Conotoxins as Tools to Understand the Physiological Function of Voltage-Gated Calcium (CaV) Channels
Abstract
:1. Introduction
1.1. Voltage-Gated Calcium Channels
1.2. CaV2.X Channels
1.3. General Properties of ω-Conotoxins
2. Classification of ω-Conotoxins That Target CaV Channels
2.1. C. geographus—GVIA
2.2. C. magus—MVIIA and MVIIC
2.3. C. striatus—SVIA and SVIB SO-3
2.4. C. catus—CVID
2.5. C. fulmen—FVIA
2.6. C. textile—TxVII and CNVIIA
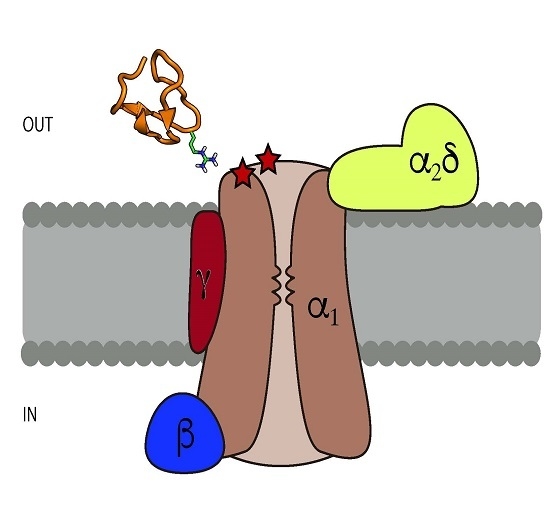
3. Structural Characteristics of ω-Conotoxins and Blockade Site on the CaV Channels
4. Therapeutic Uses of Conotoxins
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Becker, S.; Terlau, H. Toxins from cone snails: Properties, applications and biotechnological production. Appl. Microbiol. Biotechnol. 2008, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.R.; Luque, A.; Olivera, B.M.; Barrett, J.; Cruz, L.J. Peptide toxins from Conus geographus venom. J. Biol. Chem. 1981, 256, 4734–4740. [Google Scholar] [PubMed]
- Fainzilber, M.; Nakamura, T.; Lodder, J.C.; Zlotkin, E.; Kits, K.S.; Burlingame, A.L. γ-Conotoxin-PnVIIA, a γ-carboxyglutamate-containing peptide agonist of neuronal pacemaker cation currents. Biochemistry 1998, 37, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Fainzilber, M.; Gordon, D.; Hasson, A.; Spira, M.E.; Zlotkin, E. Mollusc-specific toxins from the venom of Conus textile neovicarius. FEBS J. 1991, 202, 589–595. [Google Scholar] [CrossRef]
- Rigby, A.C.; Lucas-Meunier, E.; Kalume, D.E.; Czerwiec, E.; Hambe, B.; Dahlqvist, I.; Fossier, P.; Baux, G.; Roepstorff, P.; Baleja, J.D.; et al. A conotoxin from Conus textile with unusual posttranslational modifications reduces presynaptic Ca2+ influx. Proc. Natl. Acad. Sci. USA 1999, 96, 5758–5763. [Google Scholar] [CrossRef] [PubMed]
- Buczek, O.; Wei, D.; Babon, J.J.; Yang, X.; Fiedler, B.; Chen, P.; Yoshikami, D.; Olivera, B.M.; Bulaj, G.; Norton, R.S. Structure and sodium channel activity of an excitatory I1-superfamily conotoxin. Biochemistry 2007, 46, 9929–9940. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Shon, K.-J.; Grilley, M.; Stocker, M.; Stühmer, W.; Olivera, B.M. Strategy for rapid immobilization of prey by a fish-hunting marine snail. Nature 1996, 381, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Gray, W.R.; Olivera, B.M.; Zeikus, R.D.; Kerr, L.; Yoshikami, D.; Moczydlowski, E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J. Biol. Chem. 1985, 260, 9280–9288. [Google Scholar] [PubMed]
- Sharpe, I.A.; Gehrmann, J.; Loughnan, M.L.; Thomas, L.; Adams, D.A.; Atkins, A.; Palant, E.; Craik, D.J.; Adams, D.J.; Alewood, P.F.; et al. Two new classes of conopeptides inhibit the α1-adrenoceptor and noradrenaline transporter. Nat. Neurosci. 2001, 4, 902–907. [Google Scholar] [CrossRef] [PubMed]
- England, L.J.; Imperial, J.; Jacobsen, R.; Craig, A.G.; Gulyas, J.; Akhtar, M.; Rivier, J.; Julius, D.; Olivera, B.M. Inactivation of a serotonin-gated ion channel by a polypeptide toxin from marine snails. Science 1998, 281, 575–578. [Google Scholar] [CrossRef]
