Marine Polysaccharides from Algae with Potential Biomedical Applications
Abstract
:1. Introduction
| Type of PS | Source | Structure | Action/Application | References | |
|---|---|---|---|---|---|
| Main Mono-Sugars/Disaccharide Units | Glycosidic Bonds of Backbone | ||||
| Chromophyta Dictyotales | |||||
| Heterofucans S-fucans | Canistrocarpus cervicornis a.k.a. Dictyota cervicornis | Fuc | Anticoagulant, antioxidant; anti-proliferative | [2,25] | |
| S-galactofucans | D. menstrualis | Gal, fuc, xyl, glcAc | Peripheral anti-nociceptive, anti-inflammatory, antioxidant; anticoagulant, anti-proliferative | [1,2,26] | |
| D. mertensis | antioxidant; anticoagulant, anti-proliferative | [2] | |||
| Heterofucans | Dictyopteris delicatula | Fuc | Anticoagulant, antioxidant, antitumor, anti-proliferative | [2,27] | |
| D. polypodioides | Fuc | Antitumor | [28] | ||
| S-galactofucans | Lobophora variegata | Gal, fuc | Antioxidant, anticoagulant, anti-inflammatory | [29,30] | |
| Heterofucans | Padina gymnospora | GlcAc, fuc, | (1,3)- and (1,4)-β-d-glcAc | Antioxidant, anticoagulant, anti-thrombotic, antiviral | [2,31,32] |
| S-fucan | P. tetrastromatica | Fuc, gal, xyl, glcAc | (1,2)- and (1,3)-α-fuc | [33] | |
| S-galactofucans; sPS; S-fucans | Spatoglossum schröederi | Gal, fuc, xyl; Fuc | (1,4)- and (1,3)-α-fuc | Anti-thrombotic; Peripheral anti-nociceptive; Anti-proliferative, anti-adhesive, antioxidant | [2,34,35,36,37,38] |
| Ectocarpales | |||||
| S-galactofucans | Adenocystis utricularis | Gal, fuc, rham, uronic acid | (1,3)-α-fuc | Antiviral | [39] |
| S-fucans | Cladosiphon okamuranus a.k.a. Okinawa mozuku | Fuc, glc, glcAc | (1,3)-α-l-fuc | Anti-proliferative, antiviral, anti-inflammatory, antiadhesive, antitumor, immunomodulator; angiogenic, gastroprotective, cardioprotective, restenosis preventive | [15,22,40,41,42,43,44,45,46,47] |
| S-fucoidan | C. novae-caledoniae | Fuc | Antitumor | [48] | |
| Fucans | Leathesia difformis | Fuc | Antiviral | [49] | |
| LMW-S-fucans | Nemacystus decipiens | Fuc | Anticoagulant | [50] | |
| Fucales | |||||
| S-fucans; LMW-sPS; S-Laminaran; or otherwise modified | Ascophyllum nodosum | Fuc, xyl, gal, glcAc; Glc | (1,3)- and (1,4)-α-l-fuc (alternating); (1,3)- and (1,6)-β-glc | Immunomodulatory, anti-inflammatory, anticoagulant, anti-thrombotic, anti-metastatic, antitumor, antiadhesive, restenosis preventive; Anti-thrombotic, anticoagulant, angiogenic Antitumor, anticoagulant; serum hypocholesterolaemic, hypotensive, antibacterial, immunomodulator | [15,20,51,52,53,54,55,56,57,58,59,60,61] |
| S-fucans | Fucus spp. F. vesiculosus | Fuc, xyl, gal, glcAc | (1,3)- and (1,4)-α-l-fuc (alternating) | Immunostimulant, antiviral, antitumor, antiproliferative, antiadhesive, anticoagulant, antioxidant, anti-metastatic, anti-inflammatory; anti-angiogenic, antithrombotic (except F. vesiculosus) | [2,15,62,63,64,65,66,67,68,69,70] |
| Laminaran; S-laminaran or otherwise modified | Fucus sp. | Glc | (1,3)- and (1,6)-β-glc | Antitumor, decreases liver triglyceride, cholesterol and phospholipid levels; serum hypocholesterolaemic, hypotensive, antibacterial, immunomodulator anticoagulant | [56,59,61] |
| S-fucans | Hizikia fusiforme a.k.a. Sargassum fusiforme | Fuc, gal, man, glcAc | (1,2)-α-d-man alternating with (1,4)-β-d-glcAc; some (1,4)-β-d-gal | Anticoagulant, anti-thrombotic | [71,72] |
| Fucans | Pelvetia fastigiata | Fuc | Antiviral | [73] | |
| LMW-S-fucans | P. canaliculata | Fuc | Antiviral | [74] | |
| S-fucans | Sargassum spp. | Fuc, gal, xyl, uronic acid | Prevent hyperlipidaemia, normalize dislipidaemia | [75,76,77] | |
| S-galactofucans | Sargassum sp. | Gal, fuc, rham, glcAc | (1,6)-β-d-gal and/or (1,2)-β-d-man | Antitumor | [28,62,78,79,80] |
| S-heterofucans | S.filipendula | Fuc | Antioxidant, anti-proliferative | [2,81] | |
| S-fucoidan | S. henslowianum | Fuc | Anti-proliferative, antitumor | [75] | |
| S-fucoidan | S. horneri | Fuc | (1,3)-α-l-fuc, (1,3)- and (1,4)-α-l-fuc | Antitumor, antiviral | [62,80] |
| LMW-fucoidan | S. patens | Fuc | Antiviral | [32] | |
| sPS | Turbinaria conoides | Antioxidant | [82] | ||
| Laminariales | |||||
| S-galactofucan | Costaria costata | Gal, fuc | Antitumor | [16] | |
| S-fucans | Ecklonia cava E. kurome | Fuc, rham, gal, glcAc | (1,3)- or (1,6)-, and (1,4)-α-l-fuc | Anti-proliferative, antitumor, anticoagulant, antioxidant, antithrombotic, anti-inflammatory | [16,83,84,85,86,87,88] |
| Fucoidans; laminarans | Eisenia bicyclis | Fuc; Glc | (1,3)- and (1,6)-β-d-glc | Anti-proliferative, antitumor, anticoagulant; Antitumor | [83,89,90,91] |
| Laminaran; S-laminaran or otherwise modified | Laminaria sp (or Saccharina) | Glc | (1,3)- and (1,6)-β-glc | Antitumor, anticoagulant, decreases liver triglyceride, cholesterol and phospholipid levels; serum hypocholesterolaemic, hypotensive, antibacterial, immunomodulator | [56,59,61] |
| S-fucoidans | Laminaria spp. | Fuc, xyl, man, glcAc | (1,3)-α-l-fuc | Antioxidant, anticoagulant, antithrombotic, anti-adhesive, anti-proliferative, anti-inflammatory, anti-angiogenic, anti-metastatic | [15,52,83,92,93,94,95,96] |
| S-galactofucan | L. japonica a.k.a. Saccharina japonica | Gal, fuc | (1,3)- and (1,4)-α-l-fuc (alternating) | Anti-lipidaemic, increases HDL, antiviral, antitumor, immunomodulator, antioxidant neuroprotective | [3,15,97,98,99,100,101,102] |
| Fucoidans | Lessonia vadosa | Fuc | Anticoagulant | [103] | |
| S-fucoidan | Saccharina cichorioides a.k.a. Laminaria cichorioides | Fuc | Antitumor, anticoagulant, anti-thrombotic | [104,105] | |
| S-galactofucans fucoidan | Undaria pinnatifida | Gal, fuc, xyl, uronic acid | (1,3)- and (1,4)-α-l-fuc (alternating) | Antiviral, anticoagulant, antitumor, anti-proliferative, immunomodulatory, anti-inflammatory induced osteoblastic differentiation | [3,52,69,106,107,108,109,110,111] |
| LMW-S-fucans | Anticoagulant | [112] | |||
| Laminaran; S-laminaran or otherwise modified | Glc | Anticoagulant, antitumor; serum hypocholesterolaemic, hypotensive, antibacterial, immunomodulator | [56,59,61] | ||
| Type of PS | Source | Structure | Action/ Application | References | |
|---|---|---|---|---|---|
| Main mono-Sugars/Disaccharide Units | Glycosidic Bonds of Backbone | ||||
| Rhodophyta Bangiales | |||||
| S-galactan porphyran | Porphyra spp. | Gal | (1,3)-β-d-gal or (1,4)-α-l-gal | Antitumor, hypotensive, regulates blood cholesterol | [113,114] |
| sPS | P. haitanensis | Antioxidant | [115] | ||
| Porphyran | P. yezoensis | Antitumor, immunomodulatory, hypolipidaemic | [116,117,118,119] | ||
| Ceramiales | |||||
| S-agarans | Bostrychia montagnei | Antiviral | [120] | ||
| S-agarans | Cryptopleura ramosa | Antiviral | [121] | ||
| Digenea simplex | Antiviral | [122] | |||
| Corallinales | |||||
| LMW-PS | Corallina sp. | Antiviral | [32] | ||
| Cryptonemiales | |||||
| Cryptonemia crenulata | Gal | Antiviral | [123] | ||
| S-agaran | Gloiopeltis complanata | Gal, Agal | [→3)-β-d-gal-(1→4)-3,6-α-l-Agal-(1→], and [→3)-β-d-gal-(1→4)-α-l-gal-(1→] | [114] | |
| Agaroid-carrageenan | G. furcata | Gal | 6-O-methyl-gal, 3,6Agal(1,3)-β-d-, and (1,4)-α-l-gal or (1,4)-α-l-Agal | [124] | |
| Gelidiales | |||||
| di-S-galactan | Gelidium crinale | Gal | Anticoagulant | [125] | |
| S-agarans and hybrid dl-galactans | Pterocladia capillacea | Gal | Antiviral | [126] | |
| Gigartinales | |||||
| S-agarans S-galactans | Aghardiella tenera | Gal | Antiviral | [127,128] | |
| S-λ-carrageenan | Chondrus crispus | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antiviral, anticoagulant, antithrombotic | [1,5,129,130,131] |
| LMW-sPS | C. ocellatus | Antitumor | [132] | ||
| S-galactans | Euchema cottonii | Gal | Antioxidant | [2] | |
| S-κ-carrageenan | E. spinosa | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Anticoagulant, anti-thrombotic | [5,130,131] |
| LMW-sPS | Furcellaria lumbricalis | Immunostimulant | [133] | ||
| S-galactans | Gigartina acicularis | Gal | Antioxidant | [2] | |
| S-carrageenans | G. skottsbergii | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antiviral, anticoagulant | [130,131,134,135] |
| Hybrid dl-galactans | Gymnogongrus torulosus | Gal | Antiviral | [136] | |
| LMW-PS | Hypnea charoides | Antiviral | [32] | ||
| LMW-S-carrageenans | Kappaphycus striatus | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antitumor, immunomodulator | [1,131] |
| S-λ-carrageenan | Phyllophora brodiei | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Anticoagulant, antithrombotic | [130,131,137] |
| LMW-sPS | Soliera chordalis | Immunostimulant | [138] | ||
| S-carrageenans | Stenogramme interrupta | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antiviral | [130,131,139] |
| Gracilariales | Antioxidant | [2] | |||
| sPS | Gracilaria caudata | ||||
| S-agarans S-galactans | G.corticata | Gal | Antiviral | [140] | |
| sPS | G. verrucosa | Immunomodulator | [141] | ||
| Halymeniales | |||||
S-galactan | Grateloupia indica | Gal | Anticoagulant, antithrombotic | [137] | |
| Nemaliales | |||||
| S-mannans | Nemalion helminthoides | Man | Antiviral | [142] | |
| Xylogalactans S-xylomannans | Nothogenia fastigiata | Xyl, gal Xyl, man | Antiviral, anticoagulant | [143,144,145] | |
| Nematomatales | |||||
| S-galactans | Schizymenia dubyi | Gal, uronic acid | Antiviral | [146] | |
| S-λ-carrageenan | S. pacifica | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antiviral | [130,131,147] |
| S-galactan | S. binderi | Gal | Anticoagulant | [148] | |
| Rhodymeniales | |||||
| di-S-galactan; LMW-sPS | Botryocladia occidentalis | Gal | Anticoagulant; anti-venom | [149,150] | |
| LMW-carrageenans | Champia feldmannii | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antitumor | [130,131,151] |
| Sebdeniales | |||||
| S-xylomannans | Sebdenia polydactyla | Xyl, man | Antiviral | [152] | |
| Type of PS | Source | Structure | Action/ Application | References | |
|---|---|---|---|---|---|
| Main Mono-Sugars/Disaccharide Units | Glycosidic Bonds of Backbone | ||||
| Chlorophyta Bryopsidales | |||||
| sPS, including S-galactans | Caulerpa spp. | Antioxidant, anticoagulant, antithrombotic; antiviral, anti-proliferative, antitumor | [2,153,154] | ||
| sPS and derivatives | C. cupressoides | Gal, man, xyl | Anti-inflammatory, antinociceptive | [8,155,156] | |
| LMW-PS sPS | C. racemosa | Gal, glc, ara, uronic acid | Antiviral; antitumor | [32,154,157] | |
| S-arabinogalactans | Codium spp. | Gal, ara | (1,3)-β-d-gal | Anticoagulant, antithrombotic, antiviral | [124,153,158,159,160,161] |
| S-pyrulylated-galactans | C. isthmocladum | (1,3)-β-d-gal | Antioxidant, anticoagulant, anti-proliferative | [2,162] | |
| Ulotrichales | |||||
| S-mannans | Capsosiphon fulvescens | Man, glcAc, gal | Immunomodulator | [163] | |
| S-rhamnans and LMW-S-rhamnans | Monostroma latissimum | Rham | (1,3)-α-l-rham, and (1,3)-α-l-rham or (1,2)-α-l-rham or (1→2,3)-α-l-rham | Antiviral, anticoagulant | [164,165,166,167,168] |
| S-rhamnans | M. nitidum | Rham, glc | Anticoagulant, antithrombotic, hepatoprotective, antitumor, immnunomodulator | [165,166,169,170,171] | |
| Ulvales | |||||
| Rhamnans | Enteromorpha intestinalis | Rham, xyl, glcAc | Antitumor, immunomodulator | [172,173] | |
| LMW-sPS | E. linza | Anticoagulant | [174] | ||
| S-ulvans and derivatives | E. prolifera | Immunomodulator, antioxidant, hypolipidaemic | [124,175,176,177] | ||
| S-ulvans and derivatives | Ulva spp. | Rham, xyl, glc, glcAc, IduAc | Anti-adhesive, antiproliferative, hepatoprotective | [178,179] | |
| sPS | U. conglobata | Rham, uronic acid | Anticoagulant | [180] | |
| sPS | U. fasciata | rham | Antioxidant. antitumor | [181] | |
| S-galactans sPS | U. lactuca | Rham, xyl, glcAc | Antioxidant, anti-proliferative, hypocholesterolaemic, hepatoprotective, antitumor; Antiviral, anti-inflammatory, antinociceptive | [90,182,183,184,185,186,187,188,189] | |
| S-ulvans | U. pertusa | Rham, xyl, glcAc, iduAc | [→4)-β-d-GlcAc-(1,4)-α-l-rham3S-(1→], and [→4)-α-l-IduAc-(1,4)-α-l-rham3S-(1→] | Antioxidant, anti-proliferative, hypocholesterolaemic | [90,182,183,184,185] |
