Determination of the Halogenated Skeleton Constituents of the Marine Demosponge Ianthella basta
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Chemical Characterization of the I. basta Skeleton and Comparison with A. cavernicola
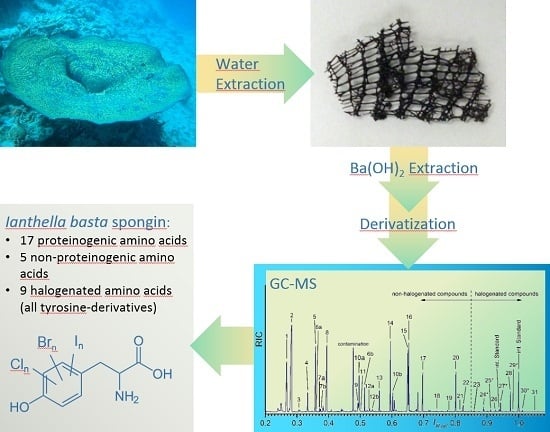
2.2. Skeletal Amino Acid Composition
2.2.1. GC-MS Analysis of the Skeletal Amino Acid Composition
2.2.2. Comparison of the Found Amino Acid Composition with the Literature
3. Materials and Methods
3.1. Sponge Samples
3.2. Extraction of the Skeletons
3.3. Light Microscopy
3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
3.5. Extraction of the Chitin-Based Scaffold
3.6. Estimation of the Content of Other Saccharides
3.7. Determination of Calcium, Silicon and Sulfur Contents by ICP-OES
3.8. Ba(OH)2 Extraction of the Amino Acids
3.9. GC-MS Measurements of the Skeleton Extracts
4. Conclusions
- The composition of non-halogenated amino acids in I. basta is similar to that of A. cavernicola. Abundant amino acids such as glycine and hydroxyproline confirm the collagenous nature of the I. basta spongin in analogy to all other investigated sponges.
- I. basta exhibits a similar variety of halogenated amino acids as already observed for A. cavernicola—in contrast to the Dictyoceratida sponges H. equina and S. officinalis obliqua. It is, therefore, tempting to speculate that this variety of halogenated amino acids is characteristic for the order Verongida.
- The differences in amino acid composition in the sponge skeletons of I. basta and A. cavernicola clearly show that the spongin in the skeletons of Verongid sponges is similar, but also exhibits some characteristic differences.
- Further investigations of the amino acid composition of other sponge samples should be performed in the future to include a larger set of different sponge species into this comparison.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arrieta, J.M.; Arnaud-Haond, S.; Duarte, C.M. What lies underneath: Conserving the oceans’ genetic resources. Proc. Natl. Acad. Sci. USA 2010, 107, 18318–18324. [Google Scholar] [CrossRef] [PubMed]
- MarinLit, The Royal Society of Chemistry. Available online: http://pubs.rsc.org/marinlit/ (accessed on 31 August 2016).
- Storch, V.; Welsch, U. Kükenthal—Zoologisches Praktikum, 27th ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Müller, W.E.G. Origin of Metazoa: Sponges as living fossils. Naturwissenschaften 1998, 85, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-W.; Chen, J.-Y.; Hua, T.-E. Precambrian sponges with cellular structures. Science 1998, 279, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, P.R. Sponges; University of California Press: Berkley, CA, USA, 1978. [Google Scholar]
- Reiswig, H.M. Particle feeding in natural populations of three marine demosponges. Biol. Bull. 1971, 141, 568–591. [Google Scholar] [CrossRef]
- Rohde, S.; Schupp, P.J. Allocation of chemical and structural defenses in the sponge Melophlus sarasinorum. J. Exp. Mar. Biol. Ecol. 2011, 399, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rohde, S.; Nietzer, S.; Schupp, P.J. Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS ONE 2015, 10, e0132236. [Google Scholar] [CrossRef] [PubMed]
- Thoms, C.; Schupp, P.J. Chemical Defense Strategies in Sponges: A Review. In Porifera Research: Biodiversity, Innovation and Sustainability; Custódio, M.R., Lôbo-Hajdu, G., Hajdu, E., Muricy, G., Eds.; Série Livros 28; Museu Nacional: Rio de Janeiro, Brazil, 2007; pp. 627–637. [Google Scholar]
