Antimicrobial Action of Compounds from Marine Seaweed
Abstract
:1. Introduction
2. Bioactive Compounds
2.1. Polysaccharides and Derived Oligosaccharides
2.1.1. Alginates
2.1.2. Carrageenans
2.1.3. Agar
2.1.4. Galactans
2.1.5. Laminarans
2.1.6. Fucoidans/Fucans
2.1.7. Ulvans
2.2. Lipids, Fatty Acids Ans Sterols
- Phospholipids are located in extra-chloroplast membranes and account for 10%–20% of total lipids in algae. They are characterized by higher contents of n-6 fatty acids, and the major fatty acids present are oleic, palmitic, stearic, arachidonic and eicosapentanoic acids. The most dominant phospholipid in algae is phosphatidylglycerol in green algae, phosphatidylcholine in red algae, and phosphatidylcholine and phosphatidylethanolamine in brown algae.
- Glycolipids are located in photosynthetic membranes and constitute more than half of the lipids in the main algal groups. They are characterized by high n-3 polyunsaturated fatty acids. Three major types of glycolipids are monogalactosyldiacylglycerides, digalactosyldiacylglycerides, and sulfoquinovosyldiacylglycerides [31].
- Triacylglicerol is the most prevalent neutral lipid, their content ranging from 1% to 97% with a function of storage and energy reservoir.
2.3. Phenolic Compounds
2.4. Pigments
2.5. Other Compounds
2.5.1. Lectins
2.5.2. Alkaloids
2.5.3. Terpenes
2.5.4. Halogenated Compounds
3. Assesment of Antimicrobial Activity
3.1. In Vitro Assays
3.1.1. Diffusion Agar Tests
3.1.2. Growth Inhibition Assay
3.1.3. Minimun Inhibitory Concentration (MIC) Determination
3.1.4. Other Studies
3.2. In Vivo Assays
4. Production of Antimicrobials from Seaweed
4.1. Factors Affecting the Content of Antimicrobials in Seaweed
4.2. Extraction of Antimicrobials from Seaweed
4.2.1. Solvent Extraction
4.2.2. Alternative Solvents
4.2.3. Sequential Extraction and Purification
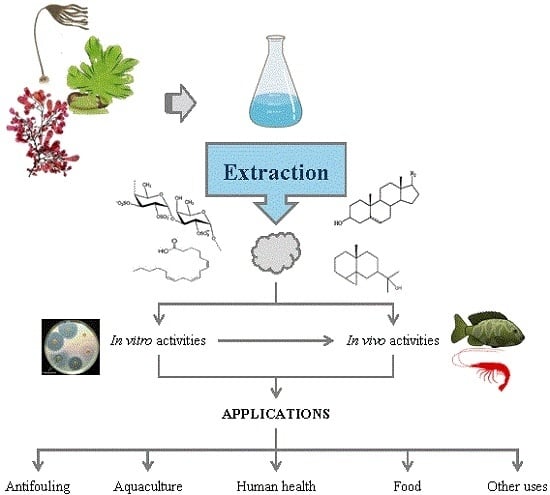
5. Applications
5.1. Biofouling
5.2. Aquaculture
5.3. Human Health
5.3.1. Antibacterial and Antifungical
Acne
Oral Microbes
5.3.2. Antiprotozoals
5.3.3. Antivirals
5.4. Food
5.5. Other Uses
6. Conclusions and Future Trends
Acknowledgments
Conflicts of Interest
References
- Bhadury, P.; Wright, P.C. Exploitation of marine algae: Biogenic compounds for potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Hellio, C.; Marechal, J.P.; Veron, B.; Bremer, G.; Clare, A.S.; Le Gal, Y. Seasonal variation of antifouling activities of marine algae from the Brittany coast (France). Mar. Biotechnol. 2004, 6, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Águila-Ramírez, R.N.; Arenas-González, A.; Hernández-Guerrero, C.J.; González-Acosta, B.; Borges-Souza, J.M.; Veron, B.; Pope, J.; Hellio, C. Antimicrobial and antifouling activities achieved by extracts of seaweeds from Gulf of California, Mexico. Hidrobiologica 2012, 22, 8–15. [Google Scholar]
- Wijesinghe, W.A.J.P.; Athukorala, Y.; Jeon, Y.J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar] [CrossRef]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar]
- Namvar, F.; Tahir, P.M.; Mohamad, R.; Mahdavi, M.; Abedi, P.; Najafi, T.F.; Rahman, H.S.; Jawaid, M. Biomedical Properties of Edible Seaweed in Cancer Therapy and Chemoprevention Trials: A Review. Nat. Prod. Commun. 2013, 8, 1811–1820. [Google Scholar] [PubMed]
- Kazlowska, K.; Hsu, T.; Hou, C.C.; Yang, W.C.; Tsai, G.J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentate. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Thanigaivel, S.; Hindu Vidhya, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Differential solvent extraction of two seaweeds and their efficacy in controlling Aeromonas salmonicida infection in Oreochromis mossambicus a novel therapeutic approach. Aquaculture 2015, 433, 56–64. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.L.; Chan, Y.S.; Yin, C.M.; Dan, X.L.; Wang, H.X.; Fang, E.F.; Lam, S.K.; Ngai, P.H.K.; et al. Antifungal and antiviral products of marine organisms. Appl. Microbiol. Biotechnol. 2014, 98, 3475–3494. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antivir. Res. 2010, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Kumari, P.; Reddy, C.R.K. Antimicrobial compounds from seaweed-associated bacteria and fungi. Appl. Microbiol. Biotechnol. 2015, 99, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Valliappan, K.; Sun, W.; Li, Z. Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Appl. Microbiol. Biotechnol. 2014, 98, 7365–7377. [Google Scholar] [CrossRef] [PubMed]
- Vatsos, I.N.; Rebours, C. Seaweed extracts as antimicrobial agents in aquaculture. J. Appl. Phycol. 2015, 27, 2017–2035. [Google Scholar] [CrossRef] [Green Version]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghannam, N.; Rajauria, G. Antimicrobial activity of compounds isolated from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 287–306. [Google Scholar]
- Fernandes, D.R.P.; de Oliveira, V.P.; Valentin, Y.Y. Seaweed biotechnology in Brazil: Six decades of studies on natural products and their antibiotic and other biological activities. J. Appl. Phycol. 2014, 26, 1923–1937. [Google Scholar] [CrossRef]
- Gouveia, V.; Seca, A.M.L.; Barreto, M.C.; Pinto, D.C.G.A. Di- and sesquiterpenoids from Cystoseira genus: Structure, intra-molecular transformations and biological activity. Mini Rev. Med. Chem. 2013, 13, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Osborne, S.; Masci, P.; Gobe, G. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Dominguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I. Chemical structures of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 23–86. [Google Scholar]
- Vera, J.; Castro, J.; González, A.; Moenne, A. Review: Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Kraan, S. Algal polysaccharides, novel applications and outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C.-F., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Cardoso, M.S.; Carvalho, G.L.; Silva, J.P.; Rodrigues, S.M.; Pereira, R.O.; Pereira, L. Bioproducts from seaweeds: A review with special focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Tutor, M.A.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar]
