet, C.; Gaubert, J.; Deslandes, É.; Turgeon, S.L.; Beaulieu, L. Bioassay-guided fractionation approach for determination of protein precursors of proteolytic bioactive metabolites from macroalgae. J. Appl. Phycol. 2014, 27, 2059–2074. [Google Scholar] [CrossRef]
- Lin, H.C.; Chou, S.T.; Chuang, M.Y.; Liao, T.Y.; Tsai, W.S.; Chiu, T.H. The effects of Caulerpa microphysa enzyme-digested extracts on ACE-inhibitory activity and in vitro anti-tumour properties. Food Chem. 2012, 134, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Nakano, T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef]
- Ramirez-Higuera, A.; Quevedo-Corona, L.; Paniagua-Castro, N.; Chamorro-Ceballos, G.; Milliar-Garcia, A.; Jaramillo-Flores, M.E. Antioxidant enzymes gene expression and antihypertensive effects of seaweeds Ulva linza and Lessonia trabeculata in rats fed a high-fat and high-sucrose diet. J. Appl. Phycol. 2014, 26, 597–605. [Google Scholar] [CrossRef]
- Wada, K.; Nakamura, K.; Tamai, Y.; Tsuji, M.; Sahashi, Y.; Watanabe, K.; Ohtsuchi, S.; Yamamoto, K.; Ando, K.; Nagata, C. Seaweed intake and blood pressure levels in healthy pre-school Japanese children. Nutr. J. 2011, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Teas, J.; Baldeón, M.E.; Chiriboga, D.E.; Davis, J.R.; Sarriés, A.J.; Braverman, L.E. Could dietary seaweed reverse the metabolic syndrome? Asia Pac. J. Clin. Nutr. 2009, 18, 145–154. [Google Scholar] [PubMed]
- Hata, Y.; Nakaijima, K.; Uchida, J.; Hidaka, H.; Nakano, T. Clinical effects of brown seaweed, Undaria pinnatifida (wakame), on blood pressure in hypertensive subjects. J. Clin. Biochem. Nutr. 2001, 30, 43–53. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Abdalla, M.S.; Eskander, E.F.; Al-Khadragy, M.F.; Massoud, M.N. Hypolipidemic influence of Sargassum subrepandum: Mechanism of action. Eur. Rev. Med. Pharmacol. Sci. 2012, 16 (Suppl. 3), 112–120. [Google Scholar] [PubMed]
- Chen, Y.Y.; Ji, W.; Du, J.R.; Yu, D.K.; He, Y.; Yu, C.X.; Li, D.S.; Zhao, C.Y.; Qiao, K.Y. Preventive effects of low molecular mass potassium alginate extracted from brown algae on DOCA salt-induced hypertension in rats. Biomed. Pharmacother. 2010, 64, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Noda, H.; Amano, H.; Nishino, T.; Nishizawa, K. Study on Antihypertensive and Antihyperlipidemic Effects of Marine Algae. Fish. Sci. 1994, 60, 83–88. [Google Scholar]
- Krotkiewski, M.; Aurel, M.; Holm, G.; Grimby, G.; Szczepanik, J. Effects of a sodium-potassium ion-exchanging seaweed preparation in mild hypertension. Am. J. Hypertens. 1991, 4, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a Renin Inhibitory Peptide from the Red Seaweed Palmaria palmata as a Functional Food Ingredient Following Confirmation and Characterization of a Hypotensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mugica, A.; Batista-Gonzalez, A.E.; Mondejar, D.; Soto-López, Y.; Brito-Navarro, V.; Vázquez, A.M.; Brömme, D.; Zaldívar-Muñoz, C.; Vidal-Novoa, A.; e Silva, A.M.D.O.; et al. Inhibition of LDL-oxidation and antioxidant properties related to polyphenol content of hydrophilic fractions from seaweed Halimeda Incrassata (Ellis) Lamouroux. Brazilian J. Pharm. Sci. 2012, 48, 31–37. [Google Scholar] [Green Version]
- Kim, T.H.; Ku, S.K.; Lee, T.; Bae, J.S. Vascular barrier protective effects of phlorotannins on HMGB1-mediated proinflammatory responses in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Adiponectin: Action, regulation and association to insulin sensitivity. Obes. Rev. 2005, 6, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Preetha, S.P.; Devaraj, H. Role of sulphated polysaccharides from Sargassum Wightii in control of diet-induced hyperlipidemia and associated inflammatiory complications in rats. Eur. J. Inflamm. 2010, 8, 23–30. [Google Scholar]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–233. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.B.; Sweeney, T.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. The effect of dietary Laminaria-derived laminarin and fucoidan on nutrient digestibility, nitrogen utilisation, intestinal microflora and volatile fatty acid concentration in pigs. J. Sci. Food Agric. 2010, 90, 430–437. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Cifuentes, A.; Ibanez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Mendis, E.; Kim, S.K. Present and future prospects of seaweeds in developing functional foods. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volme 64, pp. 1–15. [Google Scholar]
- Cofrades, S.; Serdaroğlu, M.; Jiménez-Colmenero, F. Design of healthier foods and beverages containing whole algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 609–633. [Google Scholar]
- Cofrades, S.; López-López, I.; Jiménez-Colmenero, F. Applications of seaweed in meat-based functional foods. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.-K., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2012; pp. 491–499. [Google Scholar]
- Lee, D.S.; Shin, M.K. Functional Meat Sauce Containing Polymann Having Effect of Reducing Neutral Lipids and Cholesterol Without Any Adverse Effect as Main Component. KR-20030045232-A, 2005. [Google Scholar]
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; López-López, I.; Solas, M.T.; Bravo, L.; Jiménez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Bastida, S.; Ruiz-Capillas, C.; Bravo, L.; Larrea, M.T.; Sánchez-Muniz, F.; Cofrades, S.; Jiménez-Colmenero, F. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci. 2009, 83, 492–498. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Cofrades, S.; Ruiz-Capillas, C.; Jiménez-Colmenero, F. Design and nutritional properties of potential functional frankfurters based on lipid formulation, added seaweed and low salt content. Meat Sci. 2009, 83, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.R.; Choi, S.H. Quality Characteristics of the Hamburger Patties with Sea Tangle (Laminaria japonica) Powder and/or Cooked Rice. Korean J. Food Sci. Anim. Resour. 2012, 32, 77–83. [Google Scholar] [CrossRef]
- Choi, Y.S.; Choi, J.H.; Han, D.J.; Kim, H.Y.; Kim, H.W.; Lee, M.A.; Chung, H.J.; Kim, C.J. Effects of Laminaria japonica on the physico-chemical and sensory characteristics of reduced-fat pork patties. Meat Sci. 2012, 91, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Choi, J.H.; Choi, Y.S.; Han, D.J.; Kim, H.Y.; Lee, M.A.; Kim, S.Y.; Kim, C.J. Effects of sea tangle (Lamina japonica) Powder on quality characteristics of breakfast sausages. Korean J. Food Sci. Anim. Resour. 2010, 30, 55–61. [Google Scholar] [CrossRef]
- Schultz Moreira, A.R.; Benedí, J.; González-Torres, L.; Olivero-David, R.; Bastida, S.; Sánchez-Reus, M.I.; González-Muñoz, M.J.; Sánchez-Muniz, F.J. Effects of diet enriched with restructured meats, containing Himanthalia elongata, on hypercholesterolaemic induction, CYP7A1 expression and antioxidant enzyme activity and expression in growing rats. Food Chem. 2011, 129, 1623–1630. [Google Scholar] [CrossRef]
- Schultz Moreira, A.R.; Benedi, J.; Bastida, S.; Sánchez-Reus, I.; Sánchez-Muniz, F.J. Nori- and sea spaghetti- but not wakame-restructured pork decrease the hypercholesterolemic and liver proapototic short-term effects of high-dietary cholesterol consumption. Nutr. Hosp. 2013, 28, 1422–1429. [Google Scholar] [PubMed]
- Schultz Moreira, A.R.; Olivero-David, R.; Vázquez-Velasco, M.; González-Torres, L.; Benedí, J.; Bastida, S.; Sánchez-Muniz, F.J. Protective Effects of Sea Spaghetti-Enriched Restructured Pork Against Dietary Cholesterol: Effects on Arylesterase and Lipoprotein Profile and Composition of Growing Rats. J. Med. Food 2014, 17, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.R.S.; Garcimartín, A.; Bastida, S.; Jiménez-escrig, A.; Rupérez, P.; Green, B.D.; Rafferty, E.; Sánchez-muniz, F.J.; Benedí, J. Effects of Undaria pinnatifida, Himanthalia elongata and Porphyra umbilicalis extracts on in vitro α-glucosidase activity and glucose diffusion. Nutr. Hosp. 2014, 29, 1434–1446. [Google Scholar] [PubMed]