- Thoms, C.; Schupp, P.J. Activated chemical defenses in marine sponges: A case study on Aplysinella rhax. J. Chem. Ecol. 2008, 34, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Thoms, C.; Ebel, R.; Proksch, P. Activated chemical defense in Aplysina sponges revisited. J. Chem. Ecol. 2006, 32, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Putz, A.; Ortlepp, S.; Kjer, J.; Bayer, M. Bioactive natural products from marine sponges and fungal endophytes. Phytochem. Rev. 2010, 9, 475–489. [Google Scholar] [CrossRef]
- Wehner, R.; Gehring, W.J. Zoologie, 24th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2007; pp. 698–699. [Google Scholar]
- Gazave, E.; Lapébie, P.; Renard, E.; Vacelet, J.; Rocher, C.; Ereskovsky, A.V.; Lavrov, D.V.; Borchiellini, C. Molecular Phylogeny Restores the Supra-Generic Subdivision of Homoscleromorph Sponges (Porifera, Homoscleromorpha). PLoS ONE 2010, 5, e14290. [Google Scholar] [CrossRef] [PubMed]
- Gazave, E.; Lapébie, P.; Ereskovsky, A.V.; Vacelet, J.; Renard, E.; Cárdenas, P.; Borchiellini, C. No longer Demospongiae: Homoscleromorpha formal nomination as a fourth class of Porifera. Hydrobiologia 2012, 687, 3–10. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; de Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global diversity of sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergquist, P.R.; Cook, S.D.C. Order Verongida. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 1081–1096. [Google Scholar]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Uriz, M.J.; Turon, X.; Becerro, M.A.; Agell, G. Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrastructural patterns, and biological functions. Microsc. Res. Tech. 2003, 62, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Spongin. In Biological Materials of Marine Origin—Invertebrates, Biologically-Inspired Systems (Book 1); Gorb, S.N., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 245–256. [Google Scholar]
- Junqua, S.; Robert, L.; Garrone, R.; de Ceccatty, M.P.; Vacelet, J. Biochemical and morphological studies on collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.-Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of collagens. Anat. Rec. 2002, 268, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Römpp Online: Spongin. Available online: https://roempp.thieme.de (accessed on 25 August 2016).
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Hackman, R.H. Studies on chitin IV the occurrence of complexes in which chitin and protein are covalently linked. Aust. J. Biol. Sci. 1960, 13, 568–577. [Google Scholar] [CrossRef]
- Blackwell, J.; Weih, M.A. Structure of chitin-protein complexes: Ovipositor of the ichneumon fly Megarhyssa. J. Mol. Biol. 1980, 137, 49–60. [Google Scholar] [CrossRef]
- Blackwell, J.; Germinario, L.T.; Weih, M.A. Chitin-Protein Complexes: Ordered Biopolymer Composites. In Biological Activities of Polymers, ACS Symposium Series; Carraher, C.E., Jr., Gebelein, C.G., Eds.; American Chemical Society: Washington, DC, USA, 1982; pp. 149–162. [Google Scholar]