- Alves, A.; Sousa, R.A.; Reis, R.L. A practical perspective on ulvan extracted from green algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Plouguerné, E.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell. Infect. Microbiol. 2014, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Sidana, J. Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 181–204. [Google Scholar]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Kraan, S. Pigments and minor compounds in algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 205–251. [Google Scholar]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Thakur, S.R.; Bansal, P. Algal lectins as promising biomolecules for biomedical research. Crit. Rev. Microbiol. 2015, 41, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.; Dan, X.; Ng, T.B. Marine lectins and their medicinal applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755–3773. [Google Scholar] [CrossRef] [PubMed]
- Güven, K.S.; Percot, A.; Sezik, E. Alkaloids in Marine Algae. Mar. Drugs 2010, 8, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef] [PubMed]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive compounds from seaweeds: Cosmetic applications and future development. Adv. Bot. Res. 2014, 71, 345–379. [Google Scholar]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ely, R.; Supriya, T.; Naik, C.G. Antimicrobial activity of marine organisms collected off the coast of South East India. J. Exp. Mar. Biol. Ecol. 2004, 309, 121–127. [Google Scholar] [CrossRef]
- Pinteus, S.; Alves, C.; Monteiro, H.; Araújo, E.; Horta, A.; Pedrosa, R. Asparagopsis armata and Sphaerococcus coronopifolius as a natural source of antimicrobial compounds. World J. Microbiol. Biotechnol. 2015, 31, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Kolsi, R.B.A.; Frikha, D.; Jribi, I.; Hamza, A.; Feki, L.; Belghith, K. Screening of antibacterial and antifongical activity in marine macroalgae and Magnoliophytea from coast of Tunisia. Int. J. Pharm. Pharm. Sci. 2015, 7, 47–51. [Google Scholar]
- Cavallo, R.A.; Acquaviva, M.I.; Stabili, L.; Cecere, E.; Petrocelli, A.; Narracci, M. Antibacterial activity of marine macroalgae against fish pathogenic Vibrio species. Cent. Eur. J. Biol. 2013, 8, 646–653. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Chaiebb, K.; Bouraoui, A. Evaluation of antimicrobial activity of organic fractions of six marine algae from tunisian Mediterranean coasts. Int. J. Pharm. Pharm. Sci. 2012, 4, 534–537. [Google Scholar]
- Bansemir, A.; Blume, M.; Schröder, S.; Lindequist, U. Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture 2006, 252, 79–84. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Desbiens, M.; Saint-Louis, R.; Zatylny-Gaudin, C.; Thibault, S. Evidence of antibacterial activities in peptide fractions originating from snow crab (Chionoecetes opilio) by-products. Probiotics Antimicrob. Proteins 2010, 2, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Bouhlal, R.; Riadi, H.; Martínez, J.; Bourgougnon, N. The antibacterial potential of the seaweeds (Rhodophyceae) of the Strait of Gibraltar and the Mediterranean coast of Morocco. Afr. J. Biotechnol. 2010, 9, 6365–6372. [Google Scholar]
- Thanigaivel, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture 2014, 433, 467–475. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vijayakumar, S.; Gopinath, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. In vivo and in vitro antimicrobial activity of Azadirachta indica (Lin) against Citrobacter freundii isolated from naturally infected Tilapia (Oreochromis mossambicus). Aquaculture 2015, 437, 252–255. [Google Scholar] [CrossRef]
- Shanmughapriya, S.; Manilal, A.; Sujith, S.; Selvin, J. Seghal Kiran, G.; Natarajaseenivasan, K. Antimicrobial activity of seaweeds extracts against multiresistant pathogens. Ann. Microbiol. 2008, 58, 535–541. [Google Scholar] [CrossRef]
- García-Bueno, N.; Decottignies, P.; Turpin, V.; Dumay, J.; Paillard, C.; Stiger-Pouvreau, V.; Kervarec, N.; Pouchus, Y.F.; Marín-Atucha, A.A.; Fleurence, J. Seasonal antibacterial activity of two red seaweeds, Palmaria palmata and Grateloupia turuturu, on European abalone pathogen Vibrio harveyi. Aquat. Living Resour. 2014, 27, 83–89. [Google Scholar] [CrossRef]
- Cox, S.; Turley, G.H.; Rajauria, G.; Abu-Ghannam, N.; Jaiswal, A.K. Antioxidant potential and antimicrobial efficacy of seaweed (Himanthalia elongata) extract in model food systems. J. Appl. Phycol. 2014, 26, 1823–1831. [Google Scholar] [CrossRef]
- Dubber, D.; Harder, T. Extracts of Ceramium rubrum, Mastocarpus stellatus and Laminaria digitata inhibit growth of marine and fish pathogenic bacteria at ecologically realistic concentrations. Aquaculture 2008, 274, 196–200. [Google Scholar] [CrossRef]
- Boisvert, C.; Beaulieu, L.; Bonnet, C.; Pelletier, E. Assessment of the antioxidant and antibacterial activities of three species of edible seaweeds. J. Food Biochem. 2015, 39, 377–387. [Google Scholar] [CrossRef]
- Bazes, S.; Silkina, A.; Defer, D.; Bernède-Bauduin, C.; Quéméner, E.; Braud, J.P.; Bourgougnon, N. Active substances from Ceramium botryocarpum used as antifouling products in aquaculture. Aquaculture 2006, 258, 664–674. [Google Scholar] [CrossRef]
- Hellio, C.; De La Broise, D.; Dufossé, L.; Le Gal, Y.; Bourgougnon, N. Inhibition of marine bacteria by extract of macroalgae: Potential use for environmentally friendly paints. Mar. Environ. Res. 2001, 52, 231–247. [Google Scholar] [CrossRef]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.D.; Blunden, G.; Tasdemir, D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phytother. Res. 2010, 24, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Bouhlal, R.; Riadi, H.; Bourgougnon, N. Antiviral activity of the extracts of Rhodophyceae from Morocco. Afr. J. Biotechnol. 2010, 9, 7968–7975. [Google Scholar]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Manilal, A.; Selvin, J.; George, S. In vivo therapeutic potentiality of red seaweed, Asparagopsis (Bonnemaisoniales, Rhodophyta) in the treatment of vibriosis in Penaeus monodon, Fabricius. Saudi J. Biol. Sci. 2012, 19, 165–175. [Google Scholar]