- Petrel, C.; Hocking, H.G.; Reynaud, M.; Upert, G.; Favreau, P.; Biass, D.; Paolini-Bertrand, M.; Peigneur, S.; Tytgat, J.; Gilles, N.; et al. Identification, structural and pharmacological characterization of τ-CnVA, a conopeptide that selectively interacts with somatostatin sst 3 receptor. Biochem. Pharmacol. 2013, 85, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Kerr, L.M.; Yoshikami, D. A venom peptide with a novel presynaptic blocking action. Nature 1984, 308, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Kaas, Q.; Westermann, J.-C.; Halai, R.; Wang, C.K.L.; Craik, D.J. ConoServer, a database for conopeptide sequences and structures. Bioinformatics 2007, 24, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Kaas, Q.; Yu, R.; Jin, A.-H.; Dutertre, S.; Craik, D.J. ConoServer: Updated content, knowledge, and discovery tools in the conopeptide database. Nucleic Acids Res. 2011, gkr886. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Rivier, J.; Scott, J.K.; Hillyard, D.R.; Cruz, L.J. Conotoxins. J. Biol. Chem. 1991, 266, 22067–22070. [Google Scholar] [PubMed]
- Adams, D.J.; Berecki, G. Mechanisms of conotoxin inhibition of N-type (Cav2.2) calcium channels. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Zamponi, G.W. Block of voltage-gated calcium channels by peptide toxins. Neuropharmacology 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ion Channels of Excitable Membranes; Sinauer: Sunderland, MA, USA, 2001; Volume 507. [Google Scholar]
- Dolphin, A.C. Voltage-gated calcium channels and their auxiliary subunits: Physiology and pathophysiology and pharmacology. J. Physiol. 2016, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.I.; Lewis, R.J. ω-conotoxins GVIA, MVIIA and CVID: SAR and clinical potential. Mar. Drugs 2006, 4, 193–214. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 1995, 80, 259–268. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Neher, E.; Moser, T. R-Type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J. Neurosci. 2000, 20, 8323–8330. [Google Scholar] [PubMed]
- Loane, D.J.; Lima, P.A.; Marrion, N. V Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. J. Cell Sci. 2007, 120, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.; Piedras-Renteria, E.S.; Smith, S.M.; Wheeler, D.B.; Lee, S.B.; Lee, T.G.; Chin, H.; Adams, M.E.; Scheller, R.H.; Tsien, R.W.; et al. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the α1A-subunit. Proc. Natl. Acad. Sci. USA 1999, 96, 15245–15250. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, H.; Kurihara, T.; Zong, S.; Kazuno, A.; Matsuda, Y.; Nonaka, T.; Han, W.; Toriyama, H.; Tanabe, T. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001, 20, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Altier, C.; Hildebrand, M.E.; Trang, T.; Salter, M.W.; Zamponi, G.W. Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014, 94, 81–140. