Resonance Rayleigh Scattering Spectra of an Ion-Association Complex of Naphthol Green B–Chitosan System and Its Application in the Highly Sensitive Determination of Chitosan
Abstract
:1. Introduction
2. Results and Discussion
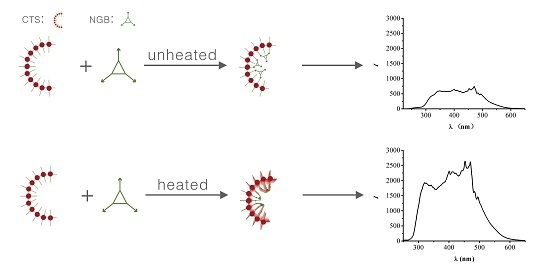
2.1. Mechanism
2.2. UV-VISAbsorption Spectra
2.3. RRS Spectra
2.4. Optimum Experimental Conditions
2.4.1. Effects of Buffer Solution
2.4.2. Effects of pH and Amount of B-R Buffer Solution
2.4.3. Effect of Concentration of Naphthol Green B
2.4.4. Effect of Reaction Temperature
2.4.5. Effects of Reaction Time and Stability
2.4.6. Effect of Addition Sequence
2.4.7. Effect of Ionic Strength
2.5. Analytical Application
2.5.1. Calibration Curves
2.5.2. Effect of the Molecular Weight of CTS
2.5.3. Effect of the Degree of Deacetylation
2.5.4. Effect of Foreign Substances
2.5.5. Precision and Recovery
3. Experimental
3.1. Materials and Reagents
3.2. Procedures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kadib, A.E. Chitosan as a Sustainable Organocatalyst: A Concise Overview. ChemSusChem 2015, 8, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Paños, I.; Acosta, N.; Heras, A. New drug delivery systems based on chitosan. Curr. Drug Discov. Technol. 2008, 5, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Q.; Wu, S.H.; Zhang, H.L.; Feng, Y.F. Development and validation of an improved Bradford method for determination of insulin from chitosan nanoparticulate systems. Pharm. Biol. 2010, 48, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Wu, S.H.; Tao, Y.; Zang, L.Q.; Su, Z.Q. Preparation and Characterization of Water-Soluble Chitosan Nanoparticles as Protein Delivery Syste. J. Nanomater. 2010, 2010, 1–5. [Google Scholar]
- Hsieh, Y.L.; Yao, H.T.; Cheng, R.S.; Chiang, M.T. Chitosan Reduces Plasma Adipocytokines and Lipid Accumulation in Liver and Adipose Tissues and Ameliorates Insulin Resistance in Diabetic Rats. J. Med. Food 2012, 15, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Borchert, H. Increased sensitivity of chitosan determination by a dye binding method. Carbohydr. Res. 2006, 341, 2978–2979. [Google Scholar] [CrossRef] [PubMed]
- Mendelovits, A.; Prat, T.; Gonen, Y.; Rytwo, G. Improved Colorimetric Determination of Chitosan Concentrations by Dye Binding. Appl. Spectrosc. 2012, 66, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.G.; Pan, S.K.; Xia, Z.Q.; Wu, S.J. A new assay of chitosan in the presence of protein by hydrolysis with commercial α-amylase. Eur. Food Res. Technol. 2011, 233, 717–719. [Google Scholar] [CrossRef]
- Zhu, X.L.; Cai, J.B.; Yang, J.; Su, Q.D. Determination of glucosamine in impure chitin samples by high-performance liquid chromatography. Carbohydr. Res. 2005, 340, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- El-Saharty, Y.S.; Bary, A.A. High-performance liquid chromatographic determination of neutraceuticals, glucosamine sulphate and chitosan, in raw materials and dosage forms. Anal. Chim. Acta. 2002, 462, 125–131. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.L.; Bu, F.; Xia, W.S. Determination of chitosan with a modified acid hydrolysis and HPLC method. Carbohydr. Res. 2013, 366, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shoer, M. A Simple Colorimetric Method for the Evaluation of Chitosan. Am. J. Anal. Chem. 2010, 1, 91–94. [Google Scholar] [CrossRef]