- Freile-Pelegrín, Y.; Morales, J.L. Antibacterial activity in marine algae from the coast of Yucatan, Mexico. Bot. Mar. 2004, 47, 140–146. [Google Scholar] [CrossRef]
- Trigui, M.; Gasmi, L.; Zouari, I.; Tounsi, S. Seasonal variation in phenolic composition, antibacterial and antioxidant activities of Ulva rigida (Chlorophyta) and assessment of antiacetylcholinesterase potential. J. Appl. Phycol. 2013, 25, 319–328. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.C.; Narracci, M.; Cecere, E.; Petrocelli, A. Biotechnological potential of the seaweed Cladophora rupestris (Chlorophyta, Cladophorales) lipidic extract. New Biotechnol. 2014, 31, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Reinecke, D.L.; van Staden, J. Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J. Appl. Phycol. 2007, 19, 271–276. [Google Scholar] [CrossRef]
- Choi, J.S.; Ha, Y.M.; Lee, B.B.; Moon, H.E.; Cho, K.K.; Choi, I.S. Seasonal variation of antibacterial activities in the green alga Ulva pertusa Kjellman. J. Environ. Biol. 2014, 35, 341–344. [Google Scholar] [PubMed]
- Krish, S.; Das, A. In-vitro bioactivity of marine seaweed, Cladophora rupestris. Int. J. Pharm. Biol. Sci. 2014, 5, 898–908. [Google Scholar]
- Padmakumar, K.; Ayyakkannu, K. Seasonal variation of antibacterial and antifungal activities of the extracts of marine algae from southern coasts of India. Bot. Mar. 1997, 40, 507–515. [Google Scholar] [CrossRef]
- Tanniou, A.; Vandanjon, L.; Incera, M.; Serrano Leon, E.; Husa, V.; Le Grand, J.; Nicolas, J.L.; Poupart, N.; Kervarec, N.; Engelen, A.; et al. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230. [Google Scholar] [CrossRef]
- El Amraoui, B.; El Amraoui, M.; Cohen, N.; Fassouane, A. Anti-Candida and anti-Cryptococcus antifungal produced by marine microorganisms. J. Mycol. Med. 2014, 24, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Gómez, L.J.; Soria-Mercado, I.E.; Guerra-Rivas, G.; Ayala-Sánchez, N.E. Antibacterial and anticancer activity of seaweeds and bacteria associated with their surface. Rev. Biol. Mar. Oceanogr. 2010, 45, 267–275. [Google Scholar] [CrossRef]
- Horta, A.; Pinteus, S.; Alves, C.; Fino, N.; Silva, J.; Fernandez, S.; Rodrigues, A.; Pedrosa, R. Antioxidant and antimicrobial potential of the Bifurcaria bifurcata epiphytic bacteria. Mar. Drugs 2014, 12, 1676–1689. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Pereira, R.; Sousa Pinto, I.; Carvalho, A.P.; Gomes, A.M. Antimicrobial activity and lipid profile of seaweed extracts from the North Portuguese Coast. Int. Food Res. J. 2013, 20, 3337–3345. [Google Scholar]
- Chambers, L.D.; Hellio, C.; Stokes, K.R.; Dennington, S.P.; Goodes, L.R.; Wood, R.J.K.; Walsh, F.C. Investigation of Chondrus crispus as a potential source of new antifouling agents. Int. Biodeterior. Biodegrad. 2011, 65, 939–946. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. Effect of processing conditions on phytochemical constituents of edible Irish seaweed Himanthalia elongate. J. Food Proc. Preserv. 2012, 36, 348–363. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, D.S.; Kang, Y.M.; Son, K.T.; Jeon, Y.J.; Kim, Y.M. Application of yeast Candida utilis to ferment Eisenia. bicyclis for enhanced antibacterial effect. Appl. Biochem. Biotechnol. 2013, 171, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Rosaline, S.D.; Sakthivelkumar, S.; Rajendran, K.; Janarthanan, S. Screening of selected marine algae from the coastal Tamil Nadu, South India for antibacterial activity. Asian Pac. J. Trop. Biomed. 2012, 2, S140–S146. [Google Scholar] [CrossRef]
- Saritha, K.; Mani, A.E.; Priyalaxmi, M.; Patterson, J. Antibacterial activity and biochemical constituents of seaweed Ulva lactuca. Glob. J. Pharmacol. 2013, 7, 276–282. [Google Scholar]
- Karthikeyan, K.; Shweta, K.; Jayanthi, G.; Prabhu, K.; Thirumaran, G. Antimicrobial and antioxidant potential of selected seaweeds from Kodinar, Southern Coast of Saurashtra, Gujarat, India. J. Appl. Pharm. Sci. 2015, 5, 35–40. [Google Scholar] [CrossRef]
- Jaswir, I.; Tope, A.H.T.; Raus, R.A.; Monsur, H.A.; Ramli, N. Study on anti-bacterial potentials of some Malaysian brown seaweeds. Food Hydrocoll. 2014, 42, 275–279. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abushady, A.M.; Elshobary, M.E. In vitro screening of antimicrobial activity of extracts of some macroalgae collected from Abu-Qir bay Alexandria, Egypt. Afr. J. Biotechnol. 2010, 9, 7203–7208. [Google Scholar]
- Kavita, K.; Singh, V.K.; Jha, B. 24-branched delta 5 sterols from Laurencia papillosa red seaweed with antibacterial activity against human pathogenic bacteria. Microbiol. Res. 2014, 169, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Al Hazzani, A.A.; Shehata, A.I.; Moubayed, N.M.S.; Al Houri, H.J. Antimicrobial and biochemical properties of selected edible brown and red marine macroalgae. J. Pure Appl. Microbiol. 2014, 8, 1275–1282. [Google Scholar]
- Horincar, V.B.; Parfene, G.; Tyagi, A.K.; Gottardi, D.; Dinica, R.; Guerzoni, M.E.; Bahrim, G. Extraction and characterization of volatile compounds and fatty acids from red and green macroalgae from the Romanian Black Sea in order to obtain valuable bioadditives and biopreservatives. J. Appl. Phycol. 2014, 26, 551–559. [Google Scholar] [CrossRef]
- Muñoz-Ochoa, M.; Murillo-Álvarez, J.I.; Zermeño-Cervantes, L.A.; Martínez-Díaz, S.; Rodríguez-Riosmena, R. Screening of extracts of algae from Baja California Sur, Mexico as reversers of the antibiotic resistance of some pathogenic bacteria. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 739–747. [Google Scholar] [PubMed]
