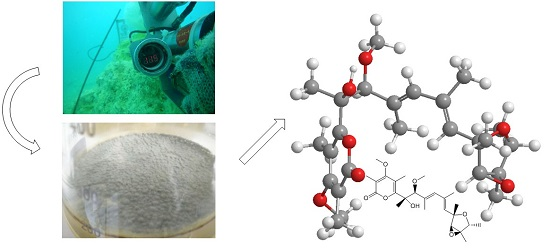
An Unusual Conformational Isomer of Verrucosidin Backbone from a Hydrothermal Vent Fungus, Penicillium sp. Y-50-10
Abstract
:1. Introduction
2. Results
Biological Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Isolation and Cultivation of the Fungal Strain
3.3. Extraction and Isolation
3.4. Computation Section
3.5. Biological Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ashforth, E.; Ren, B.; Song, F.; Dai, H.; Liu, M.; Wang, J.; Xie, Q.; Zhang, L. Bioprospecting microbial natural product libraries from the marine environment for drug discovery. J. Antibiot. 2010, 63, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Dai, H.; Tong, Y.; Ren, B.; Chen, C.; Sun, N.; Liu, X.; Bian, J.; Liu, M.; Gao, H.; et al. Trichodermaketones A–D and 7-O-methylkoninginin D from the marine fungus Trichoderma koningii. J. Nat. Prod. 2010, 73, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; An, R.; Wang, J.; Sun, N.; Zhang, S.; Hu, J.; Kuai, J. Exploring novel bioactive compounds from marine microbes. Curr. Opin. Microbiol. 2005, 8, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, M.; Burka, L.T.; Harris, T.M. Structural studies of the mycotoxin verrucosidin. J. Org. Chem. 1984, 49, 3762–3766. [Google Scholar] [CrossRef]
- Whang, K.; Cooke, R.J.; Okay, G.; Cha, J.K. Total synthesis of (+)-verrucosidin. J. Am. Chem. Soc. 1990, 112, 8985–8987. [Google Scholar] [CrossRef]
- Wilson, B.J.; Byerly, C.S.; Burka, L.T. Neurologic disease of fungal origin in three herds of cattle. J. Am. Vet. Med. Assoc. 1981, 179, 480–481. [Google Scholar] [PubMed]
- Hodge, R.P.; Harris, C.M.; Harris, T.M. Verrucofortine, a major metabolite of Penicillium verrucosum var. cyclopium, the fungus that produces the mycotoxin verrucosidin. J. Nat. Prod. 1988, 51, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.-J.; Park, H.-R.; Ryoo, I.-J.; Kim, J.-P.; Yun, B.-S.; Kim, C.-J.; Shin-ya, K.; Yoo, I.-D. Deoxyverrucosidin, a novel GRP78/Bip down-regulator, produced by Penicillium sp. J. Antibiot. 2005, 58, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Ren, B.; Wei, J.; Chen, C.; Sun, J.; Song, F.; Dai, H.; Zhang, L. Verrucosidinol and verrucosidinol acetate, two pyrone-type polyketides isolated from a marine derived fungus Penicillum aurantiogriseum. Mar. Drugs 2010, 8, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Ye, Y.; Li, C.Y.; Zeng, Y.; Zhao, P.J. Chemical constituents of endophyte Penicillium sp. DCS82 from Daphniphyllum longeracemosum. Guihaia 2013, 4, 30. [Google Scholar]
- Azumi, M.; Ishidoh, K.I.; Kinoshita, H.; Nihira, T.; Ihara, F.; Fujita, T.; Igarashi, Y. Aurovertins F–H from the Entomopathogenic Fungus Metarhizium anisopliae (1). J. Nat. Prod. 2008, 71, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Gaussian09, revision A.1; Gaussian Inc.: Wallingford, CT, USA, 2009.
- SpecDis, version 1.63; University of Wuerzburg: Wuerzburg, Germany, 2015.
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]




| Carbon Number | δC, Type a | δH (J in Hz) | Carbon Number | δC, Type a | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 166.0, C | 14 | 67.4, C | ||
| 2 | 109.4, C | 15 | 76.9, CH | 4.05, q (6.8) | |
| 3 | 170.4, C | 16 | 8.9, CH3 | 2.00, s | |
| 4 | 112.3, C | 17 | 8.8, CH3 | 2.25, s | |
| 5 | 160.5, C | 18 | 22.1, CH3 | 1.45, s | |
| 6 | 78.7, C | 19 | 13.3, CH3 | 1.79, s | |
| 7 | 90.6, CH | 3.91, s | 20 | 17.6, CH3 | 1.89, s |
| 8 | 132.7, C | 21 | 20.8, CH3 | 1.37, s | |
| 9 | 135.3, CH | 5.77, s | 22 | 12.5, CH3 | 1.47, s |
| 10 | 135.1, C | 23 | 17.8, CH3 | 1.18, d (6.8) | |
| 11 | 132.0, CH | 5.45, s | 24 | 59.7, CH3 | 3.84, s |
| 12 | 80.0, C | 25 | 55.7, CH3 | 3.25, s | |
| 13 | 67.3, CH | 3.55, s |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Shi, Y.; Auckloo, B.N.; Chen, X.; Chen, C.-T.A.; Tao, X.; Wu, B. An Unusual Conformational Isomer of Verrucosidin Backbone from a Hydrothermal Vent Fungus, Penicillium sp. Y-50-10. Mar. Drugs 2016, 14, 156. https://doi.org/10.3390/md14080156
Pan C, Shi Y, Auckloo BN, Chen X, Chen C-TA, Tao X, Wu B. An Unusual Conformational Isomer of Verrucosidin Backbone from a Hydrothermal Vent Fungus, Penicillium sp. Y-50-10. Marine Drugs. 2016; 14(8):156. https://doi.org/10.3390/md14080156
Chicago/Turabian StylePan, Chengqian, Yutong Shi, Bibi Nazia Auckloo, Xuegang Chen, Chen-Tung Arthur Chen, Xinyi Tao, and Bin Wu. 2016. "An Unusual Conformational Isomer of Verrucosidin Backbone from a Hydrothermal Vent Fungus, Penicillium sp. Y-50-10" Marine Drugs 14, no. 8: 156. https://doi.org/10.3390/md14080156
APA StylePan, C., Shi, Y., Auckloo, B. N., Chen, X., Chen, C. -T. A., Tao, X., & Wu, B. (2016). An Unusual Conformational Isomer of Verrucosidin Backbone from a Hydrothermal Vent Fungus, Penicillium sp. Y-50-10. Marine Drugs, 14(8), 156. https://doi.org/10.3390/md14080156