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.I.; Rash, L.D.; Vila-Farrés, X.; Rosengren, K.J.; Mobli, M.; King, G.F.; Alewood, P.F.; Craik, D.J.; Durek, T. Chemical Synthesis, 3D Structure, and ASIC Binding Site of the Toxin Mambalgin-2. Angew. Chem. Int. Ed. 2014, 53, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Pallaghy, P.K.; Norton, R.S.; Nielsen, K.J.; Craik, D.J. A common structural motif incorporating a cystine knot and a triple-stranded β-sheet in toxic and inhibitory polypeptides. Protein Sci. 1994, 3, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.A.; Cerutti, L.; Hulo, N.; Gattiker, A.; Falquet, L.; Pagni, M.; Bairoch, A.; Bucher, P. PROSITE: A documented database using patterns and profiles as motif descriptors. Brief. Bioinform. 2002, 3, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.A.; De Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Res. 2012, 41, D344–D347. [Google Scholar] [CrossRef] [PubMed]
- Ellinor, P.T.; Zhang, J.F.; Horne, W.A.; Tsien, R.W. Structural determinants of the blockade of N-type calcium channels by a peptide neurotoxin. Nature 1994, 372, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Gray, W.R.; Zeikus, R.; McIntosh, J.M.; Varga, J.; Rivier, J.; De Santos, V.; Cruz, L.J. Peptide neurotoxins from fish-hunting cone snails. Science 1985, 230, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- McCleskey, E.W.; Fox, A.P.; Feldman, D.H.; Cruz, L.J.; Olivera, B.M.; Tsien, R.W.; Yoshikami, D. Omega-conotoxin: Direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc. Natl. Acad. Sci. USA 1987, 84, 4327–4331. [Google Scholar] [CrossRef] [PubMed]
- Regan, L.J.; Sah, D.W.Y.; Bean, B.P. Ca2+ channels in rat central and peripheral neurons: High-threshold current resistant to dihydropyridine blockers and ω-conotoxin. Neuron 1991, 6, 269–280. [Google Scholar] [CrossRef]
- Olivera, B.M.; Cruz, L.J.; De Santos, V.; LeCheminant, G.; Griffin, D.; Zeikus, R.; McIntosh, J.M.; Galyean, R.; Varga, J. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega.-conotoxin from Conus magus venom. Biochemistry 1987, 26, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Ramilo, C.A.; Zafaralla, G.C.; Nadasdi, L.; Hammerland, L.G.; Yoshikami, D.; Gray, W.R.; Kristipati, R.; Ramachandran, J.; Miljanich, G.; Olivera, B.M. Novel alpha- and omega-conotoxins from Conus striatus venom. Biochemistry 1992, 31, 9919–9926. [Google Scholar] [CrossRef] [PubMed]
- Miljanich, G.P.; Bitner, R.S.; Bowersox, S.S.; Fox, J.A.; Valentino, K.L.; Yamashiro, D.H. Method of Treating Ischemia-Related Neuronal Damage. U.S. Patent 5,051,403 A, 24 September 1991. [Google Scholar]