- Xu, D.P.; Liu, S.P.; Liu, Z.F.; Hu, X.L. Determination of verapamil hydrochloride with 12-tungstophosphoric acid by resonance Rayleigh scattering method coupled to flow injection system. Anal. Chim. Acta 2007, 588, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.Y.; Wang, Y.; Wang, T.J.; Pang, B.; Zhao, T.T. The analytical application and spetral investigation of DNA-CPB-emodin and sensitive determination of DNA by resonance Rayleigh light scattering technique. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2013, 101, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.F.; Song, W.W.; Luo, H.Q.; Li, N.B. Detection of mercury ions (II) based on non-cross-linking aggregation of double-stranded DNA modified gold nanoparticles by resonance Rayleigh scattering method. Biosensors Bioelectron. 2015, 65, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.Q.; Yang, D.; Jiang, Z.L. A new resonance Rayleigh scattering spectral method for determination of O3 with victoria blue B. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014, 117, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.L.; Lu, Q.M.; Liu, Z.F.; Liu, S.P.; Chen, G.C.; Duan, H.; Song, D.; Wang, J.; Liu, J. Resonance Rayleigh scattering spectra of ion-association nanoparticles of [Co(4-[(5-Chloro-2-pyridyl) azo]-1, 3-diaminobenzene)2]2+-sodium dodecyl benzene sulfonate system and its analytical application. Anal. Chim. Acta 2009, 632, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Long, X.F.; Bi, S.P.; Ni, H.Y.; Tao, X.C.; Gan, N. Resonance Rayleigh scattering determination of trace amounts of Al in natural waters and biological samples based on the formation of an Al(III)–morin–surfactant complex. Anal. Chim. Acta 2004, 501, 89–97. [Google Scholar] [CrossRef]
- Cui, Z.P.; Hu, X.L.; Liu, S.P.; Liu, Z.F. A dual-wavelength overlapping resonance Rayleigh scattering method for the determination of chondroitin sulfate with nile blue sulfate. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2011, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, C.Y.; Liu, S.P.; Liu, Z.F.; Yang, J.D.; Zhu, J.H.; Qiao, M.; Duan, R.L.; Hu, X.L. A novel method for detecting allura red based on triple-wavelength overlapping resonance Rayleigh scattering. RSC Adv. 2014, 4, 37100. [Google Scholar] [CrossRef]
- Parham, H.; Saeed, S. Resonance Rayleigh scattering method for determination of ethion using silver nanoparticles as probe. Talanta 2015, 131, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Felimban, R.; Traianedes, K.; Moulton, S.E.; Wallace, G.G.; Chung, J.; Quigley, A.; Choong, P.F.M.; Myers, D.E. Chondrogenesis of Infrapatellar Fat Pad Derived Adipose Stem Cells in 3D Printed Chitosan Scaffold. PLoS ONE 2014, 9, e102638. [Google Scholar]
- Gu, B.; Zhong, H.; Li, X.M.; Wang, Y.Z.; Ding, B.C.; Cheng, Z.P.; Zhang, L.L.; Li, S.P.; Yao, C. Sensitve Determination of Proteins With Naphthol Green B by Resonance Light Scattering Technique. J. Appl. Spectrosc. 2013, 80, 486–491. [Google Scholar] [CrossRef]
- Beatriz, M.; Marian, M.; Ruth, H.; Angeles, H. Suitability of a colorimetric method for the selective determination of chitosan in dietary supplements. Food Chem. 2011, 126, 1836–1839. [Google Scholar]
- Lu, G.H.; Wang, L.R.; Wang, R.X.; Zeng, Y.; Huang, X. Determination of Chitosan by Cathodic Stripping Voltammetry. Anal. Sci. 2006, 22, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Li, N.B.; Luo, H.Q. Resonance Rayleigh scattering method for the determination of chitosan with some anionic surfactants. Luminescence 2008, 23, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Liu, S.P.; Wang, L.; Liu, Z.W.; He, Y.Q. Study on the interaction between CdSe quantum dots and chitosan by scattering spectra. J. ColloidInterface Sci. 2009, 338, 578–583. [Google Scholar] [CrossRef] [PubMed]







