Synthesis and in Vitro Antiproliferative Evaluation of Some B-norcholesteryl Benzimidazole and Benzothiazole Derivatives
Abstract
:1. Introduction

2. Results and Discussion
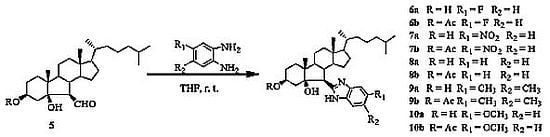
2.1. Chemistry





2.2. Biological Results and Discussion
| Compounds | Cells | |||
|---|---|---|---|---|
| HeLa | A549 | HEPG2 | HEK293T | |
| 7a | 4.2 | 66.7 | >40 | 19.1 |
| 7b | 15.9 | 28.2 | 21.2 | 20.3 |
| 8a | 3.6 | 47.2 | 21.8 | 37.2 |
| 8b | 31.2 | 50.6 | 30.3 | >80 |
| 9a | 4.9 | 61.2 | 29.6 | 53.3 |
| 9b | 4.7 | 11.9 | 4.2 | >80 |
| 10a | 7.5 | 13.1 | 4.5 | >80 |
| 10b | 2.2 | 31.2 | >40 | >80 |
| 11a | 7.5 | 14.0 | 8.4 | >80 |
| 11b | 11.8 | >80 | >40 | >80 |
| 12a | 16.6 | 27.0 | 12.2 | >100 |
| 12b | 3.7 | >80 | >80 | >100 |
| 13 | 22.0 | >80 | >80 | 58.3 |
| 15 | 20.6 | >80 | >80 | >80 |
| Compounds | SI HeLa | SI A549 | SI HEPG2 |
|---|---|---|---|
| 7a | 4.5 | - | - |
| 7b | 1.3 | - | - |
| 8a | 10.3 | - | 1.7 |
| 8b | 2.6 | 1.6 | 2.6 |
| 9a | 10.9 | - | 1.8 |
| 9b | 17.0 | 6.7 | 19.0 |
| 10a | 10.7 | 6.1 | 17.8 |
| 10b | 36.4 | 2.6 | - |
| 11a | 10.7 | 5.7 | 9.5 |
| 11b | 6.8 | - | - |
| 12a | 6.0 | 3.7 | 8.2 |
| 12b | 27.0 | - | - |
| 13 | 2.7 | - | - |
| 15 | 3.9 | - | - |
- (1)
- The 6-benzimidazole group is a better substituent than 6-benzothiazole group for increasing the antiproliferative activity of compounds (compare 9a and 13).
- (2)
- The presence of electron-withdrawing groups in the benzimidazole will decrease the cytotoxicity of the compounds and electron-donating groups show not an obvious effect for cytotoxicity of compounds (compare 7b, 8b and 9b or 9a, 10a and 11a).
- (3)
- Introduction of an isoxazolidine ring joined with ring B or an 6-imine moiety cannot increased the cytotoxicity of the compounds (compare 7a and 12a, 12b). The introduction of pyridine ring in benzimidazole deceases the cytotoxicity of the compound on HeLa and HEPG2 cells (compare 9a, 12a and 15).