- Pallaghy, P.K.; Norton, R.S. Refined solution structure of ω-conotoxin GVIA: Implications for calcium channel binding. J. Pept. Res. 1999, 53, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Mould, J.; Yasuda, T.; Schroeder, C.I.; Beedle, A.M.; Doering, C.J.; Zamponi, G.W.; Adams, D.J.; Lewis, R.J. The α2δ auxiliary subunit reduces affinity of ω-conotoxins for recombinant N-type (Cav2.2) calcium channels. J. Biol. Chem. 2004, 279, 34705–34714. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.H.; Bradley, E.K.; Miljanich, G.P.; Nadasdi, L.; Ramachandran, J.; Basus, V.J. Solution structure of. omega.-conotoxin GVIA using 2-D NMR spectroscopy and relaxation matrix analysis. Biochemistry 1993, 32, 7396–7405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Pettus, M.; Gao, D.; Phillips, C.; Bowersox, S.S. Effects of intrathecal administration of ziconotide, a selective neuronal N-type calcium channel blocker, on mechanical allodynia and heat hyperalgesia in a rat model of postoperative pain. Pain 2000, 84, 151–158. [Google Scholar] [CrossRef]
- Miljanich, G.P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yan, Z.; Liu, Z.; Wang, S.; Wu, Q.; Yu, S.; Ding, J.; Dai, Q. Molecular basis of toxicity of N-type calcium channel inhibitor MVIIA. Neuropharmacology 2016, 101, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.J.; Beck, J.C.; de Kater, A.W.; Shopp, G.M. Nonclinical safety of ziconotide: An intrathecal analgesic of a new pharmaceutical class. Int. J. Toxicol. 2007, 26, 411–421. [Google Scholar] [CrossRef] [PubMed]
- McGivern, J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Kim, J.I.; Kobayashi, K.; Kodera, Y.; Maeda, T.; Sato, K. Three-Dimensional Structure in Solution of the Calcium Channel Blocker omega.-Conotoxin MVIIA. Biochemistry 1995, 34, 10256–10265. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.J.; Thomas, L.; Lewis, R.J.; Alewood, P.F.; Craik, D.J. A consensus structure for ω-conotoxins with different selectivities for voltage-sensitive calcium channel subtypes: Comparison of MVIIA, SVIB and SNX-202. J. Mol. Biol. 1996, 263, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Smith, A.B.; Schroeder, C.I.; Yasuda, T.; Lewis, R.J. ω-conotoxin CVID inhibits a pharmacologically distinct voltage-sensitive calcium channel associated with transmitter release from preganglionic nerve terminals. J. Biol. Chem. 2003, 278, 4057–4062. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.A.; Kieffer, B.; Dejaegere, A.; Sirockin, F.; Lefèvre, J.-F. Structural and dynamic characterization of ω-conotoxin MVIIA: The binding loop exhibits slow conformational exchange. Biochemistry 2000, 39, 3908–3919. [Google Scholar] [CrossRef] [PubMed]
- Monje, V.D.; Haack, J.A.; Naisbitt, S.R.; Miljanich, G.; Ramachandran, J.; Nasdasdi, L.; Olivera, B.M.; Hillyard, D.R.; Gray, W.R. A new Conus peptide ligand for Ca channel subtypes. Neuropharmacology 1993, 32, 1141–1149. [Google Scholar] [CrossRef]
- Farr-Jones, S.; Miljanich, G.P.; Nadasdi, L.; Ramachandran, J.; Basus, V.J. Solution structure of ω-conotoxin MVIIC, a high affinity ligand of P-type calcium channels, using1H NMR spectroscopy and complete relaxation matrix analysis. J. Mol. Biol. 1995, 248, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.J.; Adams, D.; Thomas, L.; Bond, T.; Alewood, P.F.; Craik, D.J.; Lewis, R.J. Structure-activity relationships of ω-conotoxins MVIIA, MVIIC and 14 loop splice hybrids at N and P/Q-type calcium channels. J. Mol. Biol. 1999, 289, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Yang, S.; Qiao, H.; Liu, Z.; Zhou, W.; Zhang, Y.; Huang, P. SO-3, a new O-superfamily conopeptide derived from Conus striatus, selectively inhibits N-type calcium currents in cultured hippocampal neurons. Br. J. Pharmacol. 