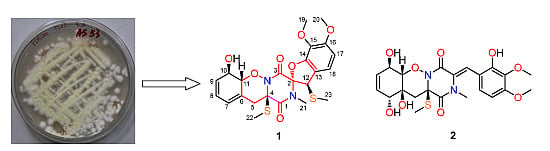
Peniciadametizine A, a Dithiodiketopiperazine with a Unique Spiro[furan-2,7'-pyrazino[1,2-b][1,2]oxazine] Skeleton, and a Related Analogue, Peniciadametizine B, from the Marine Sponge-Derived Fungus Penicillium adametzioides
Abstract
:1. Introduction

2. Results and Discussion
| No. | 1 (Acquired in DMSO-d6) | 2 (Acquired in Acetone-d6) | ||||
|---|---|---|---|---|---|---|
| δC a, Type | δH (J in Hz) b | HMBC | δC a, Type | δH (J in Hz) b | HMBC | |
| 1 | 161.7, C | 164.9, C | ||||
| 2 | 100.8, C | 129.0, C | ||||
| 3 | 158.7, C | 160.9, C | ||||
| 4 | 70.4, C | 68.1, C | ||||
| 5 | 36.8, CH2 | 3.58, d (14.0) | 1, 4, 6, 7, 11 | 33.0, CH2 | 2.09, d (15.0) | 6, 7 |
| 2.98, d (14.0) | 2.54, d (15.0) | |||||
| 6 | 129.7, C | 74.0, C | ||||
| 7 | 122.2, CH | 5.82, d (2.5) | 6, 8 | 75.2, CH | 4.40, br s | 6, 8 |
| 8 | 122.4, CH | 5.76, dd (10.0, 2.5) | 6, 10 | 130.3, CH | 5.54, br d (10.4) | 6, 10 |
| 9 | 132.2, CH | 5.69, br d (10.0) | 7, 11 | 128.9, CH | 5.60, br d (10.4) | 7, 11 |
| 10 | 69.5, CH | 4.54, br dd (13.5, 4.5) | 65.9, CH | 4.65, br s | 9 | |
| 11 | 91.4, CH | 4.92, d (13.5) | 10 | 89.8, CH | 4.00, d (8.2) | 6, 10 |
| 12 | 53.5, CH | 4.95, br s | 2, 3, 13, 14, 23 | 118.1, CH | 7.25, s | 3, 14, 18 |
| 13 | 118.3, C | 115.6, C | ||||
| 14 | 149.7, C | 150.1, C | ||||
| 15 | 132.3, C | 137.1, C | ||||
| 16 | 153.1, C | 154.8, C | ||||
| 17 | 107.0, CH | 6.76, d (8.4) | 13, 15, 16 | 104.8, CH | 6.66, d (8.7) | 13, 15 |
| 18 | 119.2, CH | 6.94, d (8.4) | 12, 14, 16 | 126.0, CH | 6.90, d (8.7) | 14, 16 |
| 19 | 60.2, CH3 | 3.73, s | 15 | 61.0, CH3 | 3.79, s | 15 |
| 20 | 56.2, CH3 | 3.80, s | 16 | 56.4, CH3 | 3.89, s | 16 |
| 21 | 28.2, CH3 | 2.78, s | 1, 2 | 35.6, CH3 | 2.91, s | 1, 2 |
| 22 | 13.2, CH3 | 2.36, s | 4 | 13.9, CH3 | 2.26, s | 4 |
| 23 | 13.0, CH3 | 1.78, s | 12 | |||
| 10-OH | 5.29, d (4.5) | |||||








3. Experimental Section
3.1. General
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Computational Section
3.6. Brine Shrimp Assay
3.7. Antimicrobial Assays
3.8. Cytotoxicity Assays
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seephonkai, P.; Kongsaeree, P.; Prabpai, S.; Isaka, M.; Thebtaranonth, Y. Transformation of an irregularly bridged epidithiodiketopiperazine to trichodermamide A. Org. Lett. 2006, 8, 3073–3075. [Google Scholar] [CrossRef] [PubMed]
- Magnus, P.; Mitchell, I.S. Hemi-thioacetal pummerer reaction for the synthesis of gliovirin benzylic sulfide models. Tetrahedron Lett. 1998, 39, 9131–9134. [Google Scholar] [CrossRef]
- Klausmeyer, P.; McCloud, T.G.; Tucker, K.D.; Cardellina, J.H., II; Shoemaker, R.H. Aspirochlorine class compounds from Aspergillus flavus inhibit azole-resistant Candida albicans. J. Nat. Prod. 2005, 68, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Li, X.M.; Liu, Y.; Wang, B.G. Penicibilaenes A and B, sesquiterpenes with a tricyclo[6.3.1.01,5]dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org. Lett. 2014, 16, 6052–6055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Mándi, A.; Li, X.M.; Du, F.Y.; Wang, J.N.; Li, X.; Kurtán, T.; Wang, B.G. Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxacage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org. Lett. 2014, 16, 4834–4837. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.M.; Meng, L.H.; Wang, B.G. N-Formyllapatin A, a new N-formylspiroquinazoline derivative from the marine-derived fungus Penicillium adametzioides AS-53. Phytochem. Lett. 2014, 10, 145–148. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, L.H.; Mándi, A.; Kurtán, T.; Li, X.M.; Liu, Y.; Li, X.; Li, C.S.; Wang, B.G. Brocaeloids A-C, 4-oxoquinoline and indole alkaloids with C-2 reversed prenylation from the mangrove-derived endophytic fungus Penicillium brocae. Eur. J. Org. Chem. 2014, 2014, 4029–4036. [Google Scholar] [CrossRef]