- Salta, M.; Wharton, J.A.; Dennington, S.P.; Stoodley, P.; Stokes, K.R. Anti-biofilm performance of three natural products against initial bacterial attachment. Int. J. Mol. Sci. 2013, 14, 21757–21780. [Google Scholar] [CrossRef] [PubMed]
- Manilal, A.; Sujith, S.; Selvin, J.; Kiran, G.S.; Shakir, C.; Lipton, A.P. Antimicrobial potential of marine organisms collected from the southwest coast of India against multiresistant human and shrimp pathogens. Sci. Mar. 2010, 74, 287–296. [Google Scholar]
- Jiang, Z.B.; Chen, Y.C.; Yao, F.; Chen, W.Z.; Zhong, S.P.; Zheng, F.C.; Shi, G.G. Antioxidant, antibacterial and antischistosomal activities of extracts from Grateloupia livida (Harv). Yamada. PLoS. ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.D.D.; Rodrigues, J.A.G.; Holanda, M.L.; Quindere, A.L.G.; de Paula, R.C.M.; Melo, V.M.M.; Benevides, N.M.B. Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilaria ornata. Braz. Arch. Biol. Technol. 2012, 55, 171–181. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracini, M.; Petrocelli, A.; Cecere, E. The lipidic extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): A potential resource for biotechnological purposes? New Biotechnol. 2012, 29, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Taskin, E.; Caki, Z.; Ozturk, M.; Taskin, E. Assessment of in vitro antitumoral and antimicrobial activities of marine algae harvested from the eastern Mediterranean sea. Afr. J. Biotechnol. 2010, 9, 4272–4277. [Google Scholar]
- Kandhasamy, M.; Arunachalam, K.D. Evaluation of in vitro antibacterial property of seaweeds of southeast coast of India. Afr. J. Biotechnol. 2008, 7, 1958–1961. [Google Scholar]
- Bianco, E.M.; de Oliveira, S.Q.; Rigotto, C.; Tonini, M.L.; Guimaraes, T.D.; Bittencourt, F.; Gouvea, L.P.; Aresi, C.; de Almeida, M.T.R.; Moritz, M.I.G.; et al. Anti-Infective potential of marine invertebrates and seaweeds from the Brazilian coast. Molecules 2013, 18, 5761–5778. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.P.; McLoughlin, P.; O’Sullivan, L.; Prieto, M.L.; Gardiner, G.E.; Lawlor, P.G.; Hughes, H. Development of a novel antimicrobial seaweed extract-based hydrogel wound dressing. Int. J. Pharm. 2013, 456, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Alves, C.; Horta, A.; Pinteus, S.; Silva, J.; Culioli, G.; Thomas, O.P.; Pedrosa, R. Antitumor and antimicrobial potential of bromoditerpenes Isolated from the red alga, Sphaerococcus coronopifolius. Mar. Drugs 2015, 13, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, T.; Vairappan, C.S. Nutritional and bioactive properties of three edible species of green algae, genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Pierre, G.; Sopena, V.; Juin, C.; Mastouri, A.; Graber, M.; Maugard, T. Antibacterial activity of a sulfated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2011, 16, 937–945. [Google Scholar] [CrossRef]
- Yuvaraj, N.; Kanmani, P.; Satishkumar, R.; Paari, K.A.; Pattukumar, V.; Arul, V. Extraction, purification and partial characterization of Cladophora glomerata against multidrug resistant human pathogen Acinetobacter baumannii and fish pathogens. World J. Fish Mar. Sci. 2011, 3, 51–57. [Google Scholar]
- Ha, Y.M.; Choi, J.S.; Lee, B.B.; Moon, H.E.; Cho, K.K.; Choi, I.S. Inhibitory effects of seaweed extracts on the growth of the vaginal bacterium Gardnerella vaginalis. J. Environ. Biol. 2014, 35, 537–542. [Google Scholar] [PubMed]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Park, N.H.; Choi, J.S.; Hwang, S.Y.; Kim, Y.C.; Hong, Y.K.; Cho, K.K.; Choi, I.S. Antimicrobial activities of stearidonic and gamma-linolenic acids from the green seaweed Enteromorpha linza against several oral pathogenic bacteria. Bot. Stud. 2013, 54, 39–47. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K.H. Antibacterial mechanism of the action of Enteromorpha linza L. essential oil against Escherichia coli and Salmonella typhimurium. Bot. Stud. 2015, 56, 13–21. [Google Scholar]
- Lee, J.H.; Eom, S.H.; Lee, E.H.; Jung, Y.J.; Kim, H.J.; Jo, M.R.; Son, K.T.; Lee, H.J.; Kim, J.H.; Lee, M.S.; et al. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible Brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Amiguet, V.T.; Jewell, L.E.; Mao, H.; Sharma, M.; Hudson, J.B.; Durst, T.; Allard, M.; Rochefort, G.; Arnason, J.T. Antibacterial properties of a glycolipid-rich extract and active principle from Nunavik collections of the macroalgae Fucus evanescens C. Agardh (Fucaceae). Can. J. Microbiol. 2011, 57, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia. elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K.H. Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef] [PubMed]
- Manilal, J.S.; Thajuddin, N.; Sujith, S.; Panikkar, M.V.N.; Idhayadhulla, A.; Kumar, R.S. Biopotentials of marine alga, Lobophora variegata collected from the shouth indian littoral. Thalassas 2012, 28, 47–54. [Google Scholar]
- Nogueira, L.F.; Morais, E.C.; Brito, M.A.; Santos, B.S.; Vale, D.L.; Lucena, B.F.; Figueredo, F.G.; Guedes, G.M.; Tintino, S.R.; Souza, C.E.; et al. Evaluation of antibacterial, antifungal and modulatory activity of methanol and ethanol extracts of Padina sanctae-crucis. Afr. Health Sci. 2014, 14, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Meillisa, A.; Siahaan, E.A.; Park, J.N.; Woo, H.C.; Chun, B.S. Effect of subcritical water hydrolysate in the brown seaweed Saccharina japonica as a potential antibacterial agent on food-borne pathogens. J. Appl. Phycol. 2013, 25, 763–769. [Google Scholar] [CrossRef]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef] [PubMed]
- Adaikalaraj, G.; Patric, R.D.; Johnson, M.; Janakiraman, N.; Babu, A. Antibacterial potential of selected red seaweeds from Manapad coastal areas, Thoothukudi, Tamil Nadu, India. Asian Pac. J. Trop. Biomed. 2012, 2, 1077–1080. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Mohabati, M.; Eslami, S.; Sohrabipour, J.; Miri, R. Biological activity and chemical constituents of red and brown algae from the Persian Gulf. Iran. J. Pharm. Res. 2013, 12, 339–348. [Google Scholar] [PubMed]