2005, 145, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tu, G.; Luo, X.; Dai, Q.; Huang, P.; Zhang, R. Three-dimensional solution structure of ω-conotoxin SO3 determined by1H NMR. Chin. Sci. Bull. 2003, 48, 1097–1102. [Google Scholar] [CrossRef]
- Lewis, R.J.; Nielsen, K.J.; Craik, D.J.; Loughnan, M.L.; Adams, D.A.; Sharpe, I.A.; Luchian, T.; Adams, D.J.; Bond, T.; Thomas, L.; et al. Novel ω-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J. Biol. Chem. 2000, 275, 35335–35344. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.I.; Doering, C.J.; Zamponi, G.W.; Lewis, R.J. N-type calcium channel blockers: Novel therapeutics for the treatment of pain. Med. Chem. 2006, 2, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.; Back, S.K.; Choi, H.-W.; Lee, J.Y.; Jung, H.H.; Ryu, J.H.; Suh, H.-W.; Na, H.S.; Kim, H.J.; et al. Analgesic effect of highly reversible ω-conotoxin FVIA on N type Ca2+ channels. Mol. Pain 2010, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Fainzilber, M.; Lodder, J.C.; van der Schors, R.C.; Li, K.W.; Yu, Z.; Burlingame, A.L.; Geraerts, W.P.M.; Kits, K.S. A novel hydrophobic omega-conotoxin blocks molluscan dihydropyridine-sensitive calcium channels. Biochemistry 1996, 35, 8748–8752. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Sasaki, T.; Sato, K.; Kohno, T. Three-dimensional solution structure of ω-conotoxin TxVII, an L-type calcium channel blocker. Biochemistry 2000, 39, 14761–14767. [Google Scholar] [CrossRef] [PubMed]
- Favreau, P.; Gilles, N.; Lamthanh, H.; Bournaud, R.; Shimahara, T.; Bouet, F.; Laboute, P.; Letourneux, Y.; Ménez, A.; Molgó, J.; et al. A new ω-conotoxin that targets N-type voltage-sensitive calcium channels with unusual specificity. Biochemistry 2001, 40, 14567–14575. [Google Scholar] [CrossRef] [PubMed]
- Violette, A.; Biass, D.; Dutertre, S.; Koua, D.; Piquemal, D.; Pierrat, F.; Stöcklin, R.; Favreau, P. Large-scale discovery of conopeptides and conoproteins in the injectable venom of a fish-hunting cone snail using a combined proteomic and transcriptomic approach. J. Proteom. 2012, 75, 5215–5225. [Google Scholar] [CrossRef] [PubMed]
- Miljanich, G.P.; Bitner, R.S.; Bowersox, S.S.; Fox, J.A.; Valentino, K.L.; Yamashiro, D.H.; Tsubokawa, M. Screening Method for Neuroprotective Compounds. U.S. Patent 5,424,218 A, 4 November 1993. [Google Scholar]
- Miljanich, G.P.; Bowersox, S.S.; Fox, J.A.; Valentino, K.L.; Bitner, R.S.; Yamashiro, D.H. Compositions for Delayed Treatment of Ischemia-Related Neuronal Damage. WO Patent 1993010145 A1, 27 May 1993. [Google Scholar]
- Bai-Song, L.; Fang, Y.; Dong, Z.; Pei-Tang, H.; Cui-Fen, H. Conopeptides from Conus striatus and Conus textile by cDNA cloning. Peptides 1999, 20, 1139–1144. [Google Scholar] [CrossRef]
