Effect of Chitosan Properties on Immunoreactivity
Abstract
:1. Introduction
2. Results
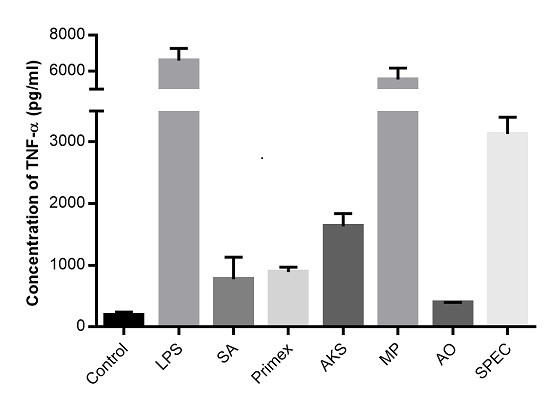
2.1. Chitosan Induced Cytokine Production
2.2. Characterization of Commercially Available Chitosan
2.3. Effect of DDA on Cytokine Release
2.4. Effect of Viscosity on Cytokine Release
2.5. Effect of Endotoxin Contamination on Cytokine Release
2.6. Effect of Endotoxin Contamination on Mouse Dendritic Cells
2.7. Effect of Endotoxin Contamination on Human Macrophage Cell Line
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Laboratory Animals
4.3. Cell Culture
4.4. Determination of Relative Viscosity, DDA and Endotoxin Content
4.5. Measurement of Immune Responses
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Al Rubeaan, K.; Rafiullah, M.; Jayavanth, S. Oral insulin delivery systems using chitosan-based formulation: A review. Expert Opin. Drug Deliv. 2016, 13, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Liu, C.; Wang, Y.; Yan, J.; Wan, H.; Fan, B. Characterization and biocompatibility of injectable microspheres-loaded hydrogel for methotrexate delivery. Carbohydr. Polym. 2016, 136, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. pH-sensitive freeze-dried chitosan—Polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef]
- Ruel-Gariepy, E.; Leclair, G.; Hildgen, P.; Gupta, A.; Leroux, J. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J. Control. Release 2002, 82, 373–383. [Google Scholar] [CrossRef]
- Vo, J.L.; Yang, L.; Kurtz, S.L.; Smith, S.G.; Koppolu, B.P.; Ravindranathan, S.; Zaharoff, D.A. Neoadjuvant immunotherapy with chitosan and interleukin-12 to control breast cancer metastasis. Oncolmmunology 2014, 3, e968001. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, B.; Zaharoff, D.A. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials 2013, 34, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.; Nagarwal, R.C.; Pandit, J.K. Lomustine loaded chitosan nanoparticles: Characterization and in vitro cytotoxicity on human lung cancer cell line L132. Chem. Pharm. Bull. 2011, 59, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, B.; Smith, S.G.; Ravindranathan, S.; Jayanthi, S.; Kumar, T.K.S.; Zaharoff, D.A. Controlling chitosan-based encapsulation for protein and vaccine delivery. Biomaterials 2014, 35, 4382–4389. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Dai, Y.; Lv, L.; Zhao, H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE 2014, 9, e84703. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.; O’Brien, F.J.; Cryan, S. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 2013, 18, 5611–5647. [Google Scholar] [CrossRef] [PubMed]
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int. J. Pharm. 2012, 427, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Tanaka, M.; Huang, Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Kweon, D.; Song, S.; Park, Y. Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomaterials 2003, 24, 1595–1601. [Google Scholar] [CrossRef]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly curable chitosan—PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.; Dias, A.M.; Leal, E.C.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Chitosan-based dressings loaded with neurotensin—An efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014, 10, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, X.; Ji, Y.; Ren, K.; Ji, J. Fast and long-acting antibacterial properties of chitosan-Ag/polyvinylpyrrolidone nanocomposite films. Carbohydr. Polym. 2012, 90, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Behera, A.K.; Lockey, R.F.; Zhang, J.; Bhullar, G.; de la Cruz, C.P.; Chen, L.C.; Leong, K.W.; Huang, S.K.; Mohapatra, S.S. Intranasal gene transfer by chitosan-DNA nanospheres protects BALB/c mice against acute respiratory syncytial virus infection. Hum. Gene Ther. 2002, 13, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Koppolu, B.; Ravindranathan, S.; Kurtz, S.L.; Yang, L.; Katz, M.D.; Zaharoff, D.A. Intravesical chitosan/interleukin-12 immunotherapy induces tumor-specific systemic immunity against murine bladder cancer. Cancer Immunol. Immunother. 2015, 64, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Ven der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001, 14, 201–207. [Google Scholar] [CrossRef]
- Yao, W.; Peng, Y.; Du, M.; Luo, J.; Zong, L. Preventative vaccine-loaded mannosylated chitosan nanoparticles intended for nasal mucosal delivery enhance immune responses and potent tumor immunity. Mol. Pharm. 2013, 10, 2904–2914. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Chondrogenic differentiation of rat MSCs on porous scaffolds of silk fibroin/chitosan blends. Biomaterials 2012, 33, 2848–2857. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Florczyk, S.J.; Leung, M.; Zhang, M. High-strength pristine porous chitosan scaffolds for tissue engineering. J. Mater. Chem. 2012, 22, 6291–6299. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Sarkar, K.; Bhattacharya, S.; Bhattacharyya, A.; Mishra, R.; Kundu, P. pH sensitive N-succinyl chitosan grafted polyacrylamide hydrogel for oral insulin delivery. Carbohydr. Polym. 2014, 112, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Nettles, D.L.; Elder, S.H.; Gilbert, J.A. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002, 8, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. In Chitosan for Biomaterials II; Springer: Berlin Heidelberg, Germany, 2011; pp. 19–44. [Google Scholar]