- Greve, H.; Kehraus, S.; Krick, A.; Kelter, G.; Maier, A.; Fiebig, H.H.; Wright, A.D.; König, G.M. Cytotoxic Bastadin 24 from the australian sponge Ianthella quadrangulata. J. Nat. Prod. 2008, 71, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Calcul, L.; Inman, W.D.; Morris, A.A.; Tenney, K.; Ratnam, J.; McKerrow, J.H.; Valeriote, F.A.; Crews, P. Additional insights on the bastadins: Isolation of analogues from the sponge Ianthella cf. reticulata and exploration of the oxime configuration. J. Nat. Prod. 2010, 73, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Lin, W.H.; Müller, W.E.G.; Kubbutat, M.; Lai, D.W.; Proksch, P. Trimeric hemibastadin congener from the marine sponge Ianthella basta. J. Nat. Prod. 2013, 76, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ortlepp, S.; Sjörgren, M.; Dahlsröm, M.; Weber, H.; Ebel, R.; Edrada, R.; Thoms, C.; Schupp, P.; Bohlin, L.; Proksch, P. Anti-fouling activity of bromotyrosine-derived sponge metabolites and synthetic analogues. Mar. Biotechnol. 2007, 9, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Ciminello, P.; Fattorusso, E.; Forino, M.; Magno, S.; Pansini, M. Chemistry of verongida Sponges VIII—Bromocompounds from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. Tetrahedron 1997, 53, 6565–6572. [Google Scholar] [CrossRef]
- Tymiak, A.A.; Rinehart, L.R., Jr. Biosynthesis of dibromotyrosine-derived antimicrobial compounds by the marine sponge Aplysina fistularis (Verongia aurea). J. Am. Chem. Soc. 1981, 103, 6763–6765. [Google Scholar] [CrossRef]
- Thomson, J.E.; Barrow, K.D.; Faulkner, D.J. Localization of two brominated metabolites, aerothionin and homoaerothionin, in spherulous cells of the marine sponge Aplysina fistularis (=Verongia thiona). Acta Zool. 1981, 64, 199–210. [Google Scholar] [CrossRef]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Distribution of brominated compounds within the sponge Aplysina aerophoba: Coupling of X-ray microanalysis with cryofixation techniques. Cell Tissue Res. 2000, 301, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tabudravu, J.N.; Eijsink, V.G.H.; Gooday, G.W.; Jaspars, M.; Komander, D.; Legg, M.; Synstad, B.; van Aalten, D.M.F. Psammaplin A, a chitinase inhibitor isolated from the fijian marine sponge Aplysinella rhax. Bioorg. Med. Chem. 2002, 10, 1123–1128. [Google Scholar] [CrossRef]
- Saper, J.; White, W.E. Amino-acid composition of sclero-protein of the sponge Hippospongia equina. Nature 1958, 181, 285–286. [Google Scholar] [CrossRef]
- Low, E.M. Halogenated amino acids of the bath sponge. J. Mar. Res. 1951, 10, 239–245. [Google Scholar]
- Ueberlein, S.; Machill, S.; Niemann, H.; Proksch, P.; Brunner, E. The skeletal amino acid composition of the marine demosponge Aplysina cavernicola. Mar. Drugs 2014, 12, 4417–4438. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, P.R.; Kelly-Borges, M. Systematics and biogeography of the genus Ianthella (Demospongiae: Verongida: Ianthellida) in the South-West Pacific. Beagle Rec. North. Territ. Mus. Arts Sci. 1995, 12, 151–176. [Google Scholar]
- Erwin, P.M.; Thacker, R.W. Phylogenetic analyses of marine sponges within the order Verongida: A comparison of morphological and molecular data. Invertebr. Biol. 2007, 126, 220–234. [Google Scholar] [CrossRef]
- Kunze, K.; Niemann, H.; Ueberlein, S.; Schulze, R.; Ehrlich, H.; Brunner, E.; Proksch, P.; van Pée, K.-H. Brominated skeletal components of the marine demosponges Aplysina cavernicola and Ianthella basta: Analytical and biochemical investigations. Mar. Drugs 2013, 11, 1271–1287. [Google Scholar] [CrossRef] [PubMed]
- Rohde, S.; Schupp, P.J. Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera). Hydrobiologia 2012, 687, 219–226. [Google Scholar] [CrossRef]
