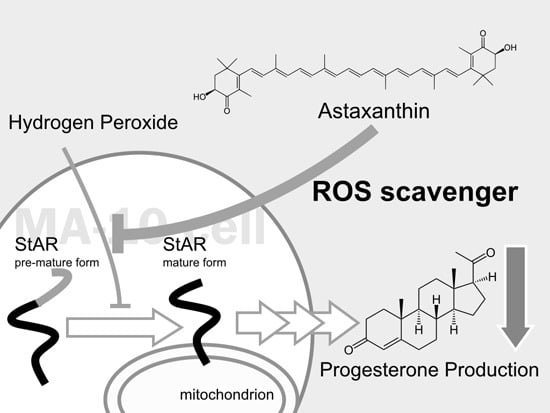
Astaxanthin Protects Steroidogenesis from Hydrogen Peroxide-Induced Oxidative Stress in Mouse Leydig Cells
Abstract
:1. Introduction

2. Results
2.1. Astaxanthin (AST) Rescues Progesterone and Testosterone Secretion from Testicular Leydig Cells Reduced by Oxidative Stress


2.2. AST Reduced ROS Levels in MA-10 Leydig Cells

2.3. AST Protects the Expression Level of Steroidogenic Proteins (StAR, P450scc, and 3β-HSD) in MA-10 Leydig Cells during Oxidative Stress