| Methods | Linear Range | Detection Limits | Investigated the Effect of the Molecular Weight of CTS | References |
|---|---|---|---|---|
| UV-VIS spectra | 10–80 µg/mL | - | N | [6] |
| UV-VIS spectra | 4.55–30.30 mg/L | 1.41 mg/mL | Y | [24] |
| Cathodic Stripping Voltammetry | 5.0 × 10−7–1.7×10−5 g/mL | 5.0 × 10−7 g/mL | N | [25] |
| RRS | 0.042–3.0 µg/mL | 1.2 µg/mL | N | [26] |
| SOS | 0.016–3.0µg/mL | 4.9 µg/mL | N | [27] |
| FDS | 0.005–3.0 µg/mL | 1.6 µg/mL | N | [27] |
| RRS | 0.10–20.0 µg/mL | 29 ng/mL | N | [27] |
| HPLC | (2–20 mg/mL) Glucosamine | - | N | [11] |
| RRS | 0.01–5.5 µg/mL | 8.87 ng/mL | Y | This paper |
| Foreign Substance | Amount Tolerated (µg/mL) | RE (%) | Foreign Substance | Amount Tolerated (µg/mL) | RE (%) |
|---|---|---|---|---|---|
| Glucose | 9600.00 | −3.59 | Fe3+ | 0.30 | −4.76 |
| β-Cyclodextrin | 120.00 | 3.33 | Mg2+ | 0.40 | −3.67 |
| Soluble starch | 100.00 | −5.51 | Ca2+ | 0.40 | 4.64 |
| VitaminC | 85.00 | −4.39 | K+ | 10.00 | 3.33 |
| Citricacid | 37.50 | −5.05 | Na+ | 5.00 | −3.38 |
| Glycine | 1600.00 | 4.69 | Cu2+ | 0.12 | 3.84 |
| l-lysine | 900.00 | −5.38 | Zn2+ | 0.80 | −5.07 |
| l-tryptophan | 250.00 | 3.42 | NH4+ | 441.85 | −5.02 |
| l-leucine | 135.00 | −4.01 |
| Cation | The Concentration of Cation (µg/mL) | Masking Agent | The Concentration of Masking Agent | RE (%) |
|---|---|---|---|---|
| Fe3+ | 3.0 | EDTA | 0.001 mol/L | −4.22 |
| Mg2+ | 2.0 | Tartaric acid | 0.08% | 4.78 |
| Ca2+ | 3.0 | Tartaric acid | 0.08% | 2.88 |
| Cu2+ | 3.0 | Tartaric acid | 0.02% | 1.31 |
| Zn2+ | 3.0 | EDTA | 0.001 mol/L | −3.04 |
| Sample | Olevy (mg/g) | AiDeLan (mg/g) |
|---|---|---|
| 1 | 920.2 | 871.5 |
| 2 | 938.5 | 871.1 |
| 3 | 930.1 | 878.4 |
| 4 | 927.2 | 876.7 |
| 5 | 922.4 | 877.2 |
| 6 | 925.6 | 871.8 |
| Average (mg/g) | 927.3 | 874.5 |
| RSD (%) | 0.70 | 0.38 |
| Sample | Found Value (µg/mL) | Added(µg/mL) | Found Value (µg/mL) (n=6) | Recoveries (%) | RSD (%) |
|---|---|---|---|---|---|
| Olevy | 0.4658 | 0.4000 | 0.8830 | 104.3 | 0.71 |
| 1.000 | 1.512 | 104.6 | |||
| 1.500 | 2.014 | 103.2 | |||
| AiDeLan | 0.4498 | 0.4000 | 0.8605 | 102.7 | 0.42 |
| 1.000 | 1.485 | 103.5 | |||
| 1.500 | 2.001 | 103.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ma, C.; Su, Z.; Bai, Y. Resonance Rayleigh Scattering Spectra of an Ion-Association Complex of Naphthol Green B–Chitosan System and Its Application in the Highly Sensitive Determination of Chitosan. Mar. Drugs 2016, 14, 71. https://doi.org/10.3390/md14040071
Zhang W, Ma C, Su Z, Bai Y. Resonance Rayleigh Scattering Spectra of an Ion-Association Complex of Naphthol Green B–Chitosan System and Its Application in the Highly Sensitive Determination of Chitosan. Marine Drugs. 2016; 14(4):71. https://doi.org/10.3390/md14040071
Chicago/Turabian StyleZhang, Weiai, Caijuan Ma, Zhengquan Su, and Yan Bai. 2016. "Resonance Rayleigh Scattering Spectra of an Ion-Association Complex of Naphthol Green B–Chitosan System and Its Application in the Highly Sensitive Determination of Chitosan" Marine Drugs 14, no. 4: 71. https://doi.org/10.3390/md14040071
APA StyleZhang, W., Ma, C., Su, Z., & Bai, Y. (2016). Resonance Rayleigh Scattering Spectra of an Ion-Association Complex of Naphthol Green B–Chitosan System and Its Application in the Highly Sensitive Determination of Chitosan. Marine Drugs, 14(4), 71. https://doi.org/10.3390/md14040071