- De Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Varahalarao, V.; Chandrasekhar, N.K. In vitro antimicrobial potentiality of some marine algae against selected phytopathogens. Biomed. Pharmacol. J. 2009, 2, 277–280. [Google Scholar]
- Gupta, S.; Cox, S.; Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N. Growth inhibition of common food spoilage and pathogenic microorganisms in the presence of brown seaweed extracts. Food Bioprocess Technol. 2012, 5, 1907–1916. [Google Scholar] [CrossRef]
- Salem, W.M.; Galal, H.; Nasr El-deen, F. Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). Afr. J. Microbiol. Res. 2011, 5, 2160–2167. [Google Scholar] [CrossRef]
- Shanmugam, J.; Devi Raman, K.; Viswanathan, S.; Nallamuthu, T. Antibacterial and antioxidant activity of red seaweeds from Kilakarai, Rameswaram, Tamilnadu, India. J. Pharm. Biomed. Sci. 2013, 32, 1386–1395. [Google Scholar]
- Patra, J.K.; Kim, S.H.; Baek, K.H. Antioxidant and free radical-scavenging potential of essential oil from Enteromorpha. linza L. prepared by microwave-assisted hydrodistillation. J. Food Biochem. 2015, 39, 80–90. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, J.-A.; Kim, K.-N.; Yoon, W.-J.; Lee, W.J.; Park, S.-Y. Antioxidative and Antimicrobial activities of Sargassum muticum extracts. J. Korean Soc. Food Sci. Nutr. 2007, 36, 663–669. [Google Scholar] [CrossRef]
- De Felício, R.; de Albuquerque, S.; Young, M.C.M.; Yokoya, N.S.; Debonsi, H.M. Trypanocidal, leishmanicidal and antifungal potential from marine red alga Bostrychia tenella J. Agardh (Rhodomelaceae, Ceramiales). J. Pharm. Biomed. Anal. 2010, 52, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Salah, K.B.H.; Ahmed, M.; Mastouri, M.; Bouraoui, A.; Aouni, M. Antibacterial and antifungal activities of brown alga Zonaria tournefortii (JV Lamouroux). Allelopathy J. 2014, 34, 143–153. [Google Scholar]
- Barreto, M.; Meyer, J.J.M. Isolation and antimicrobial activity of a lanosol derivative from Osmundaria serrata (Rhodophyta) and a visual exploration of its biofilm covering. S. Afr. J. Bot. 2006, 72, 521–528. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Suzuki, M.; Ishii, T.; Okino, T.; Abe, T.; Masuda, M. Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry 2008, 69, 2490–2494. [Google Scholar] [CrossRef] [PubMed]
- Ben Redjem, Y.; Ktari, L.; Medhioub, A.; Romdhane, M.S.; Langar, H.; El Bour, M. Antibacterial and algicidal properties of some brown seaweeds from Northern coasts of Tunisia. Life Environ. 2013, 63, 127–133. [Google Scholar]
- Mathan, S.; Subramanian, V.; Nagamony, S.; Ganapathy, K. Isolation of endophytic fungiform marine algae and its bioactivity. Int. J. Res. Pharm. Sci. 2013, 4, 45–49. [Google Scholar]
- Cortés, Y.; Hormazábal, E.; Leal, H.; Urzúa, A.; Mutis, A.; Parra, L.; Quiroz, A. Novel antimicrobial activity of a dichloromethane extract obtained from red seaweed Ceramium rubrum (Hudson) (Rhodophyta: Florideophyceae) against Yersinia ruckeri and Saprolegnia. parasitica, agents that cause diseases in salmonids. Electron. J. Biotechnol. 2014, 17, 126–131. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. Investigation of seaweed extracts as a source of treatment against bacterial fish pathogen. Aquaculture 2015, 448, 82–86. [Google Scholar] [CrossRef]
- Oliveira, S.T.L.; Veneroni-Gouveia, G.; Santos, A.C.; Sousa, S.M.N.; Veiga, M.L.; Krewer, C.C.; Costa, M.M. Ascophyllum nodosum in the diet of tilapia (Oreochromis niloticus) and its effect after inoculation of Aeromonas hydrophila. Pesqui. Vet. Bras. 2014, 34, 403–408. [Google Scholar] [CrossRef]
- Dhanya, K.I.; Swati, V.I.; Vanka, K.S.; Osborne, W.J. Antimicrobial activity of Ulva reticulata and its endophytes. J. Ocean Univ. China, 2016, 15, 363–369. [Google Scholar] [CrossRef]
- Kim, I.H.; Lee, D.G.; Lee, S.H.; Ha, J.M.; Ha, B.J.; Kim, S.K.; Lee, J.H. Antibacterial activity of Ulva lactuca against methicillin-resistant Staphylococcus aureus (MRSA). Biotechnol. Bioprocess Eng. 2007, 12, 579–582. [Google Scholar] [CrossRef]
- Oh, K.; Lee, J.H.; Chung, S.C.; Shin, J.; Shin, H.J.; Kimd, H.K.; Lee, H.S. Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Leitner, S.; Minicante, S.A.; Lass-Florl, C. The Mediterranean red alga Asparagopsis taxiformis has antifungal activity against Aspergillus species. Mycoses 2013, 56, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Mohandass, C.; Vijayaraj, A.S.; Rajasabapathy, R.; Satheeshbabu, S.; Rao, S.V.; Shiva, C.; De-Mello, L. Biosynthesis of silver nanoparticles from marine seaweed Sargassum cinereum and their antibacterial activity. Indian J. Pharm. Sci. 2013, 75, 606–610. [Google Scholar] [PubMed]
- Osman, M.E.H.; Aboshady, A.M.; Elshobary, M.E. Production and characterization of antimicrobial active substance from some macroalgae collected from AbuQir bay (Alexandria) Egypt. Afr. J. Biotechnol. 2013, 12, 6847–6858. [Google Scholar]
- Gerasimenko, N.I.; Martyyas, E.A.; Busarova, N.G. Composition of lipids and biological activity of lipids and photosynthetic pigments from algae of the families Laminariaceae and Alariaceae. Chem. Nat. Compd. 2012, 48, 737–741. [Google Scholar] [CrossRef]
- El Wahidi, M.; El Amraoui, B.; El Amraoui, M.; Bamhaoud, T. Screening of antimicrobial activity of macroalgae extracts from the Moroccan Atlantic coast. Ann. Pharm. Fr. 2015, 73, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Andrade, P.B.; Valentão, P. Screening of a marine algal extract for antifungal activities. Methods Mol. Biol. 2015, 1308, 411–420. [Google Scholar] [PubMed]
- Wei, Y.X.; Liu, Q.; Yu, J.; Feng, Q.; Zhao, L.; Song, H.P.; Wang, W.X. Antibacterial mode of action of 1,8-dihydroxy-anthraquinone from Porphyra haitanensis against Staphylococcus aureus. Nat. Prod. Res. 2015, 29, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.B.; Choi, J.S.; Moon, H.E.; Ha, Y.M.; Kim, M.S.; Cho, K.K.; Choi, I.S. Inhibition of growth and urease of Helicobacter pylori by Korean edible seaweed extracts. Bot. Sci. 2013, 91, 515–522. [Google Scholar] [CrossRef]