| LMW-S-ulvan or otherwise modified | U. pertusa | Antioxidant, hypotriglyceridaemic, decrease LDL- and increases HDL-cholesterol, immunostimulatory | [166,185,190,191] | ||
| S-PS | U. rigida | Rham, glcAc | β-d-glcAc-(1,4)-l-rham (disacharide) | Immunostimulatory | [178,192] |
| Type of PS | Source | Main Neutral Sugars | Action/Application | References |
|---|---|---|---|---|
| MICROALGAE | ||||
| Diatoms | ||||
| sPS | Cylindrotheca closterium | xyl, glc, man, rham | [193,194] | |
| sPS | Navicula salinarum | glc, xyl, gal, man | [193] | |
| s-EPS | Phaeodactylum tricornutum | glc, man, xyl, rham | Anti-adhesive | [195,196,197] |
| EPS | Haslea ostrearia | [198] | ||
| EPS | Nitzschia closterium | [199] | ||
| EPS | Skeletonema costatum | |||
| EPS | Chaetoceros spp. | rham, fuc, gal, man | [200] | |
| EPS | Amphora sp. | [201] | ||
| Chlorophytes | ||||
| sPS | Chlorella stigmatophora | glc, xyl, fuc, | Anti-inflammatory, immunomodulator | [195] |
| sPS | C. autotrophica | [202] | ||
| PS β-(1,3)-glucan | C. vulgaris | rham, gal, arab, 2-O-methyl-rham glc | Antitumor, infection preventive agent | [24,203,204] |
| EPS | Dunaliella salina | gal, glc, xyl, fru | [205] | |
| EPS | Ankistrodesmus angustus | [201] | ||
| EPS | Botryococcus braunii | gal, fuc, glc, rham | [206,207] | |
| Prasinophyte | ||||
| sPS | Tetraselmis sp. | Anti-adhesive | [202] | |
| Prymnesiophyte/haptophyte | ||||
| sPS | Isochrysis sp. | [202] | ||
| Rhodophytes | ||||
| sPS | Porphyridium sp. | xyl, gal, glc | Anti-inflammatory, immunomodulator, prevention of tumour cell growth, anti-adhesive, antiviral, biolubricant | [208,209,210,211,212,213] |
| sPS | P. cruentum | xyl, gal, glc, glcAc, 3-O-methyl-xyl | Antioxidant and free radical scavenging, immunomodulator, antiviral, antibacterial, antilipidaemic, antiglycaemic | [214,215,216,217,218,219,220,221,222] |
| sPS | P. purpureum | antiviral | [223] | |
| sPS | Rhodella reticulata | xyl, rham, 3-O-methyl-rham, 4-O-methyl-gal | Antiviral, antilipidaemic, antiglycaemic, prevention of tumour cell growth | [208,213,219], |
| R. maculata | xyl, gal, glc,3-O-methyl-xyl | [224,225] | ||
| Dinoflagellates | ||||
| sPS | Cochlodinium polykrikoides | man, gal, glc | Antiviral | [226] |
| sPS | Gyrodinium impudicum | gal | Antiviral, anti-inflammatory, immunomodulator, anti-proliferative, prevention of tumour cell growth | [23,227,228,229] |
| CYANOBACTERIA | ||||
| EPS | Aphanothece halophytica | glc, fuc, man, arab, glcAc | [230] | |
| EPS s-Spirulan | Arthrospira platensis | gal, xyl, glc, fru rham, fuc, glc, 3-O-methyl-rham | Antiviral, antibacterial, prevention of tumour cell growth Anti-proliferative, anti-adhesive, anti-metastatic | [19,223,231,232,233,234,235] |
| sPS | Anabaena, Gloethece, Nostoc Aphanocapsa, Phormidium, Synechocystis, Cyanothece | [19] |
2. Some Structural Characteristics of Polysaccharides Produced by Marine Algae
2.1. Macroalgae
2.2. Microalgae and Cyanobacteria




3. Potential Medical/Biomedical Applications of Polysaccharides from Marine Algae. Relation with Some Chemical Features of Their Structures
3.2. Anti-Inflammatory and Immunomodulatory Activities
3.3. Anti-Proliferative, Tumour Suppressor, Apoptotic and Cytotoxicity Activities
3.4. Anticoagulant and Antithrombotic Activities
3.5. Antilipidaemic (Hypocholesterolaemic and Hypotriglyreridaemic), Hypoglycaemic and Hypotensive Activities
3.6. Antiaging (Antioxidant) Activity
3.7. Nutritional Applications: Fibres (Dietary), Prebiotic and Probiotic
3.8. Other Biological Activities
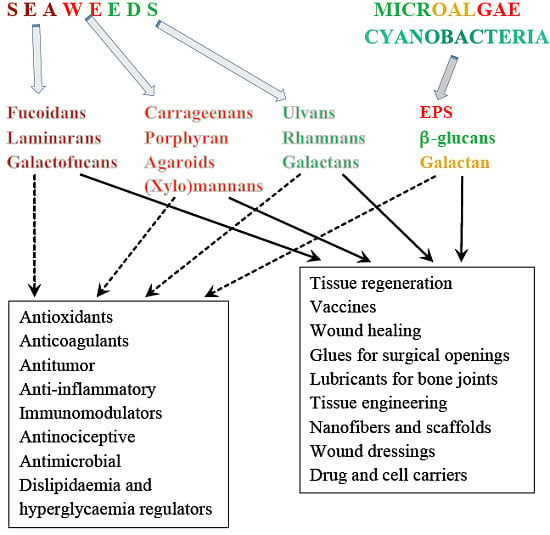
3.9. Biomedical Applications
| Groups of PSs | Possible Sources | Applications | References |
|---|---|---|---|
| Alginates | Laminaria spp, A. nodosum, Ecklonia sp., M. pyrifera, Durvillaea, Lessonia | Drugs carriers | [371] |
| Encapsulation | [372,373,374] | ||
| Scaffolds for ligaments and tissue engineering | |||
| Regeneration of tissues | |||
| Moulding in dentistry | |||
| Wound healing and dressings | [375,376,377] | ||
| Agaroids | B. montaignei, Goiopeltis spp., A. tenera, P. capillacea | Cell encapsulation | |
| Scaffolds for tissue engineering | [378] | ||
| Wound healing and dressings | [379] | ||
| Revascularization | [380] | ||
| Ulvans | Ulva rigida, Ulva spp. | Drug carriers | [381] |
| Wound dressings | [382,383] | ||
| Tissue engineering | [384] | ||
| β-glucans | A. nodosum, E. bicyclis, Fucus sp., Laminaria sp., U. pinnatifida (laminaran); C. vulgaris | Wound healing | [385,386,387] |
| Burn-wound dressings | |||
| Tissue regeneration | [388,389,390] | ||
| fucoidans | U. pinnatifida | Vaccines for immunotherapy | [299] |
| PSs from microalgae | A. platensis | Production of nanofibers | [391] |
| Gluing and soft tissue closure after surgery | [6] | ||
| Porphyridium | Lubricants for bone joints | [212,392] |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Albuquerque, I.R.L.; Cordeiro, S.L.; Gomes, D.L.; Dreyfuss, J.L.; Filgueira, L.G.A.; Leite, E.L.; Nader, H.B.; Rocha, H.A.O. Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis. Mar. Drugs 2013, 11, 2722–2740. [Google Scholar]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Seaweed as a source of novel nutraceuticals: Sulfated polysaccharides and peptides. Adv. Food Nutr. Res. 2011, 64, 325–337. [Google Scholar] [PubMed]
- Kraan, S. Algal polysaccharides, novel applications and outlook. In Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology; InTech: Rijeka, Croatia, 2012; Chapter 22; pp. 489–524. [Google Scholar]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Gomez d’Ayala, G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.M.; Banaigs, B.; Abou-Mansor, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. 2015, 125, 32–41. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Bioactivity and Applications of polysaccharides from marine microalgae. In Polysaccharides: Bioactivity and Biotechnology; Merillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 5, 541–552. [Google Scholar]
- Ermakova, S.; Sokolova, R.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Fucoidans from Brown seaweeds Sargassum hornery, Ecklonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, N.; Sakari, S.; Yamagushi, Y.; Takenaka, H. Inhibitory effects of microalgae on activation of hyaluronidase. J. Appl. Phycol. 2001, 13, 489–492. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulphated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fisher, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Smitt, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulphated polysaccharides and fucoidans from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007, 98, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, K.; Yokokura, T.; Satoh, H.; Mutai, M. Anti-tumor effect by oral administration of Chlorella extract, PCM-4 by oral admission. Gan To Kagaku Zasshi 1983, 10, 781–785. (In Japanese) [Google Scholar]
- Camara, R.B.G.; Costa, L.S.; Fidelis, G.P.; Nobre, L.T.D.B.; Dantas-Santos, N.; Cordeiro, S.L.; Costa, M.S.S.P.; Alves, L.G.; Rocha, H.A.O. Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar. Drugs 2011, 9, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, I.R.L.; Queiroz, K.C.S.; Alves, L.G.; Santos, E.A.; Leite, E.L.; Rocha, H.A.O. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res. 2004, 37, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Usov, A.I. Structural analysis of fucoidans. Nat. Prod. Commun. 2008, 3, 1639–1648. [Google Scholar]
- Sokolova, R.V.; Ermakova, S.P.; Awada, S.M.; Zvyagintseva, T.N.; Kanaan, H.M. Composition, structural characteristics, and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem. Nat. Comp. 2011, 47, 329–334. [Google Scholar] [CrossRef]
- Medeiros, V.P.; Queiroz, K.C.; Cardoso, M.L.; Monteiro, G.R.; Oliveira, F.W.; Chavante, S.F.; Guimarães, L.A.; Rocha, H.A.; Leite, E.L. Sulfated galactofucan from Lobophora variegata: Anticoagulant and anti-inflammatory properties. Biochemistry (Mosc.) 2008, 73, 1018–1024. [Google Scholar] [CrossRef]
- Paiva, A.A.; Castro, A.J.; Nascimento, M.S.; Will, L.S.; Santos, N.D.; Araújo, R.M.; Xavier, C.A.; Rocha, F.A.; Leite, E.L. Antioxidant and anti-inflammatory effect of polysaccharides from Lobophora variegata on zymosan-induced arthritis in rats. Int. Immunopharmacol. 2011, 11, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.; Alves, L.G.; de Queiroz, K.C.; Santos, M.G.; Marques, C.T.; Chavante, S.F.; Rocha, H.A.O.; Leite, E.L. Partial characterization and anticoagulant activity of a heterofucan from the brown seaweed Padina gymnospora. Braz. J. Med. Biol. Res. 2005, 38, 523–533. [Google Scholar] [PubMed]
- Zhu, W.; Ooi, V.E.C.; Chan, P.K.S.; Ang, P.O., Jr. Inhibitory effect of extracts of marine algae from Hong Kong against Herpes simplex viruses. In Proceedings of the 17th International Seaweed Symposium; Chapman, A.R.O., Anderson, R.J., Vreeland, V.J., Davison, I.R., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 159–164. [Google Scholar]
- Karmakar, P.; Ghosh, T.; Sinha, S.; Saha, S.; Mandal, P.; Ghosal, P.K.; Ray, B. Polysaccharides from the brown seaweed Padina tetrastromatica: Characterization of a sulfated fucan. Carbohyd. Polym. 2009, 78, 416–421. [Google Scholar] [CrossRef]
- Almeida-Lima, J.; Dantas-Santos, N.; Gomes, D.L.; Cordeiro, S.L.; Sabry, D.A.; Costa, L.S.; Freitas, M.L.; Silva, N.B.; Moura, C.E.B.; Lemos, T.M.A.M.; et al. Evaluation of acute and subchronic toxicity of a non-anticoagulant, but antithrombotic algal heterofucan from the Spatoglossum schröederi in wistar rats. Rev. Bras. Farmacogn. 2011, 21, 674–679. [Google Scholar] [CrossRef]
- Almeida-Lima, J.; Costa, L.S.; Silva, N.B.; Melo-Silveira, R.F.; Silva, F.V.; Felipe, M.B.M.C.; Medeiros, S.R.B.M.; Leite, E.L.; Rocha, H.A.O. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. J. Appl. Toxicol. 2010, 30, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Farias, W.R.; Lima, P.C.; Rodrigues, N.V.; Siqueira, R.C.; Amorim, R.M.; Pereira, M.G.; Assreuy, A.M. A novel antinociceptive sulphated polysaccharide of the brown marine alga Spatoglossum schröederi. Nat. Prod. Commun. 2011, 6, 863–866. [Google Scholar] [PubMed]
- Rocha, H.A.O.; Franco, C.R.C.; Trindade, E.S.; Veiga, S.S.; Leite, E.L.; Dietrich, C.P.; Nader, H.B. Fucan inhibits Chinese hamster ovary cell (CHO) adhesion to fibronectin by binding to the extracellular matrix. Planta Med. 2005, 71, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.A.O.; Moraes, F.A.; Trindade, E.S.; Franco, C.R.C.; Torquato, R.J.S.; Veiga, S.S.; Valente, A.P.; Mourão, P.A.; Leite, E.L.; Nader, H.B.; et al. Structural and haemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schröederi. An ideal antithrombotic agent? J. Biol. Chem. 2005, 280, 41278–41288. [Google Scholar] [CrossRef] [PubMed]
- Ponce, N.M.A.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Hidari, K.I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Shibata, H.; Kimura-Takagi, I.; Hashimoto, S.; Kimura, K.; Makino, T.; Aiyama, R.; Ueyama, S.; Yokokura, T. Structural study of fucoidan from Cladosiphon okamuranus Tokida. Glycoconj. J. 1999, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Wada-Funada, U.; Mano, H.; Matahira, Y.; Kawaguchi, M.; Wada, M. Proportion of murine cytotoxic T cells is increased by high molecular-weight fucoidan extracted from Okinawa mozuku (Cladosiphon okamuranus). J. Health Sci. 2005, 51, 394–397. [Google Scholar] [CrossRef]
- Teruya, T.; Konishi, T.; Uechi, S.; Tamaki, H.; Tako, M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladosiphon okamuranus Tokida in U937 cells. Int. J. Biol. Macromol. 2007, 41, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Miki, Y.; Kimura, T.; Tanaka, K.; Nakagawa, T.; Kawamukai, M.; Matsuda, H. Effects of fucoidan from Mozuku on human stomach cell lines. Food Sci. Technol. Res. 2006, 12, 218–222. [Google Scholar] [CrossRef]