- Berecki, G.; Motin, L.; Haythornthwaite, A.; Vink, S.; Bansal, P.; Drinkwater, R.; Wang, C.I.; Moretta, M.; Lewis, R.J.; Alewood, P.F.; et al. Analgesic ω-conotoxins CVIE and CVIF selectively and voltage-dependently block recombinant and native N-type calcium channels. Mol. Pharmacol. 2010, 77, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kits, K.S.; Lodder, J.C.; Van Der Schors, R.C.; Li, K.W.; Geraerts, W.P.M.; Fainzilber, M. Novel ω-Conotoxins Block Dihydropyridine-Insensitive High Voltage-Activated Calcium Channels in Molluscan Neurons. J. Neurochem. 1996, 67, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-P.; Hamid, J.; Doering, C.; Bosey, G.M.; Snutch, T.P.; Zamponi, G.W. Residue Gly1326 of the N-type calcium channel α1B subunit controls reversibility of ω-conotoxin GVIA and MVIIA block. J. Biol. Chem. 2001, 276, 15728–15735. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.I.; Smythe, M.L.; Lewis, R.J. Development of small molecules that mimic the binding of ω-conotoxins at the N-type voltage-gated calcium channel. Mol. Divers. 2004, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Duggan, P.J.; Forsyth, S.A.; Lewis, R.J.; Lok, Y.P.; Schroeder, C.I. Synthesis and biological evaluation of nonpeptide mimetics of ω-conotoxin GVIA. Bioorg. Med. Chem. 2004, 12, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Nadasdi, L.; Yamashiro, D.; Chung, D.; Tarczy-Hornoch, K.; Adriaenssens, P.; Ramachandran, J. Structure-Activity Analysis of a Conus Peptide Blocker of N-Type Neuronal Calcium Channels. Biochemistry 1995, 34, 8076–8081. [Google Scholar] [CrossRef] [PubMed]
- Menzler, S.; Bikker, J.A.; Horwell, D.C. Synthesis of a non-peptide analogue of omega-conotoxin MVIIA. Tetrahedron Lett. 1998, 39, 7619–7622. [Google Scholar] [CrossRef]
- Menzler, S.; Bikker, J.A.; Suman-Chauhan, N.; Horwell, D.C. Design and biological evaluation of non-peptide analogues of omega-conotoxin MVIIA. Bioorg. Med. Chem. Lett. 2000, 10, 345–347. [Google Scholar] [CrossRef]
- Guo, Z.-X.; Cammidge, A.N.; Horwell, D.C. Dendroid peptide structural mimetics of ω-conotoxin MVIIA based on a 2 (1H)-quinolinone core. Tetrahedron 2000, 56, 5169–5175. [Google Scholar] [CrossRef]
- Duggan, P.J.; Tuck, K.L. Bioactive mimetics of conotoxins and other venom peptides. Toxins 2015, 7, 4175–4198. [Google Scholar] [CrossRef] [PubMed]
- Layer, R.T.; Mcintosh, J.M. Conotoxins: Therapeutic Potential and Application. Mar. Drugs 2006, 4, 119–142. [Google Scholar] [CrossRef]
- Yeager, R.E.; Yoshikami, D.; Rivier, J.; Cruz, L.J.; Miljanich, G.P. Transmitter release from presynaptic terminals of electric organ: Inhibition by the calcium channel antagonist omega Conus toxin. J. Neurosci. 1987, 7, 2390–2396. [Google Scholar] [PubMed]
- Malmberg, A.B.; Yaksh, T.L. Effect of continuous intrathecal infusion of ω-conopeptides, N-type calcium-channel blockers, on behavior and antinociception in the formalin and hot-plate tests in rats. Pain 1995, 60, 83–90. [Google Scholar] [CrossRef]