- Vedhagiri, K.; Manilal, A.; Valliyammai, T.; Shanmughapriya, S.; Sujith, S.; Selvin, J.; Natarajaseeniva, K. Antimicrobial potential of a marine seaweed Asparagopsis taxiformis against Leptospira javanica isolates of rodent reservoirs. Ann. Microbiol. 2009, 59, 431–437. [Google Scholar] [CrossRef]
- Choi, J.S.; Ha, Y.M.; Joo, C.U.; Cho, K.K.; Kim, S.J.; Choi, I.S. Inhibition of oral pathogens and collagenase activity by seaweed extracts. J. Environ. Biol. 2012, 33, 115–121. [Google Scholar] [PubMed]
- Kim, Y.H.; Kim, J.H.; Jin, H.J.; Lee, S.Y. Antimicrobial activity of ethanol extracts of Laminaria japonica against oral microorganisms. Anaerobe 2013, 21, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.Z.; Buettner, C.; Phillips, R.S.; Davis, R.B.; Mukamal, K.J. n-3 fatty acids and periodontitis in US adults. J. Am. Diet. Assoc. 2010, 110, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jeong, M.R.; Choi, S.M.; Na, S.S.; Cha, J.D. Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch. Oral Biol. 2013, 58, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.M.; Sassaki, G.L.; Romanos, M.T.V.; Barreto-Bergter, E. Structural characterization and anti-HSV-1 and HSV-2 activity of glycolipids from the marine algae Osmundaria obtusiloba isolated from Southeastern Brazilian coast. Mar. Drugs 2012, 10, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Koishi, A.C.; Zanello, P.R.; Bianco, E.M.; Bordignon, J.; dos Santos, C.N.D. Screening of dengue virus antiviral activity of marine seaweeds by an in situ enzyme-linked immunosorbent assay. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, J.L.; Barbosa, J.; Cavalcanti, D.; Pereira, R.C.; Fontes, C.F.L.; Teixeira, V.L.; Souza, T.M.L.; Paixão, I.C.P. The effects of the diterpenes Isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the Herpes Simplex Type-1 Replicative Cycle. Planta Med. 2010, 76, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.; Soares, A.R.; Sigiliano, L.; Machado, F.; Kaiser, C.; Romeiro, N.; Gestinari, L.; Santos, N.; Romanos, M.T.V. In vitro anti-HMPV activity of meroditerpenoids from marine alga Stypopodium zonale (Dictyotales). Molecules 2011, 16, 8437–8450. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Karadeniz, F. Anti-HIV activity of extracts and compounds from marine algae. Adv. Food Nutr. Res. 2011, 64, 255–265. [Google Scholar] [PubMed]
- Morya, V.K.; Kim, J.; Kim, E.-K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microb. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Naesens, L.; Stephens, C.E.; Andrei, G.; Loregian, A.; De Bolle, L.; Snoeck, R.; Sowell, J.W.; De Clercq, E. Antiviral properties of new arylsulfone derivatives with activity against human betaherpesviruses. Antivir. Res. 2006, 72, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 2011, 72, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-González, R.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Ávila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nagorskaia, V.P.; Reunov, A.V.; Lapshina, L.A.; Ermak, I.M.; Barabanova, A.O. Influence of kappa/beta-carrageenan from red alga Tichocarpus crinitus on development of local infection induced by tobacco mosaic virus in Xanthinc tobacco leaves. Izv. Akad. Nauk. Ser. Biol. 2008, 3, 360–364. [Google Scholar] [PubMed]
- De S.f-Tischer, P.C.; Talarico, L.B.; Noseda, M.D.; Guimarães, S.M.; Damonte, E.B.; Duarte, M.E.R. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr. Polym. 2006, 63, 459–465. [Google Scholar] [CrossRef]
- Vijayavel, K.; Martínez, J.A. In vitro antioxidant and antimicrobial activities of two Hawaiian marine Limu: Ulva fasciata (Chlorophyta) and Gracilaria salicornia (Rhodophyta). J. Med. Food. 2010, 13, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Karutha Pandian, S. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food-borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Moroney, N.C.; O’Grady, M.N.; O’Doherty, J.V.; Kerry, J.P. Effect of a brown seaweed (Laminaria digitata) extract containing laminarin and fucoidan on the quality and shelf-life of fresh and cooked minced pork patties. Meat Sci. 2013, 94, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Siahaan, E.A.; Pendleton, P.; Woo, H.C.; Chun, B.S. Brown seaweed (Saccharina japonica) as an edible natural delivery matrix for allyl isothiocyanate inhibiting food-borne bacteria. Food Chem. 2014, 152, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Dorta, F.; Medina, C.; Ramírez, A.; Ramírez, I.; Peña-Cortés, H. Anti-phytopathogenic activities of macro-algae extracts. Mar. Drugs 2011, 9, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.; Collier, P.J. The impact and mode of action of phenolic compounds extracted from brown seaweed on mixed anaerobic microbial cultures. J. Appl. Microbiol. 2013, 114, 964–973. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Review prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef] [PubMed]



| Red Seaweed | Solvent | Organisms | Ref. |
|---|---|---|---|
| Alsidium corallinum | M | Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus | [49] |
| Ceramium rubrum | M | E. coli, Enterococcus faecalis, S. aureus | [49] |
| Ceramium virgatum | H/1:1 EE:Hp | Salmonella enteritidis, E.coli, Listeria monocytogenes, Bacillus cereus | [85] |
| Chondrocanthus acicularis | M | E. coli, K. pneumoniae, E. faecalis, S. aureus | [49] |
| Chondracanthus canaliculatus | E | S. aureus, Streptococcus pyogenes | [86] |
| Chondrus crispus | M | L. monocytogenes, Salmonella abony, E. faecalis, P. aeruginosa | [6] |
| C. crispus | 95% E | Pseudoalteromonas elyakovii, Vibrio aestuarianus, Polaribacter irgensii, Halomonas marina, Shewanella putrefaciens | [75] |