- Campbell, F.L. The detection and estimation of insect chitin; and the irrelation of “chitinization” to hardness and pigmentation of the cuticula of the american cockroach, periplaneta americana L. Ann. Entomol. Soc. Am. 1929, 22, 401–426. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.-C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Ehrlich, H.; Simon, P.; Carrillo-Cabrera, W.; Bazhenov, V.V.; Botting, J.P.; Ilan, M.; Ereskovsky, A.V.; Muricy, G.; Worch, H.; Mensch, A.; et al. Insights into chemistry of biological materials: Newly discovered silica-Aragonite-Chitin biocomposites in demosponges. Chem. Mater. 2010, 22, 1462–1471. [Google Scholar] [CrossRef]
- North, M. Principles and Applications of Stereochemistry; CRC Press: Cheltenham, UK, 1998; pp. 75–77. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Spektroskopische Daten zur Strukturaufklärung Organischer Verbindungen; 5. Auflage; Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Hunt, S.; Breuer, S.W. Isolation of a new naturally occurring halogenated amino acid: Monochloro-monobromotyrosine. Biochim. Biophys. Acta Gen. Subj. 1971, 252, 401–404. [Google Scholar] [CrossRef]
- Horton, H.R.; Moran, L.A.; Scrimgeour, K.G.; Perry, M.D.; Rawn, J.D. Biochemie; Pearson Studium Verlag: München, Germany, 2008. [Google Scholar]
- Čmelik, S. Über einen Farbstoff von Protein-Natur aus dem Schwamme Aplysina aerophoba Nardo. Hoppe-Seyler´s Z. Physiol. Chem. 1952, 289, 218–220. [Google Scholar] [CrossRef] [PubMed]



| Parameter | I. basta | A. cavernicola |
|---|---|---|
| percentage of skeleton in the sponge | 50.1 ± 20.0 wt. % | 3.1 ± 1.3 wt. % |
| percentage of chitin in the skeleton | 17.1 ± 1.4 wt. % | 8.0 ± 1.4 wt. % |
| percentage of other saccharides in the skeleton | 3–4 wt. % | 1–2 wt. % |
| content of calcium in the skeleton | 15.5 mg/g | 3.5 mg/g |
| estimated content of calcium carbonate in the skeleton | 3.9 wt. % | 0.9 wt. % |
| content of silicon in the skeleton | <1.7 mg/g | <1.7 mg/g |
| estimated content of protein in the skeleton | ≤77 wt. % | ≤90 wt. % |
| content of sulfur in the skeleton | 14.4 mg/g | 11.8 mg/g |
| halogens present in the skeleton | Br, Cl, I | Br, Cl, I |
| bromine content in the skeleton [44] | 51 ± 4 mg/g | 40 ± 3 mg/g |
| Peak | Amino Acid | Proteinogenic | Halogenated |
|---|---|---|---|
| 1 | Alanine | X | |
| 2 | Glycine | X | |
| 3 | α-Aminobutyric Acid (AABA) | ||
| 4 | Valine | X | |
| 5 | Leucine | X | |
| 6a | Serine (2 TBDMS) | X | |
| 7a | Isoleucine | X | |
| 7b | Isoleucine | X | |
| 8 | Proline | X | |
| 9 | Oxoproline | ||
| 10a | Hydroxyproline (2 TBDMS) | ||
| 11 | Methionine | X | |
| 6b | Serine (3 TBDMS) | X | |
| 12a | Threonine (3 TBDMS) | X | |
| 12b | Threonine (3 TBDMS) | X | |
| 13 | Phenylalanine | X | |
| 14 | Aspartic Acid | X | |
| 10b | Hydroxyproline (3 TBDMS) | ||
| 15 | Glutamic Acid | X | |
| 16 | Ornithine | ||
| 17 | Lysine | X | |
| 18 | Arginine | X | |
| 19 | Histidine | X | |
| 20 | Tyrosine | X | |
| 21 | Tryptophan | X | |
| 22 | Hydroxylysine | ||
| 23 | 3-Monochlorotyrosine | X | |
| 24 * | Monobromotyrosine | X | |
| 25 * | Dichlorotyrosine | X | |
| 26 | 3-Monoiodotyrosine | X | |
| 27 * | Monobromo-monochlorotyrosine | X | |
| 28 | 3,5-Dibromotyrosine | X | |
| 29 * | Monochloro-monoiodotyrosine | X | |
| 30 * | Monobromo-monoiodotyrosine | X | |
