Krishnolides A–D: New 2-Ketokhayanolides from the Krishna Mangrove, Xylocarpus moluccensis
Abstract
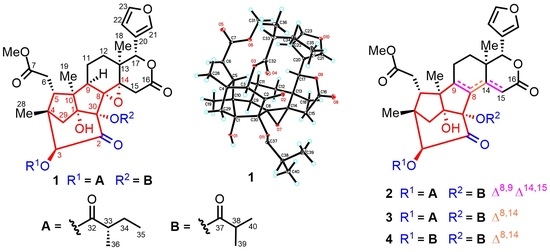
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Plant Material
3.3. Extraction and Isolation
3.4. X-ray Crystal Data for Krishnolide A (1)
3.5. HIV-Inhibitory Bioassay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural Products from True Mangrove Flora: Source, Chemistry and Bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, X.W.; Guo, Y.W. Recent Progress on the Mangrove Plants: Chemistry and Bioactivity. Curr. Org. Chem. 2016, 20, 1923–1942. [Google Scholar] [CrossRef]
- Li, W.S.; Wu, J.; Li, J.; Satyanandamurty, T.; Shen, L.; Bringmann, G. Krishnadimer A, an axially chiral non-biaryl natural product: Discovery and biomimetic synthesis. Org. Lett. 2017, 19, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.G.; Li, W.S.; Pedpradab, P.; Liu, J.J.; Wu, J.; Shen, L. Thaixylomolins O–R: Four new limonoids from the Trang mangrove, Xylocarpus moluccensis. RSC Adv. 2016, 6, 85978–85984. [Google Scholar] [CrossRef]
- Li, W.S.; Jiang, Z.P.; Shen, L.; Pedpradab, P.; Bruhn, T.; Wu, J.; Bringmann, G. Antiviral limonoids including khayanolides from the Trang mangrove plant Xylocarpus moluccensis. J. Nat. Prod. 2015, 78, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.Y.; Bruhn, T.; Katele, F.Z.; Xiao, Q.; Pedpradab, P.; Wu, J.; Bringmann, G. Thaixylomolins A-C: Limonoids featuring two new motifs from the Thai Xylocarpus moluccensis. Org. Lett. 2013, 15, 3682–3685. [Google Scholar] [CrossRef] [PubMed]
- Olmo, L.R.V.; Silva, M.F.; Das, G.F.D.; Fo, E.R.; Vieira, P.C.; Fernandes, J.B.; Marsaioli, A.J.; Pinheiro, A.L.; Vilela, E.F. Rearranged limonoids from Khaya senegalensis. Phytochemistry 1996, 42, 831–837. [Google Scholar] [CrossRef]
- Nakatani, M.; Abdelgaleil, S.A.M.; Okamura, H.; Iwagawa, T.; Sato, A.; Doe, M. Khayanolides A and B, new rearranged phragmalin limonoid antifeedants from Khaya senegalensis. Tetrahedron Lett. 2000, 41, 6473–6477. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Okamura, H.; Iwagawa, T.; Sato, A.; Miyahara, I.; Doe, M.; Nakatani, M. Khayanolides, rearranged phragmalin limonoid antifeedants from Khaya senegalensis. Tetrahedron 2001, 57, 119–126. [Google Scholar] [CrossRef]
- Nakatani, M.; Abdelgaleil, S.A.M.; Kassem, S.M.I.; Takezaki, K.; Okamura, H.; Iwagawa, T.; Doe, M.J. Three new modified limonoids from Khaya senegalensis. J. Nat. Prod. 2002, 65, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.M.; Nakatani, M.J. Antifeeding activity of limonoids from Khaya senegalensis. Appl. Entomol. 2003, 127, 236–239. [Google Scholar] [CrossRef]