- Sluka, K.A. Blockade of N-and P/Q-type calcium channels reduces the secondary heat hyperalgesia induced by acute inflammation. J. Pharmacol. Exp. Ther. 1998, 287, 232–237. [Google Scholar] [PubMed]
- Xiao, W.H.; Bennett, G.J. Synthetic omega-conopeptides applied to the site of nerve injury suppress neuropathic pains in rats. J. Pharmacol. Exp. Ther. 1995, 274, 666–672. [Google Scholar] [PubMed]
- Wang, Y.-X.; Gao, D.; Pettus, M.; Phillips, C.; Bowersox, S.S. Interactions of intrathecally administered ziconotide, a selective blocker of neuronal N-type voltage-sensitive calcium channels, with morphine on nociception in rats. Pain 2000, 84, 271–281. [Google Scholar] [CrossRef]
- Hannon, H.E.; Atchison, W.D. Omega-conotoxins as experimental tools and therapeutics in pain management. Mar. Drugs 2013, 11, 680–699. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Takahashi, M.; Ogura, A.; Kohno, T.; Kudo, Y.; Sato, K. Hydroxyl group of Tyr13 is essential for the activity of omega-conotoxin GVIA, a peptide toxin for N-type calcium channel. J. Biol. Chem. 1994, 269, 23876–23878. [Google Scholar] [PubMed]
- Lew, M.J.; Flinn, J.P.; Pallaghy, P.K.; Murphy, R.; Whorlow, S.L.; Wright, C.E.; Norton, R.S.; Angus, J.A. Structure-function relationships of ω-conotoxin GVIA Synthesis, structure, calcium channel binding, and functional assay of alanine-substituted analogues. J. Biol. Chem. 1997, 272, 12014–12023. [Google Scholar] [CrossRef] [PubMed]


| Conotoxin Family | Molecular Target | Reference |
|---|---|---|
| α (alpha) | Nicotinic acetylcholine receptors (nAChR) | [3] |
| γ (gamma) | Neuronal pacemaker cation currents (inward cation current) | [4] |
| δ (delta) | Voltage-gated sodium (Na+) channels | [5] |
| ε (epsilon) | Presynaptic calcium (Ca2+) channels or G protein-coupled presynaptic receptors | [6] |
| ι (iota) | Voltage-gated sodium (Na+) channels | [7] |
| κ (kappa) | Voltage-gated potassium (K+) channels | [8] |
| μ (mu) | Voltage-gated sodium (Na+)channels | [9] |
| ρ (rho) | Alpha1-adrenoceptors (GPCR) | [10] |
| σ (sigma) | Serotonin-gated ion channels 5-HT3 | [11] |
| τ (tau) | Somatostatin receptor | [12] |
| χ (chi) | Neuronal noradrenaline transporter | [10] |
| ω (omega) | Voltage-gated calcium (CaV) channels | [13] |
| Ca Channel | Human Gene Name | Voltage Activation | α1 Subunit | Ca Current |
|---|---|---|---|---|
| CaV1.1–1.4 | CACNA1S; CACNA1C; CACNA1D; CACNA1F | HVA | α1S, C, D, F | L |
| CaV2.1 | CACNA1A | HVA | α1A | P/Q |
| CaV2.2 | CACNA1B | HVA | α1B | N |
| CaV2.3 | CACNA1E | HVA | α1E | R |
| CaV3.1–3.3 | CACNA1G; CACNA1H; CACNA1I | LVA | α1G, H, I. | T |
| Specie Conus | ω-Conotoxin | Alternative Names | Target | Organism | IC50 | Reference |
|---|---|---|---|---|---|---|
| C. geographus | GVIA | G6a, SNX-124, | CaV2.1 | R. norvegicus | 1.05 μM 1 | [57] |
| CaV2.2 | R. norvegicus | 2.02 pM 1 | [62] | |||
| GVIB | ? | [34] | ||||
| GVIC | ? | [34] | ||||
| GVIIA | SNX-178 | CaV2.2 | R. norvegicus | 22.9 nM 1 | [64] | |