- Meng, L.H.; Li, X.M.; Lv, C.T.; Li, C.S.; Xu, G.M.; Huang, C.G.; Wang, B.G. Sulfur-containing cytotoxic curvularin macrolides from Penicillium sumatrense MA-92, a fungus obtained from the rhizosphere of the mangrove Lumnitzera racemosa. J. Nat. Prod. 2013, 76, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Makishima, D.; Akiyama, K.; Hayashi, H. New convulsive compounds, brasiliamides A and B, from Penicillium brasilianum Batista JV-379. Biosci. Biotechnol. Biochem. 2002, 66, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Silverton, J.V.; Kabuto, C.; Akiyama, T. The structure and absolute configuration of viridicatumtoxin: 2'S-(2'á,7'â,11'aâ,12'â)-7',7'a,8',11',11'a,12'-hexahydro-5',6',7'a,10',11'a,12'-hexahydroxy-3'-methoxy-2,6,6-trimethyl-7',8'-dioxospiro[2-cyelohexene-1,2'(l'H)-cyelopenta[de]naphthaeene]-9'-carboxamide methanolate. Acta Cryst. 1982, 38, 3032–3037. [Google Scholar]
- Guo, C.J.; Yeh, H.H.; Chiang, Y.M.; Sanchez, J.F.; Chang, S.L.; Bruno, K.S.; Wang, C.C.C. Biosynthetic pathway for the epipolythiodioxopiperazine acetylaranotin in Aspergillus terreus revealed by genome-based deletion analysis. J. Am. Chem. Soc. 2013, 135, 7205–7213. [Google Scholar] [CrossRef] [PubMed]
- Chankhamjon, P.; Boettger-Schmidt, D.; Scherlach, K.; Urbansky, B.; Lackner, G.; Kalb, D.; Dahse, H.M.; Hoffmeister, D.; Hertweck, C. Biosynthesis of the halogenated mycotoxin sspirochlorine in koji mold involves a cryptic amino acid conversion. Angew. Chem. Int. Ed. 2014, 53, 13409–13413. [Google Scholar] [CrossRef] [PubMed]
- Stipanovic, R.D.; Howell, C.R. The structure of gliovirin, a new antibiotic from Gliocladium virens. J. Antibiotics 1982, 35, 1326–1330. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.M.; Teuscher, F.; Li, D.L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- MacroModel: Versatile, full-featured program for molecular modeling. Schrödinger LLC. Available online: http://www.schrodinger.com/productpage/14/11/ (accessed on 31 December 2013).
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Xu, D.X.; Mándi, A.; Kurtán, T.; Li, T.J.; Schulz, B.; Zhang, W. Structure, absolute configuration, and conformational study of 12-membered macrolides from the fungus Dendrodochium sp. associated with the sea cucumber Holothuria nobilis Selenka. J. Org. Chem. 2013, 78, 7030–7047. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Varetto, U. MOLEKEL 5.4, Swiss National Supercomputing Centre: Manno, Switzerland, 2009.
- An, C.Y.; Li, X.M.; Luo, H.; Li, C.S.; Wang, M.H.; Xu, G.M.; Wang, B.G. 4-Phenyl-3,4-dihydroquinolone derivatives from Aspergillus nidulans MA-143, an endophytic fungus Isolated from the mangrove plant Rhizophora stylosa. J. Nat. Prod. 2014, 76, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, R.I.; Cavanaugh, P.F., Jr.; Kline, S.J.; Hughes, R.G., Jr.; Elliott, G.T.; Porter, C.W. Antineoplastic and antiherpetic activity of spermidine catecholamide iron chelators. Biochem. Biophys. Res. Commun. 1984, 121, 848–854. [Google Scholar] [CrossRef]
- Mosmann, T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Mándi, A.; Li, X.-M.; Meng, L.-H.; Kurtán, T.; Wang, B.-G. Peniciadametizine A, a Dithiodiketopiperazine with a Unique Spiro[furan-2,7'-pyrazino[1,2-b][1,2]oxazine] Skeleton, and a Related Analogue, Peniciadametizine B, from the Marine Sponge-Derived Fungus Penicillium adametzioides. Mar. Drugs 2015, 13, 3640-3652. https://doi.org/10.3390/md13063640
Liu Y, Mándi A, Li X-M, Meng L-H, Kurtán T, Wang B-G. Peniciadametizine A, a Dithiodiketopiperazine with a Unique Spiro[furan-2,7'-pyrazino[1,2-b][1,2]oxazine] Skeleton, and a Related Analogue, Peniciadametizine B, from the Marine Sponge-Derived Fungus Penicillium adametzioides. Marine Drugs. 2015; 13(6):3640-3652. https://doi.org/10.3390/md13063640
Chicago/Turabian StyleLiu, Yang, Attila Mándi, Xiao-Ming Li, Ling-Hong Meng, Tibor Kurtán, and Bin-Gui Wang. 2015. "Peniciadametizine A, a Dithiodiketopiperazine with a Unique Spiro[furan-2,7'-pyrazino[1,2-b][1,2]oxazine] Skeleton, and a Related Analogue, Peniciadametizine B, from the Marine Sponge-Derived Fungus Penicillium adametzioides" Marine Drugs 13, no. 6: 3640-3652. https://doi.org/10.3390/md13063640
APA StyleLiu, Y., Mándi, A., Li, X. -M., Meng, L. -H., Kurtán, T., & Wang, B. -G. (2015). Peniciadametizine A, a Dithiodiketopiperazine with a Unique Spiro[furan-2,7'-pyrazino[1,2-b][1,2]oxazine] Skeleton, and a Related Analogue, Peniciadametizine B, from the Marine Sponge-Derived Fungus Penicillium adametzioides. Marine Drugs, 13(6), 3640-3652. https://doi.org/10.3390/md13063640