- Shibata, H.; Iimuro, M.; Uchiya, N.; Kawamori, T.; Nagaoka, M.; Ueyama, S.; Hashimoto, S.; Yokokura, T.; Sugimura, T.; Wakabayashi, K. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter 2003, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Kimura-Takagi, I.; Nagaoka, M.; Hashimoto, S.; Aiyama, R.; Iha, M.; Ueyama, S.; Yokokura, T. Properties of fucoidan from Cladosiphon okamuranus Tokida in gastric mucosal protection. Biofactors 2000, 11, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.; Rajendran, M.; Pasanban, B.; Rengasamy, R. Cardioprotective activity of Cladosiphon okamuranus against isoproterenol induced myocardial infraction in rats. Phytomedicine 2010, 18, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.C.; Reynaldi, S.; Stortz, C.A.; Cerezo, A.S.; Damonte, E.B. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine 1999, 6, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, O.H.; Lee, H.H.; Lee, B.Y. A 4-week repeated oral dose toxicity study of fucoidan from the sporophyll of Undaria pinnatifida in Sprague-Dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Anastase-Ravion, S.; Carreno, M.P.; Blondin, C.; Ravion, O.; Champion, J.; Chaubet, F.; Haeffner-Cavaillon, N.; Letourneur, D. Heparin-like polymers modulate proinflammtory cytokine production by lipopolysaccharide-stimulated human monocytes. J. Biomed. Mat. Res. 2002, 60, 375–383. [Google Scholar] [CrossRef]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res 1999, 319, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Chevolot, L.; Mulloy, B.; Racqueline, J. A disaccharide repeat unit is the structure structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Colliec-Jouault, S.; Millet, J.; Helley, D.; Sinquin, C.; Fischer, A.M. Effect of low-molecular-weight fucoidan on experimental arterial thrombosis in the rabbit and rat. J. Thromb. Haemost. 2003, 1, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.A.; Szegezdi, E.; Mulloy, B.; Samali, A.; Tuohy, M.G. An unfractionated fucoidan from Ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J. Nat. Prod. 2011, 74, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Paper, D.H.; Donaldson, J.; Alban, S.; Franz, G. Characterization of a laminarin sulfate which inhibits basic fibroblast growth-factor binding and endothelial-cell proliferation. J. Cell Sci. 1995, 108, 3591–3598. [Google Scholar] [PubMed]
- Luyt, C.E.; Meddahi-Pellé, A.; Ho-Tin-Noe, B.; Colliec-Jouault, S.; Guezennec, J.; Louedec, L.; Prats, H.; Jacob, M.P.; Osborne-Pellegrin, M.; Letourneur, D.; et al. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J. Pharmacol. Exp. Therapeut. 2003, 305, 24–30. [Google Scholar] [CrossRef]
- Matou, S.; Helley, D.; Chabut, D.; Bros, A.; Fischer, A.M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res. 2002, 106, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.Q.; Elkin, M.; Aingorn, E.; Ishai-Michaeli, R.; Stein, C.A.; Vlodavsky, I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int. J. Cancer 1999, 83, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Percival, E. Glucuronoxylofucan, a cell-wall component of Ascophyllum nodosum. Carbohydr. Res. 1968, 7, 272–277. [Google Scholar] [CrossRef]
- Renn, D.W.; Noda, H.; Amano, H.; Nishino, T.; Nishizana, K. Antihypertensive and antihyperlipidemic effects of funoran. Fisch. Sci. 1994, 60, 423–427. [Google Scholar]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Beress, A.; Wassermann, O.; Tahhan, S.; Bruhn, T.; Beress, L.; Kraiselburd, E.N.; Gonzalez, L.V.; de Motta, G.E.; Chavez, P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993, 56, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from brown seaweed Fucus evanescens. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.; Pereira, M.S. Searching for alternatives to heparin: Sulfated fucans from marine invertebrates. Trends Cardiovasc. Med. 1999, 9, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.S.; Mulloy, B.; Mourão, P.A. Structure and anticoagulant activity of sulfated fucans. Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J. Biol. Chem. 1999, 274, 7656–7667. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Suzuki, H.; Wada, Y.; Kodama, T.; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-κB-dependent signaling pathways through macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2006, 343, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, G.Y.; Nam, T.J.; Deuk Kim, N.; Hyun Choi, Y. Antiproliferative activity of fucoidan was associated with the induction of apoptosis and autophagy in AGS human gastric cancer cells. J. Food Sci. 2011, 76, T77–T83. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnicka, F.; Copikova, J.; Park, Y.I. Structure and antitumor activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Yang, J.W.; Yoon, S.Y.; Oh, S.J.; Kim, S.K.; Kang, K.W. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem. Biophys. Res. Commun. 2006, 346, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, X.J.; Sun, J.L.; Xu, S.Y. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed, Hizikia fusiforme. Carbohydr. Res. 2006, 341, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, K.; Nishino, T.; Fujihara, M. Isolation and preliminary characterization of fucose-containing sulfated polysaccharides with blood-anticoagulant activity from seaweed Hizikia fusiforme. Carbohydr. Res. 1989, 194, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, P.S.; Millman, I.; Blumberg, B.S. Interaction of fucoidan from Pelvetia fastigiata with surface antigens of hepatitis B and woodchuck hepatitis viruses. Planta Med. 1989, 55, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Klarzynski, O.; Descamps, V.; Plesse, B.; Yvin, J.C.; Kloareg, B.; Fritig, B. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant Microbe Interact. 2003, 16, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.; Sathivel, A.; Devaki, T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol. Cell. Biochem. 2005, 276, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Josephine, A.; Veena, C.K.; Amudha, G.; Preetha, S.P.; Varalakshmi, P. Protective role of sulphated polysaccharides in abating the hyperlipidemic nephropathy provoked by cyclosporine A. Arch. Toxicol. 2007, 81, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 2001, 333, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. J. Appl. Phycol. 2011, 24, 715–723. [Google Scholar] [CrossRef]
- Hoshino, T.; Hayashi, T.; Hayashi, K.; Hamada, J.; Lee, J.B.; Sankawa, U. An antivirally active sulfated polysaccharide from Sargassum horneri (Turner) C. Agardh. Biol. Pharm. Bull. 1998, 21, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Costa, M.S.S.P.; Almeida-Lima, J.; Melo-Silveira, R.F.; Albuquerque, I.R.L.; et al. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar]
- Chattopadhyay, N.; Ghosh, T.; Sinha, S.; Chattopadhyay, K.; Karmakar, P.; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem. 2010, 11, 823–829. [Google Scholar] [CrossRef]
- Yamamoto, I.; Takahashi, M.; Tamura, E.; Maruyama, H.; Mori, H. Antitumor activity of edible marine algae: Effect of crude fucoidan fractions prepared from edible brown seaweed against L-1210 leukemia. Hydrobiology 1984, 116/117, 145–148. [Google Scholar] [CrossRef]
- Nishino, T.; Yokoyama, G.; Dobahi, K. Isolation, purification and characterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia kurome and their blood-anticoagulant activities. Carbohydr. Res. 1989, 186, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Aizu, Y.; Nagumo, T. The influence of sulfate content and molecular weight of a fucan sulfate from the brown seaweed Ecklonia kurome on its antithrombin activity. Thromb. Res. 1991, 64, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Kiyohara, H.; Yamada, H.; Nagumo, T. An anticoagulant fucoidan from the brown seaweed Ecklonia kurome. Phytochemistry 1991, 30, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Geng, M.Y.; Zhang, J.T.; Jiang, H.D. An in vitro study of the structure-activity relationships of sulfated polysaccharide from brown algae to its antioxidant effect. J. Asian Nat. Prod. Res. 2001, 3, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Kim, K.N.; Lee, S.H.; Ahn, G.; Cha, S.H.; Kim, A.D.; Yang, X.-D.; Kang, M.-C.; Jeon, Y.-J. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS-stimulated RAW264.7 macrophages. Carbohydr. Polym. 2011, 85, 80–85. [Google Scholar] [CrossRef]
- Takahashi, M. Studies on the mechanism of host mediated antitumor action of fucoidan from a brown alga Eisenia bicyclis. J. Jpn. Soc. Reticuloendothel. Syst. 1983, 22, 269–283. [Google Scholar]
- Usui, T.; Asari, K.; Mizuno, T. Isolation of highly purified fucoidan from Eisenia bicyclis and its anticoagulant and antitumor activities. Agric. Biol. Chem. 1980, 44, 2. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry 2010, 71, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I.; Smirnova, G.P.; Bilan, M.I.; Shashkov, A.S. Polysaccharides of algae: 53. Brown alga Laminaria saccharina (L.) Lam. as a source of fucoidan. Bioorg. Khim 1998, 24, 382–389. [Google Scholar]
- Maruyama, H.; Yamamoto, I. An antitumor fraction from an edible brown seaweed Laminaria religiosa. Hydrobiologia 1984, 116/177, 534–536. [Google Scholar] [CrossRef]
- Kitamura, K.; Matsuo, M.; Yasui, T. Enzymic degradation of fucoidan by fucoidanase from the hepatopancreas of Patinopecten yessoensis. Biosci. Biotechnol. Biochem. 1992, 56, 490–494. [Google Scholar] [CrossRef]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm. Biol. 2010, 48, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.H.; Chen, L.; Li, Z.J.; Cai, Y.P.; Lin, H.; Fang, Y. Antioxidative activities of low molecular fucoidans from kelp Laminaria japonica. Dev. Food Sci. 2004, 42, 139–145. [Google Scholar]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tian, T.C.; Shi, Y.C. Study on antivirus effect of fucoidan in vitro. J. N. Bethune Univ. Med. Sci. 1995, 21, 255–257. [Google Scholar]
- Li, D.Y.; Xu, R.Y.; Zhou, W.Z.; Sheng, X.B.; Yang, A.Y.; Cheng, J.L. Effects of fucoidan extracted from brown seaweed on lipid peroxidation in mice. Acta Nutrim. Sin. 2002, 24, 389–392. [Google Scholar]
- Wang, W.T.; Zhou, J.H.; Xing, S.T.; Guan, H.S. Immunomodulating action of marine algae sulfated polysaccharides on normal and immunosuppressed mice. Chin. J. Pharm Toxicol. 1994, 8, 199–202. [Google Scholar]
- Luo, D.; Zhan, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Athukorala, Y.; Lee, J.S.; Jeon, Y.J. Simple separation of anticoagulant sulfated galactan from red algae. J. Appl. Phycol. 2008, 20, 1053–1059. [Google Scholar] [CrossRef]
- Yoon, S.J.; Pyun, Y.R.; Hwang, J.K.; Mourão, P.A.S. A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity. Carbohydr. Res. 2007, 342, 2326–2330. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.D.; Dragar, C. Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytother. Res. 2004, 18, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Hemmingson, J.A.; Falshow, R.; Furneaux, R.H.; Thompsom, K. Structure and antiviral activity of the galactofucans sulfates extracted from Undaria pinnatifida (Phaeophyta). J. Appl. Phycol. 2006, 18, 185–193. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. Vivo 2003, 17, 245–249. [Google Scholar]
- Cho, Y.S.; Jung, W.K.; Kim, J.A.; Choi, I.W.; Kim, S.K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.Y.; You, S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2011, 16, 291–297. [Google Scholar] [CrossRef]
- Maruyamaa, H.; Tamauchib, H.; Hashimotoc, M.; Nakano, T. Suppression of Th2 immune responses by Mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005, 137, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Preobrazhenskaya, M.E.; Berman, A.E.; Mikhailov, V.I.; Ushakova, N.A.; Mazurov, A.V.; Semenov, A.V.; Usov, A.I.; Nifant’ev, N.E.; Bovin, N.V. Fucoidan inhibits leukocyte recruitment in a model peritoneal inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochem. Mol. Biol. Int. 1997, 43, 443–451. [Google Scholar] [PubMed]
- Noda, H. Health benefits and nutritional properties of nori. J. Appl. Phycol. 1993, 5, 255–258. [Google Scholar] [CrossRef]
