Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
2.1.1. Astaxanthin Identification and Measurements by Spectrophotometry

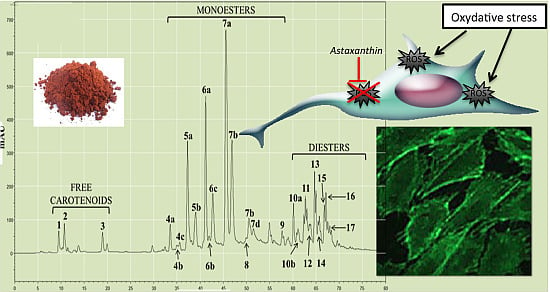
2.1.2. Characterization by HPLC-DAD and (HPLC-(APCI+)/IT-MS)


| Chromatographic Peak Number | Compound | Content in AstaP (%) | Content in AstaCO2 (%) |
|---|---|---|---|
| 1 | Astaxanthin | 0.45 | 1.70 |
| 4–9 | Monoesters | 62.6 | 76.1 |
| 10–17 | Diesters | 36.9 | 22.2 |
2.1.3. Physicochemical TEAC Assay

| Product | TEAC | ORAC | ||
|---|---|---|---|---|
| Unitless (Slope Ratio) | (mmol Trolox/g) | References | (µM TE) | |
| AstaxS | 1.32 ± 0.15 | 2.21 ± 0.25 a | 2.43 a [27] | 1.68 ± 0.25 |
| AstaP | 4.37 ± 0.33 | 0.18 ± 0.01 b | 0.1–0.25 b [28] | 8.1 ± 1.21 |
| 0.1–0.4 b [27] | ||||
| AstaCO2 | 2.37 ± 0.11 | 3.01 ± 0.14 b | 4.07 ± 0.61 | |
2.1.4. ORAC Assay
2.2. Biological Evaluation: Cellular Effects
2.2.1. Cell Viability and Morphology


2.2.2. Cellular Antioxidant Activity on Endothelial Cells
| Product | CAA (%) |
|---|---|
| AstaS | 0.3 ± 0.2 * |
| AstaP | 25.4 ± 9.5 |
| AstaCO2 | 30.4 ± 12.7 |
3. Experimental Section
3.1. Chemicals and Biological Reagents
3.2. Algae Material
3.3. Extraction of Natural Astaxanthin
3.4. Spectrophotometric Measurements and Astaxanthin Concentration Calculations
3.5. TEAC