| 31 | 3,5-Diiodotyrosine | X |
| Halogenation State | Amino Acid | Ianthella basta | Aplysina cavernicola [41] | Hippospongia equina [39] | Spongia officinalis obliqua [40] |
|---|---|---|---|---|---|
| Alanine | X | X | X | X | |
| Non-halogenated | α-Aminobutyric Acid (AABA) | X | X | X | |
| γ-Aminobutyric Acid (GABA) | X | ||||
| Arginine | X | X | X | ||
| Aspartic Acid | X | X | X | X | |
| Cystine | X | ||||
| Glutamic Acid | X | X | X | X | |
| Glycine | X | X | X | X | |
| Histidine | X | X | X | ||
| Hydroxylysine | X | ||||
| Hydroxyproline | X | X | X | X | |
| Isoleucine | X | ||||
| Leucine | X | X | X | X | |
| Lysine | X | X | X | X | |
| Methionine | X | X | X | ||
| Ornithine | X | X | X | ||
| Oxoproline | X | X | |||
| Phenylalanine | X | X | X | ||
| Proline | X | X | X | X | |
| Serine | X | X | X | ||
| Threonine | X | X | X | ||
| Tryptophan | X | X | X | X | |
| Tyrosine | X | X | X | X | |
| Valine | X | X | X | X | |
| Halogenated | Monobromohistidine | X | |||
| Monobromotyrosine | X | X | |||
| 3-Monochlorotyrosine | X | X | |||
| 3-Monoiodotyrosine | X | X | X | ||
| Monochloro-monoiodotyrosine | X | X | |||
| Monobromo-monochlorotyrosine | X | X | |||
| Monobromo-monoiodotyrosine | X | X | |||
| Dichlorotyrosine | X | X | |||
| 3,5-Dibromotyrosine | X | X | X | ||
| 3,5-Diiodotyrosine | X | X | X | X |
| Halogenation State | Amino Acids | Amounts in | |
|---|---|---|---|
| Ianthella basta | Aplysina cavernicola [41] | ||
| Non-halogenated | Glycine | ++++ | ++++ |
| Alanine | +++ | +++ | |
| Aspartic Acid | +++ | ++ | |
| Glutamic Acid | +++ | ++ | |
| Hydroxyproline | +++ | +++ | |
| Leucine | +++ | + | |
| Lysine | +++ | +++ | |
| Ornithine | +++ | +++ | |
| Proline | +++ | +++ | |
| Tyrosine | +++ | +++ | |
| Oxoproline | ++ | + | |
| Phenylalanine | ++ | + | |
| Serine | ++ | ++++ | |
| Valine | ++ | ++ | |
| Arginine | + | + | |
| α-Aminobutyric Acid (AABA) | + | + | |
| Histidine | + | ++ | |
| Hydroxylysine | + | - | |
| Isoleucine | + | - | |
| Methionine | + | + | |
| Threonine | + | +++ | |
| Tryptophan | + | + | |
| Monobromo-monochlorotyrosine | ++ | +++ | |
| 3,5-Dibromotyrosine | ++ | +++ | |
| Monobromotyrosine | + | ++ | |
| Halogenated | 3-Monochlorotyrosine | + | + |
| 3-Monoiodotyrosine | + | + | |
| Monochloro-monoiodotyrosine | + | + | |
| Monobromo-monoiodotyrosine | + | + | |
| Dichlorotyrosine | + | ++ | |
| 3,5-Diiodotyrosine | + | + | |
| Monobromohistidine | - | + | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueberlein, S.; Machill, S.; Schupp, P.J.; Brunner, E. Determination of the Halogenated Skeleton Constituents of the Marine Demosponge Ianthella basta. Mar. Drugs 2017, 15, 34. https://doi.org/10.3390/md15020034
Ueberlein S, Machill S, Schupp PJ, Brunner E. Determination of the Halogenated Skeleton Constituents of the Marine Demosponge Ianthella basta. Marine Drugs. 2017; 15(2):34. https://doi.org/10.3390/md15020034
Chicago/Turabian StyleUeberlein, Susanne, Susanne Machill, Peter J. Schupp, and Eike Brunner. 2017. "Determination of the Halogenated Skeleton Constituents of the Marine Demosponge Ianthella basta" Marine Drugs 15, no. 2: 34. https://doi.org/10.3390/md15020034
APA StyleUeberlein, S., Machill, S., Schupp, P. J., & Brunner, E. (2017). Determination of the Halogenated Skeleton Constituents of the Marine Demosponge Ianthella basta. Marine Drugs, 15(2), 34. https://doi.org/10.3390/md15020034