3. Experimental Section
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Compounds 7–11
3.1.2. Compounds 12a–12b Were Prepared Similarly According to the Procedure of 7–11, but Using 4-Trifluoromethyl-O-phenylenediamine as Reagent
3.1.3. Compound 13 Was Prepared Similarly According to the Procedure of 7–11, but Using 2-Mercaptophenylamine as Reagent
3.1.4. 3β,5β-Dihydroxy-6-(N-3′-(2-amino)pyridyl)imine-B-norcholestane (15)
3.2. Biological Assays
3.2.1. Materials
3.2.2. Cell Culture
3.2.3. Assay for Cell Viability
3.2.4. Annexin V Staining Assay
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Jemal, A.; Siegel, R.; Ward, E.C.A. Cancer Statistics, 2010. Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Miyamoto, T.; Kodama, K.; Aramaki, H.Y.R.; van Soest, R.W.M. Orostanal, a novel abeo-sterol inducing apoptosis in leukemia cell from a marine sponge, Stelletta hiwasaensis. Tetrahedron Lett. 2001, 42, 6349–6351. [Google Scholar] [CrossRef]
- Wei, X.; Rodríguez, A.D.; Wang, Y.; Franzblau, S.G. Novel ring B abeo-sterols as growth inhibitors of Mycobacterium tuberculosis isolated from a Caribbean Sea sponge, Svenzea zeai. Tetrahedron Lett. 2007, 48, 8851–8854. [Google Scholar] [CrossRef]
- Gan, C.F.; Fan, L.H.; Cui, J.G.; Huang, Y.M.; Jiao, Y.X.; Wei, W.X. Synthesis and in vitro antiproliferative evaluation of some ring B abeo-sterols. Steroids 2012, 77, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.F.; Fan, L.H.; Huang, Y.M.; Liu, Z.P.; Cui, J.G. Synthesis of novel ring B abeo-sterol derivatives and their antiproliferative activities. Med. Chem. 2013, 9, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.F.; Lin, Q.F.; Cui, J.G.; Feng, J.D.; Guo, J.N.; Liao, H.Y.; Huang, Y.M. Synthesis and in vitro antiproliferative evaluation of some novel B-norcholesterols. Steroids 2014, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kapoor, V.K.; Paul, D. Heterosteroids and drug research. In Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Springer: Amsterdam, The Netherlands, 1979; Volume 16, pp. 35–150. [Google Scholar]
- Singh, H.; Jindal, D.P.; Yadav, M.R.; Kumar, M. Heterosteroids and drug research. In Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier science publishers: Amsterdam, The Netherlands, 1991; Volume 28, pp. 233–300. [Google Scholar]
- Lakhani, N.J.; Sarkar, M.A.; Venitz, J.; Figg, W.D. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy 2003, 23, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Carlini, P.; Frassoldati, A.; Marco, S.D.; Casali, A.; Ruggeri, E.M.; Nardi, M.; Papaldo, P.; Fabi, A.; Paoloni, F.; Cognetti, F. Formestane, a steroidal aromatase inhibitor after failure of non-steroidal aromatase inhibitors (anastrozole and letrozole): Is a clinical benefit still achievable? Ann. Oncol. 2001, 12, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.; Wagstaff, A.J. Estramustine Phosphate Sodium. Am. J. Can. 2003, 2, 373–390. [Google Scholar] [CrossRef]
- Attard, G.; Reid, A.H.M.; Yap, T.A.; Raynaud, F.; Dowsett, M.; Settatree, S.; Barrett, M.; Parker, C.; Martins, V.; Folkerd, E.; et al. Phase I Clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 2008, 26, 4563–4571. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.G.; Liu, L.; Gan, C.F.; Xiao, Q.; Huang, Y.M. Recent progress in synthesis and biological activity of steroids bearing aromatic rings and heterocycles. Progress Chem. 2014, 26, 320–333. [Google Scholar]
- Ma, B.; Xiao, Z.Y.; Chen, Y.J.; Lei, M.; Meng, Y.H.; Guo, D.A.; Liu, X.; Hu, L.H. Synthesis and structure activity relationships study of cytotoxic bufalin 3-nitrogen-containing-ester derivatives. Steroids 2013, 78, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, G.L.; Zhang, T.; He, X.R.; Wu, Z.Y.; Xiao, Y.L.; Pan, Y.H.; Qiu, G.F.; Liu, P.; Hu, X.M. Synthesis, characterization and biological evaluation of some 16b-azolyl-3bamino-5a-androstane derivatives as potential anticancer agents. Eur. J. Med. Chem. 2011, 46, 3662–3674. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Zheng, Y.F.; Lu, Y.Z.; Song, C.J.; Wang, Y.G.; Yu, B.; Liu, H.M. Synthesis and biological evaluation of novel steroidal[17,16-d][1,2,4]triazolo [1,5-a]pyrimidines. Steroids 2012, 77, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Mótyán, G.; Wölfling, J.; Kovács, I.; Zupkó, I.; Frank, É. A facile access to novel steroidal 17-2′-(1′,3′,4′)-oxadiazoles, and anevaluation of their cytotoxic activities in vitro. Bioorg. Med. Chem. Lett. 2014, 24, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; Zhang, E.; Pang, L.P.; Song, L.X.; Li, Y.F.; Yu, B.; Liu, H.M. Design and synthesis of novel D-ring fused steroidal heterocycles. Steroids 2013, 78, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, W.S. The first stereoselective synthesis of orostanal isolated from a marine sponge Stelletta hiwasaensis. Tetrahedron 2003, 59, 3379–3384. [Google Scholar] [CrossRef]
- Wentworth, P., Jr.; Nieva, J.; Takeuchi, C.; Galve, R.; Wentworth, A.D.; Dilley, R.B.; DeLaria, G.A.; Saven, A.; Babior, B.M.; Janda, K.D.; et al. Evidence for ozone formation in human atherosclerotic arteries. Science 2003, 302, 1053. [Google Scholar] [CrossRef] [PubMed]
- Natalie, K.C.; Johanna, C.S.; Terry, D.B.; Wentworth, P., Jr. Adduction of cholesterol 5,6-secosterol aldehyde to membrane-bound myelin basic protein exposes an immunodominant epitope. Biochemistry 2011, 50, 2092–2100. [Google Scholar] [CrossRef]
- Lin, S.N.; Yang, L.H. A simple and efficient procedure for the synthesis of benzimidazoles using air as the oxidant. Tetrahedron Lett. 2005, 46, 4315–4319. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Qi, B.; Gan, C.; Liu, Z.; Huang, H.; Lin, Q.; Zhao, D.; Huang, Y. Synthesis and in Vitro Antiproliferative Evaluation of Some B-norcholesteryl Benzimidazole and Benzothiazole Derivatives. Mar. Drugs 2015, 13, 2488-2504. https://doi.org/10.3390/md13042488
Cui J, Qi B, Gan C, Liu Z, Huang H, Lin Q, Zhao D, Huang Y. Synthesis and in Vitro Antiproliferative Evaluation of Some B-norcholesteryl Benzimidazole and Benzothiazole Derivatives. Marine Drugs. 2015; 13(4):2488-2504. https://doi.org/10.3390/md13042488
Chicago/Turabian StyleCui, Jianguo, Binbin Qi, Chunfang Gan, Zhipin Liu, Hu Huang, Qifu Lin, Dandan Zhao, and Yanmin Huang. 2015. "Synthesis and in Vitro Antiproliferative Evaluation of Some B-norcholesteryl Benzimidazole and Benzothiazole Derivatives" Marine Drugs 13, no. 4: 2488-2504. https://doi.org/10.3390/md13042488
APA StyleCui, J., Qi, B., Gan, C., Liu, Z., Huang, H., Lin, Q., Zhao, D., & Huang, Y. (2015). Synthesis and in Vitro Antiproliferative Evaluation of Some B-norcholesteryl Benzimidazole and Benzothiazole Derivatives. Marine Drugs, 13(4), 2488-2504. https://doi.org/10.3390/md13042488