- El-Aswad, A.F.; Abdelgaleil, S.A.M.; Nakatani, M. Feeding deterrent and growth inhibitory properties of limonoids from Khaya senegalensis against the cotton leafworm, Spodoptera littoralis. Pest Manag. Sci. 2003, 60, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Odeku, O.A.; Wang, X.N.; Yue, J.M. Limonoids from the stem bark of Khaya grandifoliola. Phytochemistry 2008, 69, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Tan, J.J.; VanDerveer, D.; Wang, X.; Wargovich, M.J.; Chen, F. Khayanolides from African mahogany Khaya senegalensis (Meliaceae): A revision. Phytochemistry 2009, 70, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, S.P.; Yin, S.; Zhang, C.R.; Wu, Y.; Yue, J.M. Phytochemistry Limonoids from Khaya ivorensis. Phytochemistry 2009, 70, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, B.; Riaz, N.; Saleem, M.A.; Naveed, M.A.; Ahmed, M.; Tahir, M.N.; Pescitelli, G.; Ashraf, M.; Ejaz, S.A.; Ahmed, I.; et al. Isolation and characterization of limonoids from Kigelia africana. Z. Naturforsch. 2013, 68b, 1041–1048. [Google Scholar] [CrossRef]
- Yuan, C.M.; Zhang, Y.; Tang, G.H.; Hao, X.J. Khayseneganins A–H, limonoids from Khaya senegalensis. J. Nat. Prod. 2013, 76, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Xia, J.J.; Qiu, M.H.; Li, Z.R. Diterpenoids and limonoids from the leaves and twigs of Swietenia mahagoni. Nat. Prod. Bioprospect. 2014, 4, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Qing, X.; Huo, C.H.; Yan, H.M.; Shi, Q.W.; Sauriol, F.; Gu, Y.C.; Kiyota, H. Ivorenoids A–F: Limonoids from Khaya ivorensis. Tetrahedron 2014, 70, 3570–3575. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Q.P.; Luo, J.; Wang, J.S.; Wang, X.B.; Zhu, M.D.; Kong, L.Y. Limonoids from the stem bark of Khaya senegalensis. Chem. Pharm. Bull. 2015, 63, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Hooft, R.W.W.; Straver, L.H.; Spek, A.L. Determination of absolute structure using Bayesian statistics on Bijvoet differences. J. Appl. Cryst. 2008, 41, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Z.L.; Mi, Z.Y.; Li, X.Y.; Jia, P.P.; Zhou, J.M.; Yin, X.; You, X.F.; Yu, L.Y.; Guo, F.; et al. High-throughput assay to identify inhibitors of Vpu-mediated down-regulationof cell surface BST-2. Antivir. Res. 2011, 91, 321–329. [Google Scholar] [CrossRef] [PubMed]




| Position | 1 a | 1 b | 2 a | 3 a | 4 a |
|---|---|---|---|---|---|