- Takano, R.; Hayashi, K.; Hara, S.; Hirase, S. Funoran from the red seaweed Gloiopeltis complanata: Polysaccharides with sulphated agarose structure and their precursor structure. Carbohydr. Polym. 1995, 27, 305–311. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Li, N.; Zhou, G.F.; Lu, X.L.; Xu, Z.H.; Li, Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanensis (Rhodophyta) in aging mice. Pharmacol. Res. 2003, 48, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Nam, T.J. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006, 79, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.; Enomoto, A.; Todoh, H.; Ametani, A.; Kaminogawa, S. Activation of murine macrophages by polysaccharide fractions from marine algae (Porphyra yezoensis). Biosci. Biotechnol. Biochem. 1993, 57, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.; Ametani, A.; Tsunehiro, J.; Nomura, K.; Itoh, M.; Fukui, F.; Kaminogawa, S. Macrophage stimulation activity of the polysaccharide fraction from a marine alga (Porphyra yezoensis): Structure-function relationships and improved solubility. Biosci. Biotechnol. Biochem. 1995, 59, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Okabe, M.; Yoshimura, T.; Sumi, T.; Tachibana, H.; Yamada, K. Dietary effect of porphyran from Porphyra yezoensis on growth and lipid metabolism of Sprague-Dawley rats. Food Sci. Technol. Res. 2004, 10, 147–151. [Google Scholar] [CrossRef]
- Duarte, M.E.; Noseda, D.G.; Noseda, M.D.; Tulio, S.; Pujol, C.A.; Damonte, E.B. Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication in vitro. Phytomedicine 2001, 8, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.J.; Scolaro, L.A.; Matulewicz, M.C.; Damonte, E.B. Antiviral activity of natural sulphated galactans on herpes virus multiplication in cell culture. Planta Med. 1997, 63, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Sekine, H.; Ohonuki, N.; Sadamasu, K.; Monma, K.; Kudoh, Y.; Nakamura, H.; Okada, Y.; Okuyama, T. The inhibitory effect of the crude extract from the seaweed Dygenea simplex C. Agardh on the in vitro cytopathic activity of HIV-1 and its antigen production. Chem. Pharm. Bull. (Tokyo) 1995, 43, 1580–1584. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.M.; Faria, P.C.S.; Scolaro, L.A.; Duarte, M.E.R.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweed Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Takano, R.; Iwane-Sakata, H.; Hayashi, K.; Hara, S.; Hirase, S. Concurrence of agaroid and carrageenan chains in funoran from the red seaweed Gloiopeltis furcata Post. Et Ruprecht (Cryptonemiales, Rhodophyta). Carbohydr. Polym. 1998, 35, 81–87. [Google Scholar] [CrossRef]
- Pereira, M.G.; Benevides, N.M.B.; Melo, M.R.S.; Valente, A.P.; Melo, F.R.; Mourão, P.A.S. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr. Res. 2005, 340, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Errea, M.I.; Matulewicz, M.C.; Damonte, E.B. Antiherpetic activity of S1, an algal derived sulphated galactan. Phytother Res. 1996, 10, 410–413. [Google Scholar] [CrossRef]
- De Clercq, E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000, 20, 323–349. [Google Scholar] [CrossRef] [PubMed]
- Witvrouw, M.; Este, J.A.; Mateu, M.Q.; Reymen, D.; Andrei, G.; Snoeck, R.; Ikeda, S.; Pauwels, R.; Bianchini, N.V.; Desmyter, J.; de Clercq, E. Activity of a sulfated polysaccharide extracted from the red seaweed Aghardhiella tenera against human immunodeficiency virus and other enveloped viruses. Antivir. Chem. Chemother. 1994, 5, 297–303. [Google Scholar] [CrossRef]
- Luescher-Mattli, M. Algae, a possible source for new drugs in the treatment of HIV and other viral diseases. Curr. Med. Chem. 2003, 2, 219–225. [Google Scholar]
- Prajapati, V.D.; Mahereriya, P.M.; Jani, G.K.; Soalnki, H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Mou, H.; Xiaolu, J.; Huashi, G. A kappa-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity. J. Appl. Phycol. 2003, 15, 297–303. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G.; Zhao, X.; Ren, W.; Jiao, G.; Fang, L.; Wang, Y.; Du, G.; Tiller, C.; Girouard, G.; et al. Structural characterisation and bioactivities of hybrid carrageenan-like sulphated galactan from red alga Furcellaria lumbricalis. Food Chem. 2011, 124, 50–57. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Pujol, C.A.; Ciancia, M.; Noseda, M.D.; Matulewicz, M.C.; Damonte, E.B.; Cerezo, A.S. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: Correlation between structure and biological activity. Int. J. Biol. Macromol. 1997, 20, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.J.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir. Res. 1999, 43, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Estevez, J.M.; Carlucci, M.J.; Ciancia, M.; Cerezo, A.S.; Damonte, E.B. Novel DL-galactan hybrids from the red seaweed Gymnogongrus torulosus are potent inhibitors of herpes simplex virus and dengue virus. Antivir. Chem. Chemother. 2002, 13, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.K.; Das, A.K.; Banerji, N.; Siddhanta, A.K.; Mody, K.H.; Ramavat, B.K.; Chauhan, V.D.; Vedasiromoni, J.R.; Ganguly, D.K. A new sulfated polysaccharide with potent blood anti-coagulant activity from the red seaweed Grateloupia indica. Int. J. Biol. Macromol. 1994, 16, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Bondu, S.; Deslandes, E.; Fabre, M.S.; Berthou, C.; Yu, G. Carrageenan from Solieria chordalis (Gigartinales): Structural analysis and immunological activities of the low molecular weight fractions. Carbohydr. Polym. 2010, 81, 448–460. [Google Scholar] [CrossRef]
- Caceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zuniga, E.A. Carrageenans from chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.; Tsunehiro, J.; Nomura, K.; Itoh, M.; Fukui, F.; Ametani, A.; Kaminogawa, S. In vivo macrophage-stimulation activity of the enzyme-degraded water-soluble polysaccharide fraction from a marine alga (Gracilaria verrucosa). Biosci. Biotechnol. Biochem. 1996, 60, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Recalde, M.P.; Noseda, M.D.; Pujol, C.A.; Carlucci, M.J.; Matulewicz, M.C. Sulfated mannans from the red seaweed Nemalion helminthoides of the South Atlantic. Phytochemistry 2009, 70, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.; Neyts, J.; Pujol, C.A.; Snoeck, R.; Andrei, G.; Ikeda, S.; Witvrouw, M.; Reymen, D.; Haines, H.; Matulewicz, M.C.; et al. Antiviral activity of a sulphated polysaccharide from the red seaweed Nothogenia fastigiata. Biochem Pharmacol. 1994, 47, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S.; Coto, C.E. Herpes simplex virus-inhibitory sulfated xylogalactans from the red seaweed Nothogenia fastigiata. Chemotherapy 1996, 42, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kolender, A.A.; Pujol, C.A.; Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. The system of sulfated alpha-(1→3)-linked D-mannans from the red seaweed Nothogenia fastigiata: Structures, antiherpetic and anticoagulant properties. Carbohydr. Res. 1997, 304, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bourgougnon, N.; Roussakis, C.; Kornprobst, J.M.; Lahaye, M. Effects in vitro of sulfated polysaccharide from Schizymenia dubyi (Rhodophyta, Gigartinales) on a non-small-cell bronchopulmonary carcinoma line (NSCLC-N6). Cancer Lett. 1994, 85, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Kido, Y.; Kobayashi, N.; Motoki, Y.; Neushul, M.; Yamamoto, N. Purification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob. Agents Chemother. 1987, 31, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, E.A.; Matsuhiro, B.; Mejias, E. Preparation of a low-molecular weight fraction by free radical depolymerization of the sulfated galactan from Schizymenia binderi (Gigartinales, Rhodophyta) and its anticoagulant activity. Carbohydr. Polym. 2006, 66, 208–215. [Google Scholar] [CrossRef]
- Farias, W.R.L.; Valente, A.P.; Pereira, M.S.; Mourão, P.A.S. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red alga Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J. Biol. Chem. 2000, 275, 29299–29307. [Google Scholar] [CrossRef] [PubMed]
- Toyama, M.H.; Toyama, D.O.; Torres, V.M.; Pontes, G.C.; Farias, W.R.L.; Melo, F.R.; Oliveira, S.C.B.; Fagundes, F.H.R.; Diz Filho, E.B.S.; Cavada, B.S. Effects of Low Molecular Weight Sulfated Galactan Fragments From Botryocladia occidentalis on the Pharmacological and Enzymatic Activity of Spla2 from Crotalus durissus cascavella. Protein J. 2010, 29, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Lins, K.O.; Bezerra, D.P.; Alves, A.P.; Alencar, N.M.; Lima, M.W.; Torres, V.M.; Farias, W.R.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J. Appl. Toxicol. 2009, 29, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Pujol, C.A.; Damonte, E.B.; Sinha, S.; Ray, B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: Structural features, chemical modification and antiviral activity. Antivir. Chem. Chemother. 2009, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Hayashi, K.; Maeda, M.; Hayashi, T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004, 70, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Shao, H.; Zhang, C.; Hong, P.; Xiong, H. Separation of the polysaccharides in Caulerpa racemosa and their chemical composition and antitumor activity. J. Appl. Polym. Sci. 2008, 110, 1435–1440. [Google Scholar] [CrossRef]
- Rodrigues, J.A.G.; Oliveira Vanderlei, E.D.S.; Silva, L.M.; de Araujo, I.W.; de Queiroz, I.N.; de Paula, G.A.; Abreu, T.M.; Ribeiro, N.A.; Bezerra, M.M.; Chaves, H.V.; et al. Antinociceptive and anti-inflammatory activities of a sulfated polysaccharide isolated from the green seaweed Caulerpa cupressoides. Pharmacol. Rep. 2012, 64, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.G.; Oliveira Vanderlei, E.D.S.; Gomes Quindere, A.L.; Monteiro, V.S.; Mendes de Vasconcelos, S.M.; Barros Benevides, N.M. Antinociceptive activity and acute toxicological study of a novel sulfated polysaccharide from Caulerpa cupressoides var. lycopodium (Chlorophyta) in Swiss mice. Acta Sci. Technol. 2013, 35, 417–425. [Google Scholar]
- Chattopadhyay, K.; Adhikari, U.; Lerouge, P.; Ray, B. Polysaccharides from Caulerpa racemosa: Purification and structural features. Carbohydr. Polym. 2007, 68, 407–415. [Google Scholar] [CrossRef]
- Fernández, P.V.; Ciancia, M.; Miravalles, A.B.; Estevez, J.M. Cell wall polymer mapping in the coenocytic macroalga Codium vermilara. J. Phycol. 2010, 46, 456–465. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Hayashi, T.; Hayashi, K.; Osawa, T.; Niiya, K.; Sakuragawa, N. Activation of heparin cofactor II by calcium spirulan. J. Biol. Chem. 2000, 275, 11379–11382. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Mody, K.H.; Ramavat, B.K.; Murthy, A.S.K.; Siddhanta, A.K. Screening of Codiacean algae (Chlorophyta) of the Indian coasts for blood anticoagulant activity. Indian J. Mar. Sci. 2002, 31, 33–38. [Google Scholar]