| C. crispus | EA | S. enteridis, E. coli, P. aeruginosa, L. innocua, B. cereus, S. aureus, L. brevis. E. faecalis, Candida sp. | [74] |
| DE | E. coli, P. aeruginosa, L. innocua, B. cereus, S. aureus, L. brevis, E. faecalis, Candida sp. | ||
| 1:1 M:W | E. coli, P. aeruginosa, L. innocua, B. cereus, S. aureus, L. brevis. E. faecalis, Candida sp. | ||
| C. crispus | 95% E/T | Cobetia marina, Marinobacter hydrocarbonoclasticus | [87] |
| Corallina elongata | E, A, M | B. subtilis, S. aureus, E. coli, Salmonella typhi, K. pneumoniae, Candida albicans | [82] |
| Corallina vancouverensis | E | S. aureus, S. pyogenes | [86] |
| Falkenbergia-phase of A. taxiformis | M | B. subtilis, K. pneumonia, P. aeruginosa, S aureus, S. epidermidis, Vibrio harveyi, V. alginolyticus, V. vulnificus, V. parahaemolyticus, V. alcaligenes | [88] |
| Ganonema farinosum | E | S. aureus, S. pyogenes | [86] |
| Gelidium attenatum | M | E. coli, K. pneumoniae, E. faecalis, S. aureus | [49] |
| Gelidium micropterum | M | V. parahaemolyticus, V. alcaligenes | [88] |
| Gelidium pulchellum | M | E. coli, E. faecalis, S. aureus | [49] |
| Gelidium pusillum | M | E. coli, K. pneumonia, E. faecalis, S. aureus | [49] |
| M | V. harveyi, V. alginolyticus, V. vulnificus, V. parahaemolyticus, V. alcaligenes | [88] | |
| Gelidium robustum | E | S. aureus, S. pyogenes | [86] |
| Gelidium spinulosum | M | E. coli, E. faecalis, S. aureus | [49] |
| Gracilaria dura | 2:1 C:M | V. ordalii, V. alginolyticus | [45] |
| Gracilaria gracilis | 2:1 C:M | V. salmonicida | [45] |
| Grateloupia livida | E/PE | S. aureus, E. coli, P. aeruginosa | [89] |
| Gracilaria ornata | W | E. coli | [90] |
| Gracilaria subsecundata | E | S. aureus, S. pyogenes | [86] |
| Gracilaria vermiculophylla | EA | S. enteridis, E. coli, P. aeruginosa, L. innocua, B. cereus, S. aureus, L. brevis. E. faecalis | [74] |
| DE | S. enteridis, E. coli, P. aeruginosa, L. innocua, B. cereus, S. aureus, L. brevis. E. faecalis, Candida sp. | ||
| 1:1 M:W | B. cereus, S. aureus | ||
| Gracilariopsis longissima | 2:1 C:M | V. alginolyticus, V. vulnificus, V. ordalii, V. salmonicida | [45] |
| M/2:1 C:M | V. alginolyticus, V. fluvialis, V. vulnificus, V. cholerae non O-1 | [91] | |
| Grateloupia filicina | M | V. harveyi, V. alginolyticus, V. vulnificus, V. parahaemolyticus, V. alcaligenes | [88] |
| G. filicina | M | S.aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| Halopitys incurvus | M | E. coli, K. pneumoniae, E. faecalis, S. aureus | [49] |
| Hypnea musciformis | M | C. albicans | [92] |
| H. musciformis | M | K. pneumonia, E. faecalis, S. aureus | [93] |
| M | E. coli, K. pneumoniae, E. faecalis, S. aureus | [49] | |
| Hypnea pannosa | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| Hypnea valentiae | E | S. aureus, S. pyogenes | [86] |
| M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] | |
| Janiarubens | E, A, M | B. subtilis, S. aureus, E. coli, S. typhi, K. pneumoniae, C. albicans | [82] |
| Laurencia dendroidea | A | S. aureus, E. faecalis | [94] |
| Laurencia jonhstonii | E | S. aureus, S. pyogenes | [86] |
| Laurencia pacifica | E | S. aureus, S. pyogenes | [86] |
| Laurencia papillosa | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| M/SC | E. coli, P. aeruginosa, K. pneumoniae, Shigella flexineri | ||
| Laurencia sp. | M | P. aeruginosa, S.epidermidis, V. harveyi, V. alginolyticus, V. vulnificus, V. parahaemolyticus | [88] |
| Neorhodomela larix | E | S. aureus, S. pyogenes | [86] |
| Ochtodes secundiramea | A | S. aureus | [94] |
| Osmundaria obtusiloba | 2:1 DCM:M | P. aeruginosa | [94] |
| Plocamium cartilagineum | M | E. coli, E. faecalis, S. aureus | [49] |
| Polysiphonia lanosa | W | MRSA,S. aureus, E. cloacae, Clostridium perfringens | [95] |
| Polysphonia tuticorinensis | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| Porphyra dioica | EA | E. coli, Bacillus cereus, Lactobacillus brevis. E. faecalis, Candida sp. | [74] |
| DE | E. coli, B. cereus, L. brevis. E. faecalis, Candida sp. | ||
| 1:1 M:W | S. aureus, E. faecalis | ||
| Portieria horemanii | M | V. harveyi, V. alginolyticus, V. vulnificus, | [88] |
| Pterocladia capillacea | E, A, M | B. subtilis, S. aureus, E. coli, S. typhi, K. pneumoniae, C. albicans | [82] |
| Pterosiphonia complanata | M | E. coli, E. faecalis, S. aureus | [49] |
| Rhodymenia californica | E | S. aureus, S. pyogenes | [86] |
| Sphaerococcus coronopifolius | M | S. aureus | [96] |
| DCM/SC | S. aureus, E. coli, P. aeruginosa, C. albicans | ||
| Spyridia filamentosa | M | S. aureus, E. coli, E. faecalis | [92] |
| Green Seaweed | Extract | Organisms | Ref. |
|---|---|---|---|
| Boodlea composita | M | V. harveyi, V. alginolyticus, V. vulnificus, V. parahaemolyticus, V. alcaligenes | [88] |
| Bryopsis pennata | M | V. vulnificus, V. parahaemolyticus | [88] |
| Caulerpa lentillifera | M/EA | E. coli, Staphylococcus aureus, Streptococcus sp., Salmonella sp. | [97] |
| Caulerpa parvula | M | V. vulnificus, V. alcaligenes | [88] |
| Caulerpa racemosa | M; M/DE; M/W | E. coli, S. aureus, Streptococcus sp., Salmonella sp. | [97] |
| Chaetomorpha aerea | W | Bacilus subtilis, Micrococcus luteus, S. aureus | [98] |
| Chaetomorpha linum | 2:1 C:M | V. ordalii, V. vulnificus | [45] |
| Cladophora albida | M | V. harveyi, V. alginolyticus, V. vulnificus, V. parahaemolyticus, V. alcaligenes | [88] |
| Cladophora glomerata | M | V. fischeri, V. vulnificus, V. anguillarum, V. parahaemolyticus | [99] |
| Cladophora rupestris | M, EA, E 2:1 C:M 2:1 C:M | E. coli, Pseudomonas aeruginosa, S. aureus, V. harveyii, V. parahaemolyticus, V. alginolyticus Enterococcus sp., Streptococcus agalactiae, V. fluvialis; V. salmonicida; V. vulnificus, V. ordalii; V. cholera non-O1; V. metschnikovii V. ordalii, V. salmonicida, V. vulnificus | [68] [65] [45] |
| Cladophora sp. | E | S. aureus, Streptococcus pyogenes | [86] |
| Codium amplivesiculatum | E | S. aureus, S. pyogenes | [86] |
| Codium cuneatum | E | S. aureus, S. pyogenes | [86] |