3.6. ORAC
3.7. Chromatography: HPLC-DAD and HPLC-(APCI) Ion Trap MS
3.8. MTT Reduction Assay
3.9. Cellular Antioxidant Activity Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Touyz, R.M. Molecular mechanisms of hypertension reactive oxygen species and antioxidants: A basic science update for the clinician. Can. J. Cardiol. 2012, 28, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis is an inflammatory disease. Am. Heart J. 1999, 138, S419–S420. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H. Primary endothelial dysfunction: Atherosclerosis. J. Mol. Cell. Cardiol. 1999, 31, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Elliott, H.L. Endothelial dysfunction in cardiovascular disease: Risk factor, risk marker, or surrogate end point? J. Cardiovasc. Pharmacol. 1998, 32, S74–S77. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial function: A critical determinant in atherosclerosis? Circulation 2004, 109, II27–II33. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Szmitko, P.E. The vascular biology of peroxisome proliferator-activated receptors: Modulation of atherosclerosis. Can. J. Cardiol. 2006, 22, 12B–17B. [Google Scholar] [CrossRef] [PubMed]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58D–68D. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the Safety and Efficacy of Astaxanthin (Carophyll® Pink 10% CWS) for Salmonids and Ornamental Fish; European Food Safety Authority (EFSA): Parma, Italy, 2014; pp. 3724–3759. [Google Scholar]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in cardiovascular health and disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Kobara, N.; Higashino, S.; Giddings, J.C.; Yamamoto, J. Astaxanthin inhibits thrombosis in cerebral vessels of stroke-prone spontaneously hypertensive rats. Nutr. Res. 2011, 31, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Adluri, R.S.; Thirunavukkarasu, M.; Zhan, L.; Maulik, N.; Svennevig, K.; Bagchi, M.; Maulik, G. Cardioprotective efficacy of a novel antioxidant mix vitaepro against ex vivo myocardial ischemia-reperfusion injury. Cell Biochem. Biophys. 2013, 67, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lauver, D.A.; Booth, E.A.; White, A.J.; Poradosu, E.; Lucchesi, B.R. Sulodexide attenuates myocardial ischemia/reperfusion injury and the deposition of C-reactive protein in areas of infarction without affecting hemostasis. J. Pharmacol. Exp. Ther. 2005, 312, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.K.; King, T.J.; Fujioka, K.; Pattison, J.; Pashkow, F.J.; Tsimikas, S. Effect of an oral astaxanthin prodrug (CDX-085) on lipoprotein levels and progression of atherosclerosis in LDLR(−/−) and apoe(−/−) mice. Atherosclerosis 2012, 222, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.K.; Malinski, T.; Mason, R.P.; Kubant, R.; Jacob, R.F.; Fujioka, K.; Denstaedt, S.J.; King, T.J.; Jackson, H.L.; Hieber, A.D.; et al. Novel astaxanthin prodrug (CDX-085) attenuates thrombosis in a mouse model. Thrombosis Res. 2010, 126, 299–305. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.H.; Place, A.R.; Caldwell, R.L.; Marshall, N.J.; Cronin, T.W. A novel function for a carotenoid: Astaxanthin used as a polarizer for visual signalling in a mantis shrimp. J. Exp. Biol. 2012, 215, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Deroche, M.E.; Briantais, J.M. Absorption spectra of chlorophyll forms, β-carotene and lutein in freeze-dried chloroplasts. Photochem. Photobiol. 1974, 19, 233–240. [Google Scholar] [CrossRef]
- Miao, F.; Lu, D.; Li, Y.; Zeng, M. Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Anal. Biochem. 2006, 352, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Chang, C.L.; Lai, G.H. Reactive oxygen species scavenging activities in a chemiluminescence model and neuroprotection in rat pheochromocytoma cells by astaxanthin, β-carotene, and canthaxanthin. Kaohsiung J. Med. Sci. 2013, 29, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Jaime, L.; Rodríguez-Meizoso, I.; Cifuentes, A.; Santoyo, S.; Suarez, S.; Ibáñez, E.; Señorans, F.J. Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT Food Sci. Technol. 2010, 43, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Ceron, M.C.; Garcia-Malea, M.C.; Rivas, J.; Acien, F.G.; Fernandez, J.M.; del Rio, E.; Guerrero, M.G.; Molina, E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 74, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sakamoto, Y. Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol. Lett. 1999, 21, 265–269. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Rajaram, M.G.; Arulmurugan, P.; Baskaraboopathy, A.; Karuppasamy, K.; Jayappriyan, K.R.; Sundararaj, R.; Rengasamy, R. Antiproliferative potential of astaxanthin-rich alga Haematococcus pluvialis flotow on human hepatic cancer (HEPG2) cell line. Biomed. Prevent. Nutr. 2012, 2, 149–153. [Google Scholar] [CrossRef]