- Ciancia, M.; Quintana, I.; Vizcarguenaga, M.I.; Kasulin, L.; de Dios, A.; Estevez, J.M.; Cerezo, A.S. Polysaccharides from the green seaweeds Codium fragile and C. vermilara with controversial effects on hemostasis. Int. J. Biol. Macromol. 2007, 41, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Farias, E.H.; Pomin, V.H.; Valente, A.P.; Nader, H.B.; Rocha, H.A.; Mourão, P.A. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.S.; Kim, W.J.; Kim, S.M.; Park, J.K.; Lee, S.M.; Kim, S.O.; Synytsya, A.; Park, Y.I. Purification, characterization and immunostimulating activity of water-soluble polysaccharide isolated from Capsosiphon fulvescens. Int. Immunopharmacol. 2010, 10, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Hayashi, K.; Hayashi, T.; Sankawa, U.; Maeda, M. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med. 1999, 65, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.M.; Zhang, Q.B.; Zhao, T.T.; Chen, R.; Zhang, H.; Niu, X.Z.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.M.; Zhao, T.T.; Zhang, Q.B.; Li, Z.; Zhao, Z.Q.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Mao, W.; Li, H.; Li, Y.; Zhang, H.; Qi, X.; Sun, H.; Chen, Y.; Guo, S. Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta). Int. J. Biol. Macromol. 2009, 44, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Mao, W.J.; Fang, F.; Li, H.Y.; Sun, H.H.; Chen, Y.; Qi, X.H. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr. Polym. 2008, 71, 428–434. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Hayashi, T.; Lee, J.B.; Srisomporn, P.; Maeda, M.; Ozawa, T.; Sakuragawa, N. Inhibition of thrombin by sulfated polysaccharides isolated from green algae. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1543, 86–94. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; You, S. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 2011, 48, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.L.; Chang, C.K.; Wu, M.L.; Huang, T.C. Studies on the expression of liver detoxifying enzymes in rats fed seaweed (Monostroma nitidum). Food Chem. Toxicol. 2007, 45, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Li, X.; Li, T.; Jiang, P.; Zhang, L.; Wu, M.; Zhang, L. Characterization and anti-tumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009, 9, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Jiang, P.; Zhang, L.; Wu, M. Antitumor and immunomodulating activity of polysaccharides from Enteromorpha intestinalis. Biotechnol. Biopro. Eng. 2010, 15, 421–428. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yao, Z.; Zhao, M.; Qi, H. Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int. J. Biol. Macromol. 2013, 58, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.S.; You, S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, S.; Xing, R.; Li, K.; Li, R.; Qin, Y.; Wang, X.; Wei, Z.; Li, P. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013, 92, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Qian, L.; Zhou, Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 62, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.B.R.; Sathivel, A.; Devaki, T. Antihepatotoxic nature of Ulva reticulata (Chlorophyceae) on acetaminophen-induced hepatoxicity in experimental rats. J. Med. Food 2004, 7, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J. Appl. Phycol. 2006, 18, 9–14. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. In vitro antioxidant and antitumor activities of different sulfated polysaccharides isolated from three algae. Int. J. Biol. Macromol. 2013, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.G.; Liu, S.; Yu, H.H.; Guo, Z.Y.; Li, Z.; Li, P.C. Preparation of high-molecular weight and high-sulfate content chitosans and their potential antioxidant activity in vitro. Carbohydr. Polym. 2005, 61, 148–154. [Google Scholar] [CrossRef]
- Lahaye, M.; Ray, B. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta)-NMR analysis of ulvan oligosaccharides. Carbohydr. Res. 1996, 283, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Brunel, M.; Bonnin, E. Fine chemical structure analysis of oligosaccharides produced by an ulvan-lyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp. (Ulvales, Chlorophyta). Carbohydr. Res. 1997, 304, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.Z.; Li, N.; Liu, X.G.; Zhou, G.F.; Zhang, Q.B.; Li, P.C. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Kaeffer, B.; Benard, C.; Lahaye, M.; Blottiere, H.M.; Cherbut, C. Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial tells. Planta Med. 1999, 65, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Margret, R.J.; Kumaresan, S.; Ravikumar, S. A preliminary study on the anti-inflammatory activity of methanol extract of Ulva lactuca in rat. J. Environ. Biol. 2009, 30, 899–902. [Google Scholar] [PubMed]
- Chiu, Y.H.; Chan, Y.L.; Li, T.L.; Wu, C.J. Inhibition of Japanese encephalitis virus infection by the sulfated polysaccharide extracts from Ulva lactuca. Mar. Biotechnol. 2012, 14, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Sathivel, A.; Raghavendran, H.R.B.; Srinivasan, P.; Devaki, T. Anti-peroxidative and anti-hyperlipidemic nature of Ulva lactuca crude polysaccharide on D-Galactosamine induced hepatitis in rats. Food Chem. Toxicol. 2008, 46, 3262–3267. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Han, J.H.; Kim, C.Y.; You, S.G. Molecular characteristics and immunomodulatory activities of water-soluble sulfated polysaccharides from Ulva pertusa. J. Med. Food 2012, 15, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, X.; Zhang, J.; Duan, Y.; Wang, X.; Zhang, Q. Synthesis and antihyperlipidemic activity of acetylated derivative of ulvan from Ulva pertusa. Int. J. Biol. Macromol. 2012, 50, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Staats, N.; de Winder, B.; Stal, L.J.; Mur, L.R. Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum. Eur. J. Phycol. 1999, 34, 161–169. [Google Scholar] [CrossRef]
- Pletikapic, G.; Radic, T.M.; Zimmermann, A.H.; Svetlicic, V.; Pfannkuchen, M.; Maric, D.; Godrjan, J.; Zutic, V. AFM imaging of extracellular polymer release by marine diatom Cylindrotheca closterium (Ehrenberg) Reiman & JC Lewin. J. Mol. Recogn. 2011, 24, 436–445. [Google Scholar] [CrossRef]
- Guzman, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-Inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.W.; Percival, E. The carbohydrates of Phaeodactylum tricornutum. Part I. Preliminary examination of the organism and characterization of low molecular weight material and of a glucan. J. Chem. Soc. 1965, 1298, 7035–7041. [Google Scholar] [CrossRef]
- Ford, C.W.; Percival, E. The carbohydrates of Phaeodactylum tricornutum. Part II. A sulphated glucuronomannan. J. Chem. Soc. 1965, 1299, 7042–7046. [Google Scholar] [CrossRef]
- Rincé, Y.; Lebeau, T.; Robert, J.M. Artificial cell-immobilization: A model simulating immobilization in natural environments? J. Appl. Phycol. 1999, 11, 263–272. [Google Scholar] [CrossRef]
- Penna, A.; Berluti, S.; Penna, N.; Magnani, M. Influence of nutrient ratios on the in vitro extracellular polysaccharide production by marine diatoms from Adriatic Sea. J. Plankton Res. 1999, 21, 1681–1690. [Google Scholar] [CrossRef]
- Urbani, R.; Sist, P.; Pletikapić, G.; Radić, T.M.; Svetličić, V.; Žutic, V. Diatom Polysaccharides: Extracellular Production, Isolation and Molecular Characterization. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 12. [Google Scholar] [CrossRef]
- Chen, C.-S.; Anaya, J.M.; Zhang, S.; Spurgin, J.; Chuang, C.-Y.; Xu, C.; Miao, A.-J.; Chen, E.Y.-T.; Schwehr, K.A.; Jiang, Y.; et al. Effects of engineered nanoparticles on the assembly of exopolymeric substances from phytoplankton. PLoS ONE 2011, 6, e21865. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Murillo, M.A.; Ascencio, F. Anti-adhesive activity of sulphated exopolysaccharides of microalgae on attachment of the red sore disease-associated bacteria and Helicobacter pylori to tissue culture cells. Lett. Appl. Microbiol. 2000, 30, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yamaura, M.; Maruyama, I. Isolation and identification of 2-O-methyl-l-rhamnose and 3-O-methyl-l-rhamnose as constituents of an acidic polysaccharide of Chlorella vulgaris. Biosci. Biotechnol. Biochem. 1997, 61, 539–540. [Google Scholar] [CrossRef]
- Ogawa, K.; Ikeda, Y.; Kondo, S. A new trisaccharide, α-d-glucopyranuronosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-rhamopyranose from Chlorella vulgaris. Carbohydr. Res. 1999, 321, 128–131. [Google Scholar] [CrossRef]
- Mishra, A.; Kavita, K.; Jha, B. Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr. Polym. 2011, 83, 852–857. [Google Scholar] [CrossRef]
- Allard, B.; Guillot, J.P. The production of extracellular polysaccharides by fresh-water microalgae. Investigation of the polysaccharide components. In Biomass for Energy and Industry; Grassi, G., Delmon, B., Molle, J.F., Zibetta, H., Eds.; Elsevier Applied Science: London, UK, 1987; pp. 603–607. [Google Scholar]
- Allard, B.; Casadeval, E. Carbohydrate composition and characterization of sugars from the green alga Botryococcus braunii. Phytochemistry 1990, 29, 1875–1878. [Google Scholar] [CrossRef]
- Geresh, S.; Arad, S.M. The extracellular polysaccharides of the red microalgae: Chemistry and rheology. Bioresour. Technol. 1991, 38, 195–201. [Google Scholar] [CrossRef]
- Dubinsky, O.; Barak, Z.; Geresh, S.; Arad, S.M. Composition of the cell-wall polysaccharide of the unicellular red alga Rhodella reticulata at two phases of growth. In Recent Advances in Algal Biotechnology, Proceedings of the 5th International Conference of the Society of Applied Algology, Tiberias, Israel, 28 January–2 February 1990; US Office of Naval Research: London, UK, 1990; p. 17. [Google Scholar]
- Arad, S.M. Production of sulphated polysaccharides from red unicellular algae. In Algal Biotechnology; Stadler, T., Mollion, J., Verdus, M.C., Karamanos, Y., Morvan, H., Christiaen, D., Eds.; Elsevier Applied Science: London, UK, 1988; pp. 65–87. [Google Scholar]
- Matsui, S.M.; Muizzudin, N.; Arad, S.M.; Marenus, K. Sulfated polysaccharides from red microalgae anti-inflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Arad, S.M.; Atar, D. Viscosupplementation with Algal Polysaccharides in the Treatment of Arthritis. Patent WO/2007/066340, 2007. [Google Scholar]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Antiviral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, D.; Morales, E.; Dominguez, A.; Fábregas, J. Productividad mixotrófica del exopolisacárido sulfatado com la microalga marina Porphyridium cruentum. In Communicaciones del III Congreso Ibérico de Biotecnología—Biotec’96; Universidad de Valladolid: Valladolid, Spain, 1996; pp. 591–592. [Google Scholar]
- Heaney-Kieras, J.H. Study of the Extracellular Polysaccharide of Porphyridium cruentum. Ph.D. Thesis, University of Chicago, Chicago, IL, USA, 1972. [Google Scholar]
- Raposo, M.F.J.; Morais, A.M.M.B.; Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Gloaguen, V.; Ruiz, G.; Morvan, H.; Mouradi-Givernaud, A.; Maes, E.; Krausz, P.; Srecker, G. The extracelular polysaccharide of Porphtyridium sp.: An NMR study of lithium-resistant oligosaccharidic fragments. Carbohydr. Res. 2004, 339, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Geresh, S.; Adin, I.; Yarmolinsky, E.; Karpasas, M. Characterization of the extracellular polysaccharide of Porphyridium sp.: Molecular weight determination and rheological properties. Carbohydr. Polym. 2002, 50, 183–189. [Google Scholar] [CrossRef]
- Dubinsky, O.; Simon, B.; Karamanos, Y.; Geresh, S.; Barak, Z.; Arad, S.M. Composition of the cell wall polysaccharide produced by the unicellular red alga Rhodella reticulata. Plant Physiol. Biochem. 1992, 30, 409–414. [Google Scholar]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different molecular weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; You, W. Studies on separation of extracellular polysaccharide from Porphyridium cruentum and its anti-HBV activity in vitro. Chin. J. Mar. Drugs (Chin.) 2005, 24, 18–21. [Google Scholar]
- Radonic, A.; Thulke, S.; Achenbach, J.; Kurth, A.; Vreemann, A.; König, T.; Walter, C.; Possinger, K.; Nitsche, A. Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to Vaccinia virus. J. Antivir. Antiretrovir. 2010, 2, 51–55. [Google Scholar]
- Evans, L.V.; Callow, M.E.; Percival, E.; Fareed, V.S. Studies on the synthesis and composition of extracellular mucilage in the unicellular red alga Rhodella. J. Cell Sci. 1974, 16, 1–21. [Google Scholar] [PubMed]
- Fareed, V.S.; Percival, E. The presence of rhamnose and 3-O-methylxylose in the extracellular mucilage from the red alga Rhodella maculata. Carbohydr. Res. 1977, 53, 276–277. [Google Scholar] [CrossRef]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulphated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped virus. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.Y.; Yim, J.H.; Lee, H.K.; Pyo, S. Activation of murine peritoneal macrophages by sulphated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03): Involvement of the NF-kappa B and JNK pathway. Int. Immunopharmacol. 2006, 6, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.H.; Son, E.; Pyo, S.; Lee, H.K. Novel sulfated polysaccharide derived from red-tide microalga Gyrodinium impudicum strain KG03 with immunostimulating activity in vivo. Mar. Biotechnol. 2005, 7, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, C.K.; Rhie, K.T.; Lee, H.K. Antiviral effects of sulphated polysaccharide from the marine microalga Gyrodinium impudicum strain KG03. Mar. Biotech. 2004, 6, 17–25. [Google Scholar] [CrossRef]