| Codium simulans | E | S. aureus, S. pyogenes | [86] |
| Enteropmorpha compressa | M | K. pneumoniae, V. harveyi, V. alginolyticus, V. vulnificus, V. parahaemolyticus, V. alcaligenes | [88] |
| E. compressa | E | Gardnerella vaginalis | [100] |
| E. compressa | E, A, M | B. subtilis, S. aureus, E. coli, S. typhi, K. pneumoniae, C. albicans | [82] |
| Enteromorpha intestinalis | A | Bacillus mycoides, B. subtilis, E. coli. K. pneumonia, S. aureus, A. flavus, A. fumigatus. C. albicans, P. purpurescens, P. verrucosum | [101] |
| Enteromorpha linza | E, A, M | B. subtilis, S. aureus, E. coli, S. typhi, K. pneumoniae, C. albicans | [82] |
| E. linza | 4:1 M:W E W/DCM | Prevotella intermedia, Porphyromonas gingivalis G. vaginalis E. coli, Salmonella. typhimurium | [102] [100] [103] |
| Laurencia johnstonii | 1:1 A:M–Er | S. aureus | [3] |
| Ulva dactilifera | E | S. aureus, S. pyogenes | [86] |
| Ulva fasciata | E, A, M | B. subtilis, S. aureus, E. coli, S. typhi, K. pneumoniae, C. albicans | [82] |
| Ulva lactuca | E, A, M | B. subtilis, S. aureus, E. coli, S. typhi, K. pneumoniae, C. albicans | [82] |
| U. lactuca | 1:1 A:M–Er | S. aureus | [3] |
| U. lactuca | A | B. mycoides, B. subtilis, E. coli. K. pneumoniae, S. aureus, A. flavus, A. fumigatus. C. albicans, P. purpurescens, P. verrucosum | [101] |
| Ulva pertusa | E | G. vaginalis | [100] |
| U. pertusa | M | G. vaginalis | [67] |
| Ulva prolifera | 2:1 C:M | V. ordali | [45] |
| Brown Seaweed | Solvent | Organisms | Ref. |
|---|---|---|---|
| Chnoospora implexa | E | S. aureus, S. pyogenes | [86] |
| Cladophora rupestris | M | E. coli, S. aureus, P. aeruginosa, V. harveyii, V. parahaemolyticus, V. alginolyticus | [68] |
| C. rupestris | E | E. coli, S. aureus, P. aeruginosa, V. harveyii, V. parahaemolyticus, V. alginolyticus | [68] |
| C. rupestris | EA | E. coli, S. aureus, P. aeruginosa, V. harveyii, V. parahaemolyticus | [68] |
| Colpomenia sinuosa | E E, A, M | S. aureus, S. pyogenes B. subtilis, S. aureus, E. coli, S. typhi, K. pneumoniae, C. albicans | [86] [82] |
| Colpomenia tuberculata | E | S. aureus, Sreptococcus pyogenes | [86] |
| Cystoseira osmundacea | E | S. pyogenes | [86] |
| Cystoseira trinodis | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| Dictyopteris delicatula | E | S. aureus, S. pyogenes | [86] |
| Dictyopteris undulata | E | S. aureus, S. pyogenes | [86] |
| Dictyota dichotoma | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| Dictyota flabellata | E | S. aureus, S. pyogenes | [86] |
| Dictyota indica | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| Dictyota sp. | 2:1 DCM:M | S. aureus, Enterococcus faecalis, P. aeruginosa | [94] |
| Eisenia bicyclis | M; M/H; M/DCM; M/B | S. aureus, S. epidermidis, Propionibacterium acnes | [104] |
| E. bicyclis | M/EA | S. aureus, S. epidermidis, P. acnes, P. aeruginosa | [104] |
| Fucus evanescens | EA | Hemophilus influenzae, Legionella pneumophila, Propionibacterium acnes S. pyogenes, Clostridium difficile, methicillin-resistant S. aureus | [105] |
| Himanthalia elongata | H, E, W (PLE) | Aspergillus niger, C. albicans, E. coli, S. aureus | [106] |
| H. elongata | W, M and mixtures | L. monocytogenes, E. faecalis, P. aeruginosa, S. abony | [107] |
| H. elongata | C,DE,H/ 60% M:W/1:1 W:EA | L. monocytogenes, S. abony | [54] |
| Hydroclathrus clathratus | E | S. aureus, S. pyogenes | [86] |
| Laminaria japonica | W | E. coli, S. aureus, B. cereus | [108] |
| Lobophora variegata | M | V. alginolyticus, V. vulnificus, V. parahaemolyticus, B. subtilis B.cereus, Micrococcus luteus, S. typhimurium, Aeromonas hydrophila, E. coli | [88] [109] |
| Padina concrescens | M E | V. alginolyticus, V. vulnificus, V. parahaemolyticus, B. subtilis S. aureus, S. pyogenes | [88] [86] |
| Padina mexicana | E | S. aureus, S. pyogenes | [86] |
| Padina pavonica | M | E. coli | [92] |
| Padina sanctae crucis | M, E | E. coli, S. aureus, P. aeruginosa, Candida tropicalis, C. kruzei | [110] |
| Padina tetrastomatica | M | V. alginolyticus, V. vulnificus, V. parahaemolyticus, B. subtilis | [88] |
| Rosenvingea intrincata | E | S. aureus, S. pyogenes | [86] |
| Saccharina japonica | scW + AcH | E. coli, S. typhimurium, S. aureus, B. cereus | [111] |
| S. japonica | H, E | E. coli, L. monocytogenes, S. aureus | [112] |
| S. japonica | SC-CO2 + E, 1:1 A:E | E. coli, L. monocytogenes, B. cereus, S. aureus, C. albicans, A. brasiliensis | [112] |
| Sargassum binderi | M, A | S. aureus, B. subtilis | [81] |
| S. binderi | EA | B. subtilis | [81] |
| Sargassum flavellum | M, A, EA | B. subtilis | [81] |
| Sargassum horneri | SC-CO2 + E, 1:1 A:E | E. coli, L. monocytogenes, B. cereus, S. aureus, C. albicans, A. brasiliensis | [112] |
| S. horneri | H, E | E. coli, L. monocytogenes, S. aureus | [112] |
| Sargassum horridum | E | S. aureus, S. pyogenes | [86] |
| Sargassum myriocystum | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| Sargassum plagyophillum | M | B.subtilis, S. aureus | [81] |
| Sargassum vulgare | E, A, M | B. subtilis, S. aureus, E. coli, S. typhi, K. pneumoniae, C. albicans | [82] |
| Scytosiphon lomentaria | M | S. aureus, S. typhimurium, E. coli | [92] |
| Stoe-chospermum marginatum | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
| Turbinaria ornata | M | S. aureus, B. subtilis, E. coli, P. aeruginosa | [83] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. https://doi.org/10.3390/md14030052
Pérez MJ, Falqué E, Domínguez H. Antimicrobial Action of Compounds from Marine Seaweed. Marine Drugs. 2016; 14(3):52. https://doi.org/10.3390/md14030052
Chicago/Turabian StylePérez, María José, Elena Falqué, and Herminia Domínguez. 2016. "Antimicrobial Action of Compounds from Marine Seaweed" Marine Drugs 14, no. 3: 52. https://doi.org/10.3390/md14030052
APA StylePérez, M. J., Falqué, E., & Domínguez, H. (2016). Antimicrobial Action of Compounds from Marine Seaweed. Marine Drugs, 14(3), 52. https://doi.org/10.3390/md14030052