3. Discussion
4. Experimental Section
4.1. Reagents and Chemicals
4.2. Primary Mouse Leydig Cell Culture
4.3. Culture of MA-10 Cells
4.4. Enzyme Immunoassay for Progesterone and Testosterone
4.5. Staining of Intracellular ROS of MA-10 Cells
4.6. Western Blot Analysis
4.7. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ge, R.; Hardy, M. Regulation of Leydig Cells During Pubertal Development. In The Leydig Cell in Health and Disease; Humana Press: Totowa, NJ, USA, 2007; pp. 55–70. [Google Scholar]
- Svechnikov, K.; Izzo, G.; Landreh, L.; Weisser, J.; Soder, O. Endocrine disruptors and Leydig cell function. J. Biomed. Biotechnol. 2010, 2010, 684504. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.M.; Mahipal, S.V.; Subhashini, J.; Reddy, M.C.; Roy, K.R.; Reddy, G.V.; Reddy, P.R.; Reddanna, P. Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod. Toxicol. 2006, 22, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Diemer, T.; Allen, J.A.; Hales, K.H.; Hales, D.B. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 2003, 144, 2882–2891. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Lu, C.C.; Lin, C.S.; Wang, P.S. Antisteroidogenic actions of hydrogen peroxide on rat Leydig cells. J. Cell. Biochem. 2003, 90, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Hales, D.B.; Allen, J.A.; Shankara, T.; Janus, P.; Buck, S.; Diemer, T.; Hales, K.H. Mitochondrial function in Leydig cell steroidogenesis. Ann. N. Y. Acad. Sci. 2005, 1061, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, P.; Muthusamy, T.; Balasubramanian, K.; Arunakaran, J. Studies on the protective role of vitamin C and E against polychlorinated biphenyl (Aroclor 1254)—Induced oxidative damage in Leydig cells. Free Radic. Res. 2005, 39, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.I.; Jin, B.; Youn, P.; Park, C.; Park, J.D.; Ryu, D.Y. Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol. Appl. Pharmacol. 2007, 218, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Liu, S.Y.; Sun, H.; Wu, X.M.; Li, J.J.; Zhu, L. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, C.S.; Lee, Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011, 49, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Ranga Rao, A.; Raghunath Reddy, R.L.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J. Agric. Food Chem. 2010, 58, 8553–8559. [Google Scholar]
- Wiki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–161. [Google Scholar]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Bolin, A.P.; Macedo, R.C.; Marin, D.P.; Barros, M.P.; Otton, R. Astaxanthin prevents in vitro auto-oxidative injury in human lymphocytes. Cell Biol. Toxicol. 2010, 26, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in cardiovascular health and disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.N.; Jena, G.B. Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology 2008, 248, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Comhaire, F.H.; El Garem, Y.; Mahmoud, A.; Eertmans, F.; Schoonjans, F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: A double blind, randomized trial. Asian J. Androl. 2005, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dufau, M.L. The luteinizing hormone receptor. Annu. Rev. Physiol. 1998, 60, 461–496. [Google Scholar] [CrossRef] [PubMed]
- Hansson, V.; Skalhegg, B.S.; Tasken, K. Cyclic-AMP-dependent protein kinase (PKA) in testicular cells. Cell specific expression, differential regulation and targeting of subunits of PKA. J. Steroid Biochem. Mol. Biol. 1999, 69, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.C.; Behrman, H.R. In vivo generation of hydrogen peroxide in the rat corpus luteum during luteolysis. Endocrinology 1991, 128, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.; Carrasco, I.; Castillo, T.; Troncoso, J.L.; Videla, L.A.; Devoto, L. Functional luteolysis in response to hydrogen peroxide in human luteal cells. J. Endocrinol. 1995, 147, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Behrman, H.R.; Aten, R.F. Evidence that hydrogen peroxide blocks hormone-sensitive cholesterol transport into mitochondria of rat luteal cells. Endocrinology 1991, 128, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Aten, R.F.; Behrman, H.R. Inhibition of protein synthesis and hormone-sensitive steroidogenesis in response to hydrogen peroxide in rat luteal cells. Endocrinology 1994, 134, 588–595. [Google Scholar] [PubMed]
- Brannian, J.D.; Larson, E.A.; Kurz, S.G.; Chaput, G.M. Hydrogen peroxide suppresses low-density lipoprotein (LDL) uptake and LDL-supported steroidogenesis by porcine luteal cells. Mol. Cell Endocrinol. 1995, 111, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Abidi, P.; Zhang, H.; Zaidi, S.M.; Shen, W.J.; Leers-Sucheta, S.; Cortez, Y.; Han, J.; Azhar, S. Oxidative stress-induced inhibition of adrenal steroidogenesis requires participation of p38 mitogen-activated protein kinase signaling pathway. J. Endocrinol. 2008, 198, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Abidi, P.; Leers-Sucheta, S.; Cortez, Y.; Han, J.; Azhar, S. Evidence that age-related changes in p38 MAP kinase contribute to the decreased steroid production by the adrenocortical cells from old rats. Aging Cell 2008, 7, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Wells, J.; Clark, B.J. The effects of hydrogen peroxide on steroidogenesis in mouse Leydig tumor cells. Endocrinology 1993, 133, 2827–2832. [Google Scholar] [PubMed]
- Lee, S.Y.; Gong, E.Y.; Hong, C.Y.; Kim, K.H.; Han, J.S.; Ryu, J.C.; Chae, H.Z.; Yun, C.H.; Lee, K. ROS inhibit the expression of testicular steroidogenic enzyme genes via the suppression of Nur77 transactivation. Free Radic. Biol. Med. 2009, 47, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, P.; Muthusamy, T.; Balasubramanian, K.; Arunakaran, J. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod. Toxicol. 2008, 25, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.S.; Chen, J.C.; Sheu, S.Y.; Huang, C.C.; Kuo, Y.H.; Chiu, C.H.; Lian, W.X.; Yang, C.J.; Kaphle, K.; Lin, J.H. Isocupressic acid blocks progesterone production from bovine luteal cells. Am. J. Chin. Med. 2002, 30, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Guo, I.C.; Wu, L.S.; Lin, J.H.; Chung, B.C. Differential inhibition of progesterone synthesis in bovine luteal cells by estrogens and androgens. Life Sci. 2001, 68, 1851–1865. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Fei, C.Y.; Srinivasan, R.; Wu, L.S. Inhibitory effects of epidermal growth factor on progesterone production of ovarian granulosa cells in Tsaiya duck (Anas platyrhynchos var. domestica). Br. Poult. Sci. 2010, 51, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, L.; Lin, C.Y.; Beattie, M.C.; Liu, J.; Zirkin, B.R. Effect of glutathione redox state on Leydig cell susceptibility to acute oxidative stress. Mol. Cell Endocrinol. 2010, 323, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Crochet, S.; Ferré, P.; Foufelle, F.; Tesseraud, S.; Dupont, J. AMP-activated protein kinase activation modulates progesterone secretion in granulosa cells from hen preovulatory follicles. J. Endocrinol. 2006, 190, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Wu, L.S.; Jong, D.S. Production and application of a polyclonal peptide antiserum for universal detection of StAR protein. Chin. J. Physiol. 2008, 51, 54–61. [Google Scholar] [PubMed]
- Chiu, C.H.; Wei, H.W.; Wu, L. Generation and utilization of P450 cholesterol side-chain cleavage enzyme and 3β-hydroxysteroid dehydrogenase antibodies for universal detection. J. Immunoass. 2008, 29, 152–160. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-Y.; Lee, Y.-J.; Chou, M.-C.; Chang, R.; Chiu, C.-H.; Liang, Y.-J.; Wu, L.-S. Astaxanthin Protects Steroidogenesis from Hydrogen Peroxide-Induced Oxidative Stress in Mouse Leydig Cells. Mar. Drugs 2015, 13, 1375-1388. https://doi.org/10.3390/md13031375
Wang J-Y, Lee Y-J, Chou M-C, Chang R, Chiu C-H, Liang Y-J, Wu L-S. Astaxanthin Protects Steroidogenesis from Hydrogen Peroxide-Induced Oxidative Stress in Mouse Leydig Cells. Marine Drugs. 2015; 13(3):1375-1388. https://doi.org/10.3390/md13031375
Chicago/Turabian StyleWang, Jyun-Yuan, Yue-Jia Lee, Mei-Chia Chou, Renin Chang, Chih-Hsien Chiu, Yao-Jen Liang, and Leang-Shin Wu. 2015. "Astaxanthin Protects Steroidogenesis from Hydrogen Peroxide-Induced Oxidative Stress in Mouse Leydig Cells" Marine Drugs 13, no. 3: 1375-1388. https://doi.org/10.3390/md13031375
APA StyleWang, J. -Y., Lee, Y. -J., Chou, M. -C., Chang, R., Chiu, C. -H., Liang, Y. -J., & Wu, L. -S. (2015). Astaxanthin Protects Steroidogenesis from Hydrogen Peroxide-Induced Oxidative Stress in Mouse Leydig Cells. Marine Drugs, 13(3), 1375-1388. https://doi.org/10.3390/md13031375