Anti-Oxidative Activity of Mytiloxanthin, a Metabolite of Fucoxanthin in Shellfish and Tunicates
Abstract
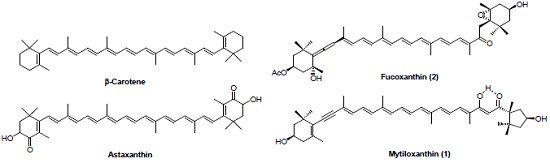
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Reagents
3.2. ESR Spin-Trapping Analysis
3.3. Inhibition of Lipid Peroxidation
3.4. Synthesis of Mytiloxanthin
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Liaaen-Jensen, S. Carotenoids in Food Chain. In Carotenoids Volume 3: Biosynthesis and Metabolism; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1998; pp. 359–371. [Google Scholar]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.B.T. Some features of the metabolism of the carotenoid pigments in the California sea mussel (Mytilis californicus). J. Biol. Chem. 1940, 136, 275–299. [Google Scholar]
- Khare, A.; Moss, G.P.; Weedon, B.C.L. Mytiloxanthin and isomytiloxanthin, two novel acetylenic carotenoids. Tetrahedron Lett. 1973, 3921–3924. [Google Scholar] [CrossRef]
- Chopra, A.K.; Khare, A.; Moss, G.P.; Weedon, B.C.L. Carotenoids and related compounds. Part 41. Structure of mytiloxanthin and synthesis of a cis isomer. J. Chem. Soc. Perkin Trans. 1 1988, 1383–1388. [Google Scholar] [CrossRef]
- Maoka, T.; Fujiwara, Y. Absolute configuration of mytiloxanthin and 9-E-mytiloxanthin. J. Jpn. Oil Chem. Soc. 1996, 45, 667–670. [Google Scholar] [CrossRef]
- Tode, C.; Yamano, Y.; Ito, M. Carotenoids and related polyenes, Part 8. Total synthesis of optically active mytiloxanthin applying the stereoselective rearrangements of tetrasubstituted epoxide. J. Chem. Soc. Perkin Trans. 1 2002, 1581–1587. [Google Scholar] [CrossRef]
- Yamano, Y.; Ohata, M.; Wada, A. Stereocontrolled total synthesis of mytiloxanthin. Carotenoid Sci. 2015, 20, 35–39. [Google Scholar]
- Partali, V.; Tangen, Y.K.; Liaaen-Jensen, S. Carotenoids in food chain studies-III. Reapportion and metabolic transformation of carotenoids in Mytilus. edulis (edible mussel). Comp. Biochem. Physiol. 1989, 92B, 239–246. [Google Scholar]
- Matsuno, T. New structures of carotenoids in marine animals. Pure Apple. Chem. 1985, 57, 659–666. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organism. Fish. Sci. 1996, 62, 134–137. [Google Scholar]
- Hirayama, O.; Nakamura, K.; Hamada, S.; Kobayashi, K. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 1994, 29, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Trevithick-Sutton, C.C.; Foote, C.S.; Collinus, M.; Trevithick, J.R. The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxy radicals: A. chemiluminesence and ESR study. Mol. Vis. 2006, 12, 1127–1135. [Google Scholar] [PubMed]
- Hama, S.; Takahashi, K.; Inai, Y.; Shirota, K.; Sakamato, R.; Yamada, A.; Tsuchidya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective effects of topical application of apoorly soluble antioxidant astaxanthin liposomat formation on ultraviolet-induced skin damage. J. Pharm Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Kuwahara, T.; Narita, M. Carotenoids of sea angels Clione limacina and Paedoclione doliiformis, from the perspective of food chain. Mar. Drugs 2014, 12, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Yamano, Y.; Tode, C.; Ito, M. Carotenoids and related polyenes, Part 5. Lewis acid-promoted stereoselective rearrangement of 5,6-epoxy carotenoid model compounds. J. Chem. Soc. Perkin Trans. 1 1998, 2569–2581. [Google Scholar] [CrossRef]
- Furuichi, N.; Hara, H.; Osaki, T.; Nakano, M.; Mori, H.; Katsumura, S. Stereocontrolled total synthesis of a polyfunctional carotenoid, peridinin. J. Org. Chem. 2004, 69, 7949–7959. [Google Scholar] [CrossRef] [PubMed]
- Yamano, Y.; Chary, V.M.; Wada, A. Stereoselective total synthesis of the acetylenic carotenoids alloxanthin and triophaxanthin. Org. Biomol. Chem. 2012, 10, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Samples Availability: Available from the authors.




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maoka, T.; Nishino, A.; Yasui, H.; Yamano, Y.; Wada, A. Anti-Oxidative Activity of Mytiloxanthin, a Metabolite of Fucoxanthin in Shellfish and Tunicates. Mar. Drugs 2016, 14, 93. https://doi.org/10.3390/md14050093
Maoka T, Nishino A, Yasui H, Yamano Y, Wada A. Anti-Oxidative Activity of Mytiloxanthin, a Metabolite of Fucoxanthin in Shellfish and Tunicates. Marine Drugs. 2016; 14(5):93. https://doi.org/10.3390/md14050093
Chicago/Turabian StyleMaoka, Takashi, Azusa Nishino, Hiroyuki Yasui, Yumiko Yamano, and Akimori Wada. 2016. "Anti-Oxidative Activity of Mytiloxanthin, a Metabolite of Fucoxanthin in Shellfish and Tunicates" Marine Drugs 14, no. 5: 93. https://doi.org/10.3390/md14050093
APA StyleMaoka, T., Nishino, A., Yasui, H., Yamano, Y., & Wada, A. (2016). Anti-Oxidative Activity of Mytiloxanthin, a Metabolite of Fucoxanthin in Shellfish and Tunicates. Marine Drugs, 14(5), 93. https://doi.org/10.3390/md14050093