- Moreira, A.S.; González-Torres, L.; Olivero-David, R.; Bastida, S.; Benedi, J.; Sánchez-Muniz, F.J. Wakame and Nori in Restructured Meats Included in Cholesterol-enriched Diets Affect the Antioxidant Enzyme Gene Expressions and Activities in Wistar Rats. Plant Foods Hum. Nutr. 2010, 65, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Olivero-David, R.; Schultz-Moreira, A.; Vázquez-Velasco, M.; González-Torres, L.; Bastida, S.; Benedí, J.; Isabel Sanchez-Reus, M.; José González-Muñoz, M.; Sánchez-Muniz, F.J. Effects of Nori- and Wakame-enriched meats with or without supplementary cholesterol on arylesterase activity, lipaemia and lipoproteinaemia in growing Wistar rats. Br. J. Nutr. 2011, 106, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Kim, H.-H. Effects of the sea tangle-added patty on postprandial blood glucose and lipid profiles in borderline-hyperlipidemic adults. FASEB J. 2013, 27, 1079.22. [Google Scholar]
- Fitzgerald, C.; Gallagher, E.; Doran, L.; Auty, M.; Prieto, J.; Hayes, M. Increasing the health benefits of bread: Assessment of the physical and sensory qualities of bread formulated using a renin inhibitory Palmaria palmata protein hydrolysate. LWT—Food Sci. Technol. 2014, 56, 398–405. [Google Scholar] [CrossRef]
- Hall, A.C.; Fairclough, A.C.; Mahadevan, K.; Paxman, J.R. Ascophyllum nodosum enriched bread reduces subsequent energy intake with no effect on post-prandial glucose and cholesterol in healthy, overweight males. A pilot study. Appetite 2012, 58, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Suzuki, N.; Nagashima, T. Angiotensin I-converting enzyme inhibitory activities of beverages made from sea algae and commercially available tea extracts. J. Food Agric. Environ. 2006, 4, 17–19. [Google Scholar]
- Fu, X.; Gao, Y.; Li, L.; Wang, J.; Xue, C.; Xu, J.; Yang, Q. Beverage Containing Water Insoluble Dietary Fiber Useful for Preventing and/or Treating e.g., Cardiovascular Disease, Diabetes and Gallstone, Comprises Algae Dietary Fiber, Citric Acid, Sugar, Fruit Juice, Plant Hardener and Water. CN 101427835 A, 13 May 2009. [Google Scholar]
- Kim, K.S. Beverage Composition Using Sea Weed Fusiforme and Onion for the Prevention of Hypertension. WO 2008032958 A1, 20 March 2008. [Google Scholar]
- Lee, D.S.; Shin, M.K. Functional Beverage Useful for Cardiovascular Disease and Liver Function Containing Polymann. KR 2005003746 A, 2005. [Google Scholar]
- Nagai, T.; Yukimoto, T. Preparation and functional properties of beverages made from sea algae. Food Chem. 2003, 81, 327–332. [Google Scholar] [CrossRef]
- Kim, Y.M.; Byun, J.Y.; Namgung, B.; Jo, J.H.; Do, J.R.; In, J.P. Studies on Functional Salt Fortified with Seaweed Components. Food and Agriculture Organization of the United Nations, 2007. Available online: http://agris.fao.org/agris-search/search.do?recordID=KR2008001412 (accessed on 6 November 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838-6865. https://doi.org/10.3390/md13116838
Cardoso SM, Pereira OR, Seca AML, Pinto DCGA, Silva AMS. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Marine Drugs. 2015; 13(11):6838-6865. https://doi.org/10.3390/md13116838
Chicago/Turabian StyleCardoso, Susana M., Olívia R. Pereira, Ana M. L. Seca, Diana C. G. A. Pinto, and Artur M. S. Silva. 2015. "Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods" Marine Drugs 13, no. 11: 6838-6865. https://doi.org/10.3390/md13116838
APA StyleCardoso, S. M., Pereira, O. R., Seca, A. M. L., Pinto, D. C. G. A., & Silva, A. M. S. (2015). Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Marine Drugs, 13(11), 6838-6865. https://doi.org/10.3390/md13116838