| GVIIB | ? | [34] | ||||
| C. magus | MVIIA | M7a, SNX-111, Ziconotide, Prialt® | CaV2.1 | R. norvegicus | 156 nM 1 | [62] |
| CaV2.2 | H. sapiens | 7.96 nM 2 | [59] | |||
| MVIIB | SNX-159 | CaV2.2 | R. norvegicus | 101 pM 1 | [65] | |
| MVIIC | M7c, SNX-230 | CaV2.1 | R. norvegicus | 600 pM 1 | [57] | |
| CaV2.2 | R. norvegicus | 7.0 nM 1 | [57] | |||
| MVIID | SNX-238 | ? | [52] | |||
| C. striatus | SVIA | S6a, SNX-157 | CaV2.2 | R. norvegicus | 1.46 μM 1 | [65] |
| SVIB | S6b, SNX-183 | CaV2.1 | [38] | |||
| CaV2.2 | R. norvegicus | 1.09 nM 1 | [65] | |||
| SO-3 | CaV2.2 | 160 nM 2 | [45] | |||
| SO-4 | ? | [66] | ||||
| SO-5 | ? | [66] | ||||
| C. catus | CVIA | C6a, catus-C1b | CaV2.1 | R. norvegicus | 850 nM 1 | [57] |
| CaV2.2 | R. norvegicus | 560 pM 1 | [57] | |||
| CVIB | C6b | CaV2.1 CaV2.2 | R. norvegicus R. norvegicus | 11 nM 1 7.7 nM 1 12 nM 2 | [57] [57] [67] | |
| CVIC | C6c | CaV2.1 | R. norvegicus | 31 nM 1 | [57] | |
| CaV2.2 | R. norvegicus | 7.6 nM 1 | [57] | |||
| CVID | AM-336, AM336, leconotide | CaV2.1 | R. norvegicus | 55 μM 1 | [57] | |
| CaV2.2 | R. norvegicus | 70 pM 1 | [57] | |||
| CVIE | CaV2.2 | R. norvegicus | 2.6 nM 2 0.12 nM 2 | [67] | ||
| CVIF | C6f | CaV1.2 | R. norvegicus | >3 μM 2 | [67] | |
| CaV1.3 | R. norvegicus | >3 μM 2 | [67] | |||
| CaV2.2 | R. norvegicus | 19.9 nM/ | [67] | |||
| 0.1 nM 2 | [67] | |||||
| CaV2.3 | R. norvegicus | >3 μM 2 | [67] | |||
| C. fulmen | FVIA | CaV2.2 | H. sapiens | 11.5 nM 2 | [59] | |
| C. radiatus | RVIA | R6a | CaV2.2 | R. norvegicus | 229 nM 1 | [39] |
| C. textile | TxVII | L-type | [60] | |||
| C. consors | CnVIIA | Cn7a, CnVIIH | CaV2.1 | R. norvegicus | 179 nM 1 | [62] |
| CaV2.2 | R. norvegicus | 2.3–3.7 pM 1 | [62] | |||
| CnVIIB | CnVIIG | ? | [63] | |||
| CnVIIC | CnVIIE | ? | [63] | |||
| C. pennaceus | PnVIA | Pn6a | ? | Lymnaea stagnalis | ~5 μM2 | [68] |
| PnVIB | Pn6b | ? | Lymnaea stagnalis | ~5 μM2 | [68] | |
| C. tulipa | TVIA | SNX-185 | CaV2.2 | R. norvegicus | 228 pM 1 | [65] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, D.; Gonzalez, W.; Fissore, R.A.; Carvacho, I. Conotoxins as Tools to Understand the Physiological Function of Voltage-Gated Calcium (CaV) Channels. Mar. Drugs 2017, 15, 313. https://doi.org/10.3390/md15100313
Ramírez D, Gonzalez W, Fissore RA, Carvacho I. Conotoxins as Tools to Understand the Physiological Function of Voltage-Gated Calcium (CaV) Channels. Marine Drugs. 2017; 15(10):313. https://doi.org/10.3390/md15100313
Chicago/Turabian StyleRamírez, David, Wendy Gonzalez, Rafael A. Fissore, and Ingrid Carvacho. 2017. "Conotoxins as Tools to Understand the Physiological Function of Voltage-Gated Calcium (CaV) Channels" Marine Drugs 15, no. 10: 313. https://doi.org/10.3390/md15100313
APA StyleRamírez, D., Gonzalez, W., Fissore, R. A., & Carvacho, I. (2017). Conotoxins as Tools to Understand the Physiological Function of Voltage-Gated Calcium (CaV) Channels. Marine Drugs, 15(10), 313. https://doi.org/10.3390/md15100313