- Vande Vord, P.J.; Matthew, H.W.T.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.K.; Doviner, V.; Orkin, B.; Kleinstern, J.; Srebnik, M.; Nissan, A.; Rubinstein, A. Biocompatibility evaluation of crosslinked chitosan hydrogels after subcutaneous and intraperitoneal implantation in the rat. J. Biomed. Mater. Res. A 2007, 83, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosic, L.; Calabro, D.; Avallone, B.; Balsamo, G. Chitosan mediated stimulation of macrophage function. Biomaterials 1994, 15, 1215–1220. [Google Scholar] [CrossRef]
- Suzuki, K.; Okawa, Y.; Hashimoto, K.; Suzuki, S.; Suzuki, M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol. Immunol. 1984, 28, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Ishihara, C.; Ukei, S.; Tokura, S.; Azuma, I. Stimulation of cytokine production in mice using deacetylated chitin. Vaccine 1986, 4, 151–156. [Google Scholar] [CrossRef]
- Nishimura, K.; Nishimura, S.; Seo, H.; Nishi, N.; Tokura, S.; Azuma, I. Effect of multiporous microspheres derived from chitin and partially deacetylated chitin on the activation of mouse peritoneal macrophages. Vaccine 1987, 5, 136–140. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, L.; Yu, Q. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.; Liu, C.; Wang, J. The effect of water-soluble chitosan on macrophage activation and the attenuation of mite allergen-induced airway inflammation. Biomaterials 2008, 29, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Villiers, C.; Chevallet, M.; Diemer, H.; Couderc, R.; Freitas, H.; Van Dorsselaer, A.; Marche, P.N.; Rabilloud, T. From secretome analysis to immunology: Chitosan induces major alterations in the activation of dendritic cells via a TLR4-dependent mechanism. Mol. Cell Proteom. 2009, 8, 1252–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Y.; Li, S.; Wang, W.; Wang, S.; Zou, M.; Guo, Y.; Fan, J.; Du, Y.; Zhang, J. The effects of chitosan oligosaccharide on the activation of murine spleen CD11c dendritic cells via Toll-like receptor 4. Carbohydr. Polym. 2011, 83, 1075–1081. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, L.; Yu, Z.; Feng, J.; Yu, Q. Role of mannose receptor in oligochitosan-mediated stimulation of macrophage function. Int. Immunopharmacol. 2005, 5, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Bueter, C.L.; Lee, C.K.; Rathinam, V.A.; Healy, G.J.; Taron, C.H.; Specht, C.A.; Levitz, S.M. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J. Biol. Chem. 2011, 286, 35447–35455. [Google Scholar] [CrossRef] [PubMed]
- Lieder, R.; Gaware, V.S.; Thormodsson, F.; Einarsson, J.M.; Ng, C.H.; Gislason, J.; Masson, M.; Petersen, P.H.; Sigurjonsson, O.E. Endotoxins affect bioactivity of chitosan derivatives in cultures of bone marrow-derived human mesenchymal stem cells. Acta Biomater. 2013, 9, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, Y.M. Chitosan-based vaccine adjuvants: Incomplete characterization complicates preclinical and clinical evaluation. Expert Rev. Vaccines 2014, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.C.; Ulevitch, R.J. The effects of bacterial endotoxins on host mediation systems. A review. Am. J. Pathol. 1978, 93, 526–618. [Google Scholar] [PubMed]
- Barbosa, J.N.; Amaral, I.F.; Aguas, A.P.; Barbosa, M.A. Evaluation of the effect of the degree of acetylation on the inflammatory response to 3D porous chitosan scaffolds. J. Biomed. Mater. Res. A 2010, 93, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Schwende, H.; Fitzke, E.; Ambs, P.; Dieter, P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 1996, 59, 555–561. [Google Scholar] [PubMed]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef] [PubMed]






| Chitosan | Molecular Weight Provided by Manufacturer (kDa) | Amount of Endotoxin (EU/mg) | DDA (%) | Viscosity (cP) |
|---|---|---|---|---|
| Sigma-Aldrich | 50–190 | 1.48 ± 0.05 | 74 ± 0.1 | 265 ± 5 |
| Primex | Not provided | 0.22 ± 0.06 | 80 ± 0.3 | 95 ± 25 |
| AK Scientific | Not provided | 1.44 ± 0.03 | 96 ± 0.4 | 65 ± 20 |
| MP Biomedicals | Not provided | 2.27 ± 0.03 | 88 ± 0.9 | 19 ± 6 |
| Acros Organics | 100–300 | 1.09 ± 0.05 | 98 ± 0.4 | 58 ± 16 |
| Spectrum chemicals | Not provided | 3.45 ± 0.04 | 92 ± 0.4 | 13 ± 5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravindranathan, S.; Koppolu, B.P.; Smith, S.G.; Zaharoff, D.A. Effect of Chitosan Properties on Immunoreactivity. Mar. Drugs 2016, 14, 91. https://doi.org/10.3390/md14050091
Ravindranathan S, Koppolu BP, Smith SG, Zaharoff DA. Effect of Chitosan Properties on Immunoreactivity. Marine Drugs. 2016; 14(5):91. https://doi.org/10.3390/md14050091
Chicago/Turabian StyleRavindranathan, Sruthi, Bhanu Prasanth Koppolu, Sean G. Smith, and David A. Zaharoff. 2016. "Effect of Chitosan Properties on Immunoreactivity" Marine Drugs 14, no. 5: 91. https://doi.org/10.3390/md14050091
APA StyleRavindranathan, S., Koppolu, B. P., Smith, S. G., & Zaharoff, D. A. (2016). Effect of Chitosan Properties on Immunoreactivity. Marine Drugs, 14(5), 91. https://doi.org/10.3390/md14050091