- Li, P.; Liu, Z.; Xu, R. Chemical characterization of the released polysaccharides from the cyanobacterium Aphanothece halophytica GR02. J. Appl. Phycol. 2001, 13, 71–77. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.J.A.; del Olmo, L.M.B.; Benito, P.B. Antiviral activities of polysaccharides from natural sources. Stud. Nat. Prod. Chem. 2005, 30, 393–418. [Google Scholar]
- Lee, J.-B.; Hayashi, T.; Hayashi, K.; Sankawa, U. Structural analysis of calcium spirulan (Ca-SP)-derived oligosaccharides using electrospray ionization mass spectrometry. J. Nat. Prod. 2000, 63, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kaji, T.; Okabe, M.; Shimada, S.; Yamamoto, C.; Fujiwara, Y.; Lee, J.-B.; Hayashi, T. Sodium spirulan as a potent inhibitor of arterial smooth muscle cell proliferation in vitro. Life Sci. 2004, 74, 1–9. [Google Scholar] [CrossRef]
- Challouf, R.; Trabelsi, L.; Dhieb, R.B.; El Abed, O.; Yahia, A.; Ghozzi, K.; Ammar, J.B.; Omran, H.; Ouada, H.B. Evaluation of cytotoxicity and biological activities in extracellular polysaccharides released by cyanobacterium Arthrospira platensis. Braz. Arch. Biol. Technol. 2011, 54, 831–838. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar]
- Pomin, V.H. Fucanomics and Galactanomics: Marine Distribution, Medicinal Impact, Conceptions, and Challenges. Mar. Drugs 2012, 10, 793–811. [Google Scholar] [CrossRef]
- Anderson, N.S.; Dolan, T.C.S.; Rees, D.A. Carrageenans. Part VII. Polysaccharides from Eucheuma spinosum and Eucheuma cottonii. The covalent structure of λ-carrageenan. J. Chem. Soc. Perkin. Trans. I 1973, 19, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Hiroe, M.; Noda, S.; Asai, I.; Ikeda, S.; Nishinari, K. Influence of molecular structure imaged with atomic force microscopy on the rheological behavior of carrageenan aqueous systems in the presence or absence of cations. Food Hydrocolloid 2007, 21, 617–629. [Google Scholar] [CrossRef]
- Li, H.; Mao, W.; Zhang, X.; Qi, X.; Chen, Y.; Chen, Y.; Chena, Y.; Xua, J.; Zhaoa, C.; Houa, Y.; et al. Structural characterization of an anticoagulant-active sulfated polysaccharide isolated from green alga Monostroma latissimum. Carbohydr. Polym. 2011, 85, 394–400. [Google Scholar] [CrossRef]
- Bilan, M.I.; Vinogradova, E.V.; Shashkov, A.S.; Usov, A.I. Structure of a highly pyruvylated galactan sulfate from the Pacific green alga Codium yezoense (Bryopsidales, Chlorophyta). Carbohydr. Res. 2007, 342, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Lee, J.B.; Hayashi, K.; Hayashi, T. Isolation of sulfated galactan from Codium fragile and its antiviral effect. Biol. Pharm. Bull. 2009, 32, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Karnjanapratum, S.; Cho, M.; Kim, J.K.; You, S. Molecular characteristics and biological activities of anionic macromolecules from Codium fragile. Int. J. Biol. Macromol. 2013, 59, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Hayashi, T.; Hayashi, K.; Sankawa, U.; Maeda, M.; Nemoto, T.; Nakanishi, H. Further purification and structural analysis of calcium spirulan from Spirulina platensis. J. Nat. Prod. 1998, 61, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Pignolet, O.; Jubeau, S.; Vaca-Garcia, C.; Michaud, P. Highly valuable microalgae: Biochemical and topological aspects. J. Ind. Microbiol. Biotechnol. 2013, 40, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Heaney-Kieras, J.H.; Chapman, D. Structural studies on the extracellular polysaccharide of the red alga Porphyridium cruentum. Carbohydr. Res. 1976, 52, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Geresh, S.; Dubinsky, O.; Arad, S.M.; Christian, D.; Glaser, R. Structure of 3-O-(α-d-glucopyranosyluronic acid)-l-galactopyranose, an aldobiuronic acid isolated from the polysaccharides of various unicellular red algae. Carbohydr. Res. 1990, 208, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Nagumo, T. The sulfate-content dependence of the anticoagulant activity of a fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr. Res. 1991, 214, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Pereira, M.S.; Valente, A.P.; Tollefsen, D.M.; Pavao, M.S.G.; Mourao, P.A.S. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II-dependent anticoagulant activity. Glycobiology 2005, 15, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Witvrouw, M.; de Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.J.; Tissot, B.; Chevolot, L.; Adjadj, E.; Du, Y.; Curmi, P.A.; Daniel, R. NMR characterization and molecular modeling of fucoidan showing the importance of oligosaccharide branching in its anticomplementary activity. Glycobiology 2010, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.J.; Mateu, C.G.; Artuso, M.C.; Scolaro, L.A. Polysaccharides from red algae: Genesis of a renaissance. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 20; pp. 535–554. Available online: http://www.intechopen.com/books/the-complex-world-of-polysaccharides/polysaccharides-from-red-algae-genesis-of-a-renaissance (accessed on 2 February 2015). [CrossRef]
- Baba, M.; Snoeck, R.; Pauwels, R.; de Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.S.A.; Mohamed, S.F.; Ali, F.M.; El-Sayed, O.H. Chemical Structure and Antiviral Activity of Water-soluble Sulfated Polysaccharides from Sargassum latifolium. J. Appl. Sci. Res. 2007, 3, 1178–1185. [Google Scholar]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Muller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, 671–680. [Google Scholar] [CrossRef]
- Zeitlin, L.; Whaley, K.J.; Hegarty, T.A.; Moench, T.R.; Cone, R.A. Tests of vaginal microbicides in the mouse genital herpes model. Contraception 1997, 56, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Oehninger, S.; Clark, G.F.; Acosta, A.A.; Hodgen, G.D. Nature of the inhibitory effect of complex saccharide moieties on the tight binding of human spermatozoa to the human zona pellucida. Fertil. Steril. 1991, 55, 165–169. [Google Scholar] [PubMed]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Antiviral effect of the red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]
- Esko, J.D.; Selleck, S.B. Order out of chaos: Assembly of ligand binding sites in heparin sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef] [PubMed]
- Heaney-Kieras, J.; Roden, L.; Chapman, D.J. The covalent linkage of protein to carbohydrate in the extracellular protein-polysaccharide from the red alga Porphyridium cruentum. Biochemistry 1977, 165, 1–9. [Google Scholar]
- Tabarsa, M.; Park, G.M.; Shin, I.S.; Lee, E.; Kim, J.K.; You, S. Structure-activity relationships of sulphated glycoproteins from Codium fragile on nitric oxide releasing capacity from RAW264.7 cells. Mar. Biotechnol. 2015, 17, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Activity of Porphyridium sp. polysaccharide against Herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Met. 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Li, L.-Y.; Li, L.-Q.; Guo, C.-H. Evaluation of in vitro antioxidant and antibacterial activities of Laminaria japonica polysaccharides. J. Med. Plants Res. 2010, 4, 2194–2198. [Google Scholar]
- Miroshnichenko, V.A.; Yansons, T.Y.; Polushin, O.G. Differentiated approach to the treatment of gastroduodenal pathology with the use of bioactive substances of marine hydrocele. In New Biomedical Technologies to Use of Dietary Supplements; Ivanov, E.M., Ed.; IMKVL Siberian Branch, Ross. Akad. Med. Nank.: Vladivostok, Russia, 1998; pp. 146–150. [Google Scholar]
- Shanmugam, M.; Mody, K.H. Heparinoid-active sulfated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. 2000, 79, 1672–1683. [Google Scholar]
- Pierre, G.; Sopena, V.; Juin, C.; Mastouri, A.; Graber, M.; Mangard, T. Antibacterial activity of a sulphated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol. Bioproc. Eng. 2011, 16, 937–945. [Google Scholar] [CrossRef]
- Yamashita, S.; Sugita-Konishi, Y.; Shimizu, M. In vitro bacteriostatic effects of dietary polysaccharides. Food Sci. Technol. Res. 2001, 7, 262–264. [Google Scholar] [CrossRef]
- Rice, P.J.; Adams, E.L.; Ozment-Skelton, T.; Gonzalez, A.J.; Goldman, M.P.; Lockhart, B.E.; Barker, L.A.; Breuel, K.F.; Deponti, W.K.; Kalbfleisch, J.H.; et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exper. Ther. 2005, 314, 1079–1086. [Google Scholar] [CrossRef]
- Verma, M.S.; Gu, F.X. 1,3-β-Glucans: Drug delivery and pharmacology. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 21; pp. 555–572. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Xing, Y.; Zhu, H.; Shen, J.; Tian, J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia–reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011, 49, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Gueniche, F.; Bertaud, A.F.; Tchen, S.I.; Fioretti, F.; Jouault, S.C.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Kim, A.J.; Kim, Y.O.; Hwang, J.K. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J. Med. Food 2005, 8, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.C.; Kim, W.J.; Kim, S.Y.; Kim, S.M.; Chung, M.K.; Park, J.W.; Suh, H.H.; Lee, K.B.; Park, Y.I. Immunomodulating activity of a fucoidan isolated from Korean Undaria pinnatifida sporophyll. Algae 2007, 22, 333–338. [Google Scholar] [CrossRef]
- Chen, Z.; Soo, M.Y.; Srinivasan, N.; Tan, B.K.H.; Chan, S.H. Activation of macrophages by polysaccharide-protein complex from Licium barbarum. Phytother. Res. 2009, 23, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapratum, S.; Tabarsa, M.; Cho, M.; You, S.G. Characterization and immunomodulatory activities of sulfated polysaccharides from Capsosiphon fulvescens. Int. J. Biol. Macromol. 2012, 51, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, X.Z.; Wen, Z.Y. Sulfated polysaccharides and immune response: Promoter or inhibitor? Panminerva Med. 2008, 50, 177–183. [Google Scholar] [PubMed]
- Tsuji, R.F.; Hoshino, K.; Noro, Y.; Tsuji, N.M.; Kurokawa, T.; Masuda, T.; Akira, S.; Nowak, B. Suppression of allergic reaction by lambda-carrageenan: Toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin. Exp. Allergy 2003, 33, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Heneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Ahn, G.N.; Jee, Y.H.; Kim, G.Y.; Kim, S.H.; Ha, J.H.; Kang, J.-S.; Lee, K.-W.; Jeon, Y.-J. Antiproliferative activity of sulfated polysaccharide isolated from an enzymatic digest of Ecklonia cava on the U-937 cell line. J. Appl. Phycol. 2009, 21, 307–314. [Google Scholar] [CrossRef]
- Chen, W.S.; Lazar, C.S.; Poenie, M.; Tsien, R.Y.; Gill, G.N.; Rosenfeld, M.G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. Nature 1987, 328, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Ermakova, S.P.; Choi, H.K.; Kusaykin, M.I.; Shevchenko, N.M.; Zvyagintseva, T.N.; Choi, H.S. Fucoidan from Laminaria cichorioides inhibits AP-1 transactivation and cell transformation in the mouse epidermal JB6 cells. Mol. Carcinog. 2008, 47, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y.S. Antitumor properties of non-starch polysaccharides: Fucoidans and Chitosans. Rus. J. Mar. Biol. 2010, 36, 321–330. [Google Scholar] [CrossRef]
- Yamamoto, I.; Nagumo, T.; Yagi, K.; Tominaga, H.; Aoki, M. Antitumor effect of seaweeds, 1. Antitumor effect of extract from Sargassum and Laminaria. Jpn. J. Exp. Med. 1974, 44, 543–546. [Google Scholar] [PubMed]