| 3 | 5.68 s | 5.44 s | 5.51 s | 5.22 s | 5.21 s |
| 5 | 2.37 m | 2.16 br d (8.0) | 2.63 m | 2.49 c | 2.48 c |
| 6a | 2.22 br d (14.0) | 2.36 c | 2.41 c | 2.30 br d (12.8) | 2.32 br d (12.8) |
| 6b | 2.43 br d (14.0) | 2.42 m | 2.41 c | 2.47 c | 2.48 c |
| 9 | 2.07 m | 1.97 m | 2.58 m | 2.59 m | |
| 11α | 1.36 c | 1.41 c | 2.20 m | 1.77 m | 1.78 m |
| 11β | 1.36 c | 1.19 m | 2.50 m | 1.50 c | 1.50 c |
| 12α | 1.28 m | 1.13 m | 1.59 m | 1.44 m | 1.44 m |
| 12β | 1.34 c | 1.17 c | 1.45 m | 1.49 m | 1.50 c |
| 15α | 3.06 d (19.0) | 3.15 d (19.0) | 6.45 br s | 3.62 dd (20.0, 3.8) | 3.62 dd (20.0, 3.8) |
| 15β | 3.28 d (19.0) | 3.00 d (19.0) | 3.73 dd (20.0, 3.2) | 3.74 dd (20.0, 3.2) | |
| 17 | 5.25 s | 5.06 s | 5.08 s | 5.10 s | 5.13 s |
| 18 | 1.13 s | 1.02 s | 1.05 s | 1.05 s | 1.05 s |
| 19 | 1.08 s | 0.98 s | 1.04 s | 1.07 s | 1.07 s |
| 21 | 7.59 br s | 7.69 br s | 7.47 br s | 7.48 br s | 7.47 br s |
| 22 | 6.45 br s | 6.52 br s | 6.45 br s | 6.43 br s | 6.42 br s |
| 23 | 7.43 br s | 7.75 br s | 7.43 br s | 7.43 br s | 7.42 br s |
| 28 | 1.05 s | 0.97 s | 1.04 s | 0.98 s | 0.98 s |
| 29pro-S | 2.13 d (12.6) | 2.09 d (12.6) | 1.97 d (12.6) | 2.03 d (12.8) | 2.03 d (12.8) |
| 29pro-R | 2.62 d (12.6) | 2.43 d (12.6) | 2.65 d (12.6) | 2.50 d (12.8) | 2.50 d (12.8) |
| 7-OMe-31 | 3.70 s | 3.63 s | 3.70 s | 3.66 s | 3.66 s |
| 3-OAcyl | |||||
| 33 | 2.43, m | 2.35 c | 2.53 m | 2.49 c | 2.65 m |
| 34 | 1.52 m | 1.46 m | 1.51 m | 1.51 c | 1.19 d (7.2) |
| 1.70 m | 1.55 m | 1.73 m | 1.68 m | ||
| 35 | 0.93 t (7.2) | 0.80 t (7.2) | 0.95 t (7.2) | 0.91 t (7.2) | 1.20 d (7.2) |
| 36 | 1.15 d (7.2) | 1.04 d (7.2) | 1.23 d (7.2) | 1.15 d (7.2) | |
| 30-OAcyl | |||||
| 38 | 2.66 m | 2.63 m | 2.66 m | 2.71 m | 2.72 m |
| 39 | 1.21 d (7.0) | 1.09 d (7.2) | 1.20 d (7.0) | 1.20 d (7.0) | 1.20 d (7.0) |
| 40 | 1.21 d (7.0) | 1.10 d (7.2) | 1.20 d (7.0) | 1.26 d (7.0) | 1.26 d (7.0) |
| 1-OH | 5.86 s |
| Position | 1 a | 1 b | 2 a | 3 a | 4 a |
|---|---|---|---|---|---|
| 1 | 85.5 qC | 85.0 qC | 88.6 qC | 86.2 qC | 86.2 qC |
| 2 | 203.2 qC | 205.9 qC | 197.9 qC | 199.2 qC | 199.4 qC |
| 3 | 83.1 CH | 83.4 CH | 79.1 CH | 79.9 CH | 80.2 CH |
| 4 | 40.6 qC | 40.8 qC | 41.9 qC | 40.5 qC | 40.6 qC |
| 5 | 42.0 CH | 42.4 CH | 46.4 CH | 39.4 CH | 39.4 CH |
| 6 | 33.5 CH2 | 33.4 CH2 | 34.1 CH2 | 34.3 CH2 | 34.3 CH2 |
| 7 | 172.7 qC | 173.6 qC | 173.4 qC | 172.9 qC | 172.9 qC |
| 8 | 73.5 qC | 73.6 qC | 124.6 qC | 132.2 qC | 132.3 qC |
| 9 | 51.3 qC | 51.2 qC | 161.9 qC | 46.8 CH | 46.7 CH |
| 10 | 53.1 qC | 53.5 qC | 60.5 qC | 56.4 qC | 56.4 qC |