- Girard-Lalancette, K.; Pichette, A.; Legault, J. Sensitive cell-based assay using dcfh oxidation for the determination of pro- and antioxidant properties of compounds and mixtures: Analysis of fruit and vegetable juices. Food Chem. 2009, 115, 720–726. [Google Scholar] [CrossRef]
- Oueslati, S.; Trabelsi, N.; Boulaaba, M.; Legault, J.; Abdelly, C.; Ksouri, R. Evaluation of antioxidant activities of the edible and medicinal suaeda species and related phenolic compounds. Ind. Crops Prod. 2012, 36, 513–518. [Google Scholar] [CrossRef]
- Yang, H.-L.; Chen, S.-C.; Lin, K.-Y.; Wang, M.-T.; Chen, Y.-C.; Huang, H.-C.; Cho, H.-J.; Wang, L.; Kumar, K.J.S.; Hseu, Y.-C. Antioxidant activities of aqueous leaf extracts of toona sinensis on free radical-induced endothelial cell damage. J. Ethnopharmacol. 2011, 137, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.I.; Mokhova, E.N. Mechanism of induction of oxidative stress in liver mitochondria by low concentrations of tert-butyl hydroperoxide. Biochem. Biokhimiia 2013, 78, 75–79. [Google Scholar] [CrossRef]
- Kucera, O.; Endlicher, R.; Rousar, T.; Lotkova, H.; Garnol, T.; Drahota, Z.; Cervinkova, Z. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxid. Med. Cell Longev. 2014, 2014, 752506. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Guerra, B.A.; Otton, R. Impact of the carotenoid astaxanthin on phagocytic capacity and ROS/RNS production of human neutrophils treated with free fatty acids and high glucose. Int. Immunopharmacol. 2011, 11, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Takahashi, M.; Mishima, T.; Mizu, M.; Takara, K.; Wada, K. Antioxidant activity of sugarcane molasses against 2,2′-azobis(2-amidinopropane) dihydrochloride-induced peroxyl radicals. Food Chem. 2013, 141, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Esteve, M.J.; Frígola, A. Orac and teac assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Nishigaki, I.; Rajendran, P.; Venugopal, R.; Ekambaram, G.; Sakthisekaran, D.; Nishigaki, Y. Cytoprotective role of astaxanthin against glycated protein/iron chelate-induced toxicity in human umbilical vein endothelial cells. Phytother. Res. PTR 2010, 24, 54–59. [Google Scholar] [CrossRef]
- Juni, R.P.; Duckers, H.J.; Vanhoutte, P.M.; Virmani, R.; Moens, A.L. Oxidative stress and pathological changes after coronary artery interventions. J. Am. Coll. Cardiol. 2013, 61, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, M.I.; Ostojic, M.; Beleslin, B.; Sagic, D.; Mangovski, L.; Stojkovic, S.; Nedeljkovic, M.; Orlic, D.; Milosavljevic, B.; Topic, D.; et al. Differential effects of drug-eluting stents on local endothelium-dependent coronary vasomotion. J. Am. Coll. Cardiol. 2008, 51, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Togni, M.; Raber, L.; Cocchia, R.; Wenaweser, P.; Cook, S.; Windecker, S.; Meier, B.; Hess, O.M. Local vascular dysfunction after coronary paclitaxel-eluting stent implantation. Int. J. Cardiol. 2007, 120, 212–220. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs 2015, 13, 2857-2874. https://doi.org/10.3390/md13052857
Régnier P, Bastias J, Rodriguez-Ruiz V, Caballero-Casero N, Caballo C, Sicilia D, Fuentes A, Maire M, Crepin M, Letourneur D, et al. Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Marine Drugs. 2015; 13(5):2857-2874. https://doi.org/10.3390/md13052857
Chicago/Turabian StyleRégnier, Philippe, Jorge Bastias, Violeta Rodriguez-Ruiz, Noelia Caballero-Casero, Carmen Caballo, Dolores Sicilia, Axelle Fuentes, Murielle Maire, Michel Crepin, Didier Letourneur, and et al. 2015. "Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity" Marine Drugs 13, no. 5: 2857-2874. https://doi.org/10.3390/md13052857
APA StyleRégnier, P., Bastias, J., Rodriguez-Ruiz, V., Caballero-Casero, N., Caballo, C., Sicilia, D., Fuentes, A., Maire, M., Crepin, M., Letourneur, D., Gueguen, V., Rubio, S., & Pavon-Djavid, G. (2015). Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Marine Drugs, 13(5), 2857-2874. https://doi.org/10.3390/md13052857