- Yamamoto, I.; Nagumo, T.; Takahashi, M.; Fujihara, M.; Suzuki, Y.; Iizima, N. Antitumor effect of seaweeds, 3. Antitumor effect of an extract from Sargassum kjellmanianum. Jpn. J. Exp. Med. 1981, 51, 187–189. [Google Scholar]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, N.; Zhao, T.; Qi, H.; Xu, Z.; Li, Z. Fucoidan inhibits the development of proteinuria in active Heymann nephritis. Phytother. Res. 2005, 19, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Teruya, T.; Tatemoto, H.; Konishi, T.; Tako, M. Structural characteristics and in vitro macrophage activation of acetyl fucoidan from Cladosiphon okamuranus. Glycoconj. J. 2009, 26, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.; Srinivasan, P.; Rekha, S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int. Immunopharmacol. 2011, 11, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk sea Fucus evanescens brown alga. Bull. Exper. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Namikoshi, M. Bioactive compounds produced by cyanobacteria. J. Int. Microbiol. Biotechnol. 1996, 17, 373–384. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.Y.; Kim, J.C.; Lee, Y.J.; You, S.G. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Lee, K.W.; Kim, S.K.; Jeon, Y.J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Uehara, T.; Harada, N.; Sekiguchi, M.; Hiraoka, A. Heparinoid-active sulfated polysaccharide from Monostroma-nitidum and their distribution in the Chlorophyta. Phytochemistry 1991, 30, 3611–3614. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsuura, Y.; Hori, K.; Miyazawa, K. An anticoagulant proteoglycan from the marine green alga, Codium pugniformis. J. Appl. Phycol. 2000, 12, 9–14. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsuura, Y.; Bacic, A.; Liao, M.L.; Hori, K.; Miyazawa, K. Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. Int. J. Biol. Macromol. 2001, 28, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mao, W.; Hou, Y.; Gao, Y.; Qi, X.; Zhao, C.; Chen, Y.; Chen, Y.; Li, N.; Wang, C. Preparation, structure and anticoagulant activity of a low molecular weight fraction produced by mild acid hydrolysis of sulfated rhamnan from Monostroma latissimum. Bioresour. Technol. 2012, 114, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.G.; Oliveira Vanderlei, E.D.S.; Bessa, E.F.; Magalhaes, F.D.A.; Monteiro de Paula, R.C.; Lima, V.; Barros Benevides, N.M. Anticoagulant activity of a sulfated polysaccharide isolated from the green seaweed Caulerpa cupressoides. Braz. Arch. Biol. Technol. 2011, 54, 691–700. [Google Scholar] [CrossRef]
- Rodrigues, J.A.G.; Lino de Queiroz, I.N.; Gomes Quindere, A.L.; Vairo, B.C.; de Souza Mourao, P.A.; Barros Benevides, N.M. An antithrombin-dependent sulfated polysaccharide isolated from the green alga Caulerpa cupressoides has in vivo anti- and prothrombotic effects. Cienc. Rural 2011, 41, 634–639. [Google Scholar] [CrossRef]
- Melo, F.R.; Pereira, M.S.; Foguel, D.; Mourão, P.A.S. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.F.; Dore, C.M.P.G.; Marques, C.T.; Nascimento, M.S.; Benevides, N.M.B.; Rocha, H.A.O.; Chavante, S.F.; Leite, E.L. Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans. Carbohydr. Polym. 2010, 79, 26–33. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Hayashi, T.; Hayashi, K.; Osawa, T.; Niiya, K.; Sakuragawa, N. Heparin cofactor II-dependent antithrombin activity of calcium spirulan. Blood Coagul. Fibrinol. 1996, 7, 554–560. [Google Scholar] [CrossRef]
- Grauffel, V.; Koareg, B.; Mabeau, S.; Duran, P.; Jozefonvicz, J. New natural polysaccharides with potent antithrombotic activity: Fucans from brown algae. Biomaterials 1989, 10, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Kindness, G.; Williamson, F.B.; Long, W.F. Effect of polyanetholesulfonic acid and xylan sulphate on antithrombin III activity. Biochem. Biophys. Res. Com. 1979, 13, 1062–1068. [Google Scholar] [CrossRef]
- Qiu, X.; Amarasekara, A.; Doctor, V. Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydr. Polym. 2006, 63, 224–228. [Google Scholar] [CrossRef]
- Jung, W.K.; Athukorala, Y.; Lee, Y.J.; Cha, S.H.; Lee, C.H.; Vasanthan, T.; Choi, K.-S.; Yoo, S.-H.; Kim, S.-K.; Jeon, Y.J. Sulfated polysaccharide purified from Ecklonia cava accelerates antithrombin III-mediated plasma proteinase inhibition. J. Appl. Phycol. 2007, 19, 425–430. [Google Scholar] [CrossRef]
- Nishino, T.; Nagumo, T. Anticoagulant and antithrombotic activities of oversulfated fucans. Carbohydr. Res. 1992, 229, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Minix, R.; Doctor, V.M. Interaction of fucoidan with proteases and inhibitors of coagulation and fibrinolysis. Thromb. Res. 1997, 87, 419–429. [Google Scholar] [CrossRef] [PubMed]
- De Candia, E.; de Cristofaro, R.; Landolfi, R. Thrombin-induced platelet activation is inhibited by high- and low-molecular-weight heparin. Circulation 1999, 99, 3308–3314. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.Z.; Zhang, Q.B.; Li, N.; Xu, Z.H.; Wang, Y.M.; Li, Z.E. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Qi, H.; Huang, L.; Liu, X.; Liu, D.; Zhang, Q.; Liu, S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Lahaye, M. Marine algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some “sea vegetables”. J. Sci. Food Agric. 1991, 54, 587–594. [Google Scholar] [CrossRef]
- Li, Z.J.; Xue, C.H.; Lin, H. The hypolipidemic effects and antioxidative activity of sulfated fucan on the experimental hyperlipidemia in rats. Acta Nutrim. Sin. 1999, 21, 280–283. [Google Scholar]
- Li, D.Y.; Xu, Z.; Zhang, S.H. Prevention and cure of fucoidan of L. japonica on mice with hypercholesterolemia. Food Sci. 1999, 20, 45–46. [Google Scholar]
- Ren, D.; Noda, H.; Amano, H.; Nishino, T.; Nishizawa, K. Study on antihypertensive and antihyperlipidemic effects of marine algae. Fish. Sci. 1994, 60, 33–40. [Google Scholar]
- Inoue, N.; Yamano, N.; Sakata, K.; Nagao, K.; Hama, Y.; Yanagita, T. The sulfated polysaccharide porphyran reduces apolipoprotein B100 secretion and lipid synthesis in HepG2 cells. Biosci. Biotechnol. Biochem. 2009, 73, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Panlasigui, L.N.; Baello, O.Q.; Dimatangal, J.M.; Dumelod, D.B. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac. J. Clin. Nutr. 2003, 12, 209–214. [Google Scholar] [PubMed]
- Reddy, B.S.; Watanabe, K.; Sheinfil, A. Effect of dietary wheat bran, alfalfa, pectin and carrageenan on plasma cholesterol and fecal bile acid and neutral sterol excretion in rats. J. Nutr. 1980, 110, 1247–1254. [Google Scholar] [PubMed]
- Ginzberg, A.; Cohen, M.; Sod-Moriah, U.A.; Shany, S.; Rosenshtrauch, A.; Arad, S.M. Chickens fed with biomass of the red microalga Porphyridium sp. have reduced blood cholesterol levels and modified fatty acids composition in egg yolk. J. Appl. Phycol. 2000, 12, 325–330. [Google Scholar] [CrossRef]
- Dvir, I.; Maislos, M.; Arad, S.M. Feeding rodents with red microalgae. In Dietary Fiber, Mechanisms of Action in Human Physiology and Metabolism; Cherbut, C., Barry, J.L., Lairon, D., Durand, M., Eds.; John Libbey Eurotext: Paris, France, 1995; pp. 86–91. [Google Scholar]
- Dvir, I.; Stark, A.H.; Chayoth, R.; Madar, Z.; Arad, S.M. Hycholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp. in rats. Nutrients 2009, 1, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, L.; Yu, Y.; Lin, W.; Chen, B.; Li, M. Reduction in the blood glucose level of exopolysaccharide of Porphyridium cruentum in alloxan-induced diabetic mice. J. Fujian Norm. Univ. 2006, 22, 77–80. (In Chinese) [Google Scholar]
- Glore, S.R.; van Treeck, D.; Knehans, A.W.; Guild, M. Soluble fiber in serum lipids: A literature review. J. Am. Diet. Assoc. 1994, 94, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Marlett, J. Dietary fibre and cardiovascular disease. In Handbook of Dietary Fibers; Cho, S.S., Dreher, M.D., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 17–30. [Google Scholar]
- Dvir, I.; Chayoth, R.; Sod-Moriah, U.; Shany, S.; Nyska, A.; Stark, A.H.; Madar, Z.; Arad, S.M. Soluble polysaccharide of red microalga Porphyridium sp. alters intestinal morphology and reduces serum cholesterol in rats. Br. J. Nutr. 2000, 84, 469–476. [Google Scholar] [PubMed]
- Lupescu, N.; Geresh, S.; Arad, S.M.; Bernstein, M.; Glaser, R. Structure of some sulfated sugars isolated after acid hydrolysis of the extracellular polysaccharide of Porphyridium sp. unicellular red alga. Carbohydr. Res. 1991, 210, 349–352. [Google Scholar] [CrossRef]
- Guillon, F.; Champ, M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 2000, 33, 233–245. [Google Scholar] [CrossRef]
- Barcelo, A.; Claustre, J.; Moro, F.; Chayvaille, J.A.; Cuber, J.C.; Plaisancie, P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 2000, 46, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Oakenfull, D. Physicochemical properties of dietary fiber: Overview. In Handbook of Dietary Fibers; Cho, S.S., Dreher, M.D., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 195–206. [Google Scholar]
- Ohta, T.; Sasaki, S.; Oohori, T.; Yoshikawa, S.; Kurihara, H. β-Glucosidase inhibitory activity of a 70% methanol extract from ezoishe (Pelvetia babingtonii de Toni) and its effect on the elevation of blood glucose level in rats. Biosci. Biotechnol. Biochem. 2002, 66, 1552–1554. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Y.; Xue, C.H.; Ning, Y.; Li, Z.J.; Xu, J.C. Acute antihypertensive effects of fucoidan oligosaccharides prepared from Laminaria japonica on renovascular hypertensive rat. J. Ocean Univ. Qingdao 2004, 34, 560–564. [Google Scholar]
- Costa, M.S.S.P.; Costa, L.S.; Cordeiro, S.L.; Almeida-Lima, J.; Dantas-Santos, N.; Magalhaes, K.D.; Sabry, D.A.; Lopes Albuquerque, I.R.; Pereira, M.R.; Leite, E.L. Evaluating the possible anticoagulant and antioxidant effects of sulfated polysaccharides from the tropical green alga Caulerpa cupressoides var. flabellata. J. Appl. Phycol. 2012, 24, 1159–1167. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, D.S.; Athukorala, Y.; Jeon, Y.J.; Senevirathne, M.; Rha, C.K. Antioxidant activity of sulfated polysaccharides isolated from Sargassum fulvellum. J. Food Sci. Nutr. 2007, 12, 65–73. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, H.-S.; Kang, I.-J.; Won, M.-H.; You, S.G. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Bergman, M.; van Moppes, D.; Grossman, S.; Arad, S.M. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Chen, B.; You, B.; Huang, J.; Yu, Y.; Chen, W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2010, 26, 833–840. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Yu, S.; Yin, L.; Zhao, M.; Han, Z. Synthesized oversulfated and acetylated derivatives of polysaccharide extracted from Enteromorpha linza and their potential antioxidant activity. Int. J. Biol. Macromol. 2011, 49, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xue, C.H.; Li, B.F. Study of antioxidant activities of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2008, 20, 431–436. [Google Scholar] [CrossRef]
- Bernal, P.; Llamas, M.A. Promising biotechnological applications of antibiofilm exopolysaccharides. Microb. Biotechnol. 2012, 5, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, O. Spirulina, the edible microorganism (algae, single-cell protein). Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [PubMed]
- Marceliano, M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer: London, UK, 2009. [Google Scholar]
- Franco, J.M.; Raymundo, A.; Sousa, I.; Gallegos, C. Influence of Processing Variables on the Rheological and Textural Properties of Lupin Protein-Stabilized Emulsions. J. Agric. Food Chem. 1998, 46, 3109–3115. [Google Scholar] [CrossRef]
- Raymundo, A.; Franco, J.; Gallegos, C.; Empis, J.; Sousa, I. Effect of thermal denaturation of lupin protein on its emulsifying properties. Nahrung 1998, 42, 220–224. [Google Scholar] [CrossRef]
- Raymundo, A.; Gouveia, L.; Batista, A.P.; Empis, J.; Sousa, I. Fat mimetic capacity of Chlorella vulgaris biomass in oil-in-water food emulsions stabilized by pea protein. Food Res. Int. 2005, 38, 961–965. [Google Scholar] [CrossRef]