| 11 | 16.7 CH2 | 16.5 CH2 | 20.6 CH2 | 18.5 CH2 | 18.6 CH2 |
| 12 | 30.1 CH2 | 30.3 CH2 | 30.7 CH2 | 31.3 CH2 | 31.3 CH2 |
| 13 | 38.1 qC | 37.9 qC | 37.8 qC | 41.1 qC | 41.1 qC |
| 14 | 65.4 qC | 65.1 qC | 151.7 qC | 139.4 qC | 139.3 qC |
| 15 | 35.2 CH2 | 35.5 CH2 | 113.8 CH | 32.8 CH2 | 32.9 CH2 |
| 16 | 169.1 qC | 169.0 qC | 165.4 qC | 169.5 qC | 169.5 qC |
| 17 | 76.9 CH | 76.9 CH | 80.2 CH | 80.3 CH | 80.3 CH |
| 18 | 16.2 CH3 | 16.6 CH3 | 16.3 CH3 | 16.6 CH3 | 16.7 CH3 |
| 19 | 16.2 CH3 | 16.0 CH3 | 13.7 CH3 | 15.6 CH3 | 15.6 CH3 |
| 20 | 119.8 qC | 120.3 qC | 120.3 qC | 120.4 qC | 120.4 qC |
| 21 | 141.7 CH | 141.7 CH | 141.2 CH | 141.3 CH | 141.3 CH |
| 22 | 109.5 CH | 110.0 CH | 110.1 CH | 110.0 CH | 110.0 CH |
| 23 | 143.4 CH | 144.6 CH | 143.0 CH | 143.1 CH | 143.0 CH |
| 28 | 19.1 CH3 | 18.8 CH3 | 21.2 CH3 | 19.9 CH3 | 19.9 CH3 |
| 29 | 42.9 CH2 | 43.3 CH2 | 41.2 CH2 | 42.9 CH2 | 42.9 CH2 |
| 30 | 86.3 qC | 87.2 qC | 92.6 qC | 91.4 qC | 91.4 qC |
| 7-OMe-31 | 51.9 CH3 | 52.1 CH3 | 52.0 CH3 | 51.7 CH3 | 51.8 CH3 |
| 3-OAcyl | |||||
| 32 | 175.3 qC | 175.2 qC | 175.7 qC | 175.6 qC | 176.0 qC |
| 33 | 40.9 CH | 40.8 CH | 40.5 CH | 40.7 CH | 33.9 CH |
| 34 | 26.8 CH2 | 26.7 CH2 | 26.6 CH2 | 27.0 CH2 | 18.5 CH3 |
| 35 | 11.3 CH3 | 11.6 CH3 | 11.6 CH3 | 11.5 CH3 | 18.9 CH3 |
| 36 | 16.1 CH3 | 16.3 CH3 | 16.5 CH3 | 16.3 CH3 | |
| 30-OAcyl | |||||
| 37 | 175.4 qC | 175.6 qC | 175.9 qC | 177.3 qC | 177.4 qC |
| 38 | 33.5 CH | 33.2 CH | 33.8 CH | 33.6 CH | 33.6 CH |
| 39 | 18.7 CH3 | 19.0 CH3 | 18.8 CH3 | 19.1 CH3 | 19.1 CH3 |
| 40 | 18.8 CH3 | 18.7 CH3 | 19.1 CH3 | 18.9 CH3 | 19.1 CH3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Satyanandamurty, T.; Shen, L.; Wu, J. Krishnolides A–D: New 2-Ketokhayanolides from the Krishna Mangrove, Xylocarpus moluccensis. Mar. Drugs 2017, 15, 333. https://doi.org/10.3390/md15110333
Zhang Q, Satyanandamurty T, Shen L, Wu J. Krishnolides A–D: New 2-Ketokhayanolides from the Krishna Mangrove, Xylocarpus moluccensis. Marine Drugs. 2017; 15(11):333. https://doi.org/10.3390/md15110333
Chicago/Turabian StyleZhang, Qun, Tirumani Satyanandamurty, Li Shen, and Jun Wu. 2017. "Krishnolides A–D: New 2-Ketokhayanolides from the Krishna Mangrove, Xylocarpus moluccensis" Marine Drugs 15, no. 11: 333. https://doi.org/10.3390/md15110333
APA StyleZhang, Q., Satyanandamurty, T., Shen, L., & Wu, J. (2017). Krishnolides A–D: New 2-Ketokhayanolides from the Krishna Mangrove, Xylocarpus moluccensis. Marine Drugs, 15(11), 333. https://doi.org/10.3390/md15110333