- Coura, C.O.; de Araújo, I.W.; Vanderlei, E.S.; Rodrigues, J.A.; Quinderé, A.L.; Fontes, B.P.; de Queiroz, I.N.; de Menezes, D.B.; Bezerra, M.M.; e Silva, A.A.; et al. Antinociceptive and anti-inflammatory activities of sulphated polysaccharides from the red seaweed Gracilaria cornea. Basic Clin. Pharmacol. Toxicol. 2012, 110, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Assreuy, A.M.S.; Gomes, D.M.; Silva, M.S.J.; Torres, V.M.; Siqueira, R.C.L.; Pires, A.F.; Criddle, D.N.; Alencar, N.M.N.; Cavada, B.S.; Sampaio, A.H.; et al. Biological effects of a sulfated-polysaccharide isolated from the marine red algae Champia feldmannii. Biol. Pharm. Bull. 2008, 31, 691–695. [Google Scholar]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Chabut, D.; Fischer, A.M.; Colliec-Jouault, S.; Laurendeau, I.; Matou, S.; Le Bonniec, B.; Helley, D. Low molecular weight fucoidan and heparin enhance the basic fibroblast growth factor-induced tube formation of endothelial cells through heparan sulfate-dependent alpha 6 overexpression. Mol. Pharmacol. 2003, 64, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Itsuko, K.; Hideyuki, S.; Masato, N.; Shusuke, H.; Haruji, S.; Teruo, Y. Antiulcer agent and adhesion inhibitor for Helicobacter pylori. Patent EP0645143 B1, 21 September 1994. [Google Scholar]
- Saito, A.; Yoneda, M.; Yokohama, S.; Okada, M.; Haneda, M.; Nakamura, K. Fucoidan prevents concanavalin A-induced liver injury through induction of endogenous IL-10 in mice. Hepatol. Res. 2006, 35, 190–198. [Google Scholar] [PubMed]
- Kawano, N.; Egashira, Y.; Sanada, H. Effect of dietary fiber in edible seaweeds on the development of d-galactosamine-induced hepatopathy in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2007, 53, 446–450. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P.; Sundarapandiyan, R. Renal peroxidative changes mediated by oxalate: The protective role of fucoidan. Life Sci. 2006, 79, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Rajesh, N.G.; Varalakshmi, P. Mitochondrial dysfunction in an animal model of hyperoxaluria: A prophylactic approach with fucoidan. Eur. J. Pharmacol. 2008, 579, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P. Effect of sulphated polysaccharides on erythrocyte changes due to oxidative and nitrosative stress in experimental hyperoxaluria. Hum. Exp. Toxicol. 2007, 26, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P. Physico-chemical alterations of urine in experimental hyperoxaluria: A biochemical approach with fucoidan. J. Pharm. Pharmacol. 2007, 59, 419–527. [Google Scholar] [CrossRef] [PubMed]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P. Beneficial role of sulfated polysaccharides from edible seaweed Fucus vesiculosus in experimental hyperoxaluria. Food Chem. 2007, 100, 1552–1559. [Google Scholar] [CrossRef]
- Liu, J.C.; Zheng, F.L.; Liu, Y.P. Effect of fucoidan on renal interstitial fibrosis in adenine-induced chronic renal failure in rats. Nephrology 2008, 13, 158. [Google Scholar] [CrossRef]
- Angulo, Y.; Lomonte, B. Inhibitory effect of fucoidan on the activities of crotaline snake venom myotoxic phospholipases A(2). Biochem. Pharmacol. 2003, 66, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Alves, A.; Popa, E.G.; Reys, L.L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ige, O.O.; Umoru, L.E.; Aribo, S. Natural Products: A Minefield of Biomaterials. ISRN Mater. Sci. 2012, 2012. [Google Scholar] [CrossRef]
- Fundueanu, G.; Esposito, E.; Mihai, D.; Carpov, A.; Desbrieres, J.; Rinaudo, M.; Nastruzzi, C. Preparation and characterization of Ca-alginate microspheres by a new emulsification. Int. J. Pharm. 1998, 170, 11–21. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 7, 106–126. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocolloid 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Kong, H.; Mooney, D. Polysaccharide- based hydrogels in tissue engineering. In Polysacharides. Structural Diversity and Functional Versatility, 2nd ed.; Dumitriu, S., Ed.; Marcel Dekker: New York, NY, USA, 2005; pp. 817–837. [Google Scholar]
- Qin, Y. The characterization of alginate wound dressing with different fiber and textile structures. J. Appl. Polym. Sci. 2006, 100, 2516–2520. [Google Scholar] [CrossRef]
- Qin, Y. Alginate fibbers: An overview of the production processes and applications in wound management. Polym. Int. 2008, 57, 171–108. [Google Scholar] [CrossRef]
- Rinaudo, M. Biomaterials based on a natural polysaccharide: Alginate. TIP. Revista Especializada en Ciencias Químico-Biológicas 2014, 17, 92–96. [Google Scholar]
- Gong, Y.; He, L.; Li, J.; Zhou, Q.; Ma, Z.; Gao, C.; Shen, J. Hydrogel-filled polylactide porous scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Yang, W.; Mao, X.; Mou, S.; Tang, S. Agar/collagen membrane as skin dressing for wounds. Biomed. Mater. 2008, 3, 1–7. [Google Scholar] [CrossRef]
- Nakayama, Y.; Tsujinaka, T. Acceleration of robust “biotube” vascular graft fabrication by in-body tissue architecture technology using a novel eosin Y-releasing mold. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Toskas, G.; Hund, R.-D.; Laourine, E.; Cherif, C.; Smyrniotopoulos, V.; Roussis, V. Nanofibers based on polysaccharides from the green seaweed Ulva rigida. Carbohydr. Polym. 2011, 84, 1093–1102. [Google Scholar] [CrossRef]
- Alves, A.; Duarte, A.R.C.; Mano, J.F.; Sousa, R.A.; Reis, R.L. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J. Supercrit. Fluids 2012, 65, 32–38. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. Processing of degradable ulvan 3D porous structures for biomedical applications. J. Biomed. Mater. Res. A 2013, 101, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Chiellini, F. Ulvan as a New Type of Biomaterial from Renewable Resources: Functionalization and Hydrogel Preparation. Macromol. Chem. Phys. 2010, 211, 821–832. [Google Scholar] [CrossRef]
- Browder, W.; Williams, D.; Lucore, P.; Pretus, H.; Jones, E.; Mcnamee, R. Effect of Enhanced Macrophage Function on Early Wound-Healing. Surgery 1988, 104, 224–230. [Google Scholar] [PubMed]
- Kofuji, K.; Huang, Y.; Tsubaki, K.; Kokido, F.; Nishikawa, K.; Isobe, T.; Murata, Y. Preparation and evaluation of a novel wound dressing sheet comprised of beta-glucan-chitosan complex. React. Funct. Polym. 2010, 70, 784–789. [Google Scholar] [CrossRef]
- Delatte, S.J.; Evans, J.; Hebra, A.; Adamson, W.; Othersen, H.B.; Tagge, E.P. Effectiveness of beta-glucan collagen for treatment of partial-thickness burns in children. J. Ped. Sur. 2001, 36, 113–118. [Google Scholar] [CrossRef]
- Sezer, A.D.; Hatipoğlu, F.; Cevher, E.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. Chitosan films containing fucoidan as a wound dressing for dermal burn healing: Preparation and in vitro/in vivo evaluation. AAPS PharmSciTech 2007, 8, E94–E101. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, T.; Martin, A.M. Wound treatment with Sorban-an alginate fibre dressing. Biomaterials 1983, 4, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Motta, G.J. Calcium alginate topical wound dressings: A new dimension in the cost-effective treatment for exudating dermal wounds and pressure sores. Ostomy Wound Manag. 1989, 25, 52–56. [Google Scholar]
- De Morais, M.G.; Stillings, C.; Dersch, R.; Rudisile, M.; Pranke, P.; Costa, J.A.V.; Wendorff, J. Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour. Technol. 2010, 101, 2872–2876. [Google Scholar] [CrossRef] [PubMed]
- Arad, S.M.; Rapoport, L.; Moshkovich, A.; van Moppes, D.; Karpasan, M.; Golan, R.; Golan, Y. Superior biolubricant from a species of red microalga. Langmuir 2006, 2, 7313–7317. [Google Scholar] [CrossRef]
- Johnson, A.S.; O'Sullivan, E.; D’Aoust, L.N.; Omer, A.; Bonner-Weir, S.; Fisher, R.J.; Weir, G.C.; Colton, C.K. Quantitative assessment of islets of langerhans encapsulated in alginate. Tissue Eng. Part C Methords 2011, 17, 435–449. [Google Scholar] [CrossRef]
- Tagler, D.; Tu, T.; Smith, R.M.; Anderson, N.R.; Tingen, C.M.; Woodruff, T.K.; Shea, L.D. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng. Part A 2012, 18, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The Influence of Hydrogel Modulus on the Proliferation and Differentiation of Encapsulated Neural Stem Cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Jeon, O.; Krebs, M.D.; Hill, M.C.; Alsberg, E. Biodegradable photo-crosslinked alginate nanofibre scaffolds with tuneable physical properties, cell adhesivity and growth factor release. Eur. Cells Mater. 2012, 24, 331–343. [Google Scholar]
- Zhou, H.; Xu, H.H.K. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 2011, 32, 7503–7513. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, H.H.K.; Zhou, H.; Weir, M.D.; Chen, Q.; Trotman, C.A. Human umbilical cord stem cell encapsulation in novel macroporous and injectable fibrin for muscle tissue engineering. Acta Biomater. 2013, 9, 4688–4697. [Google Scholar] [CrossRef] [PubMed]
- Khanarian, N.T.; Jiang, J.; Wan, L.Q.; Mow, V.C.; Lu, H.H. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng. Part A 2012, 18, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Hatch, A.; Hansmann, G.; Murthy, S.K. Engineered alginate hydrogels for effective microfluidic capture and release of endothelial progenitor cells from whole blood. Langmuir 2011, 27, 4257–4264. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; James, D.; Li, H.; Butler, J.; Rafii, S.; Rabbany, S. Generation of stable co-cultures of vascular cells in a honeycomb alginate scaffold. Tissue Eng. Part A 2010, 16, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, H.; Shahgasempour, S.; Naimi-Jamal, M.R.; Petrovi, H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int. J. Nanomed. 2011, 6, 2133–2141. [Google Scholar] [CrossRef]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.; Duan, B.; Malone, E.; Wu, J.; Girardi, L.N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012, 4. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Novotna, K.; Parizek, M. Polysaccharides as Cell Carriers for Tissue Engineering: The Use of Cellulose in Vascular Wall Reconstruction. Physiol. Res. 2014, 63, 29–47. [Google Scholar]
- Petrulyte, S. Advanced textile materials and biopolymers in wound management. Dan. Med. Bull. 2008, 55, 72–77. [Google Scholar] [PubMed]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell delivery systems using alginate-carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.Y.; Son, S.; Lee, S.J.; You, D.G.; Yhee, J.Y.; Park, J.H.; MSwierczewska, M.; Lee, S.; Kwon, I.C.; Kim, S.H.; et al. Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: Sequential delivery of doxorubicin and Bcl-2 siRNA. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Ghadban, A.1.; Albertin, L.; Rinaudo, M.; Heyraud, A. Biohybrid glycopolymer capable of ionotropic gelation. Biomacromolecules 2012, 13, 3108–3119. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Yang, M. Evaluation of glucan/poly(vinyl alcohol) blend wound dressing using rat models. Int. J. Pharm. 2008, 346, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, B.; Freguin-Bouilland, C.; Lallemand, F.; Henry, J.P.; Litzler, P.Y.; Marie, J.P.; Richard, V.; Thuillez, C.; Plissonnier, D. Low molecular weight fucan prevents transplant coronaropathy in rat cardiac allograft model. Transpl. Immunol. 2006, 16, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Fréguin-Bouilland, C.; Alkhatib, B.; David, N.; Lallemand, F.; Henry, J.P.; Godin, M.; Thuillez, C.; Plissonnier, D. Low molecular weight fucoidan prevents neointimal hyperplasia after aortic allografting. Transplantation 2007, 83, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967-3028. https://doi.org/10.3390/md13052967
De Jesus Raposo MF, De Morais AMB, De Morais RMSC. Marine Polysaccharides from Algae with Potential Biomedical Applications. Marine Drugs. 2015; 13(5):2967-3028. https://doi.org/10.3390/md13052967
Chicago/Turabian StyleDe Jesus Raposo, Maria Filomena, Alcina Maria Bernardo De Morais, and Rui Manuel Santos Costa De Morais. 2015. "Marine Polysaccharides from Algae with Potential Biomedical Applications" Marine Drugs 13, no. 5: 2967-3028. https://doi.org/10.3390/md13052967
APA StyleDe Jesus Raposo, M. F., De Morais, A. M. B., & De Morais, R. M. S. C. (2015). Marine Polysaccharides from Algae with Potential Biomedical Applications. Marine Drugs, 13(5), 2967-3028. https://doi.org/10.3390/md13052967