Metabolic Profiling as a Screening Tool for Cytotoxic Compounds: Identification of 3-Alkyl Pyridine Alkaloids from Sponges Collected at a Shallow Water Hydrothermal Vent Site North of Iceland
Abstract
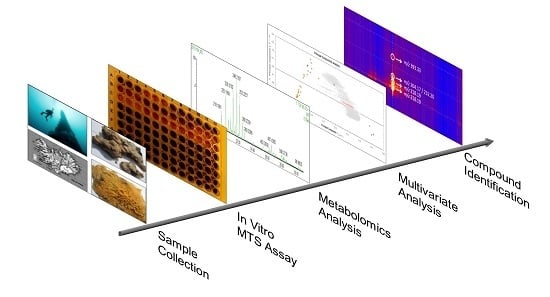
:1. Introduction
2. Results
2.1. Metabolic Profiling
2.2. Characterization of 3-APAs in Haliclona rosea Extracts
2.3. Cyclostellettamine P by Ion Mobility Mass Spectrometry
2.4. 3-APAs in Haliclona rosea
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Collection, Identification and Extraction of Sponges
4.3. Partition of Haliclona rosea Extracts and Isolation of Cyclostellettamines
4.4. MTS Assay—Measurement of Cell Viability (Cytotoxicity)
4.5. Mass Spectrometry Based Metabolomics
4.6. Data Processing and Analysis
4.7. Ion Mobility Mass Spectrometry
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, M.B. Mid-Ocean ridges. In Igneous Petrogenesis; Wilson, M., Ed.; Springer: Dordrecht, The Netherlands, 1989. [Google Scholar]
- Lebar, M.D.; Heimbegner, J.L.; Baker, B.J. Cold-water marine natural products. Nat. Prod. Rep. 2007, 24, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Marteinsson, V.T.; Kristjansson, J.K.; Kristmannsdottir, H.; Dahlkvist, M.; Saemundsson, K.; Hannington, M.; Petursdottir, S.K.; Geptner, A.; Stoffers, P. Discovery and description of giant submarine smectite cones on the seafloor in Eyjafjordur, northern Iceland, and a novel thermal microbial habitat. Appl. Environ. Microbiol. 2001, 67, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, J.R. Marine invertebrate chemical defenses. Chem. Rev. 1993, 93, 1911–1922. [Google Scholar] [CrossRef]
- Lopanik, N.B. Chemical defensive symbioses in the marine environment. Funct. Ecol. 2013, 28, 328–340. [Google Scholar] [CrossRef]
- Pawlik, J.R.; Loh, T.L.; McMurray, S.E.; Finelli, C.M. Sponge communities on Caribbean coral reefs are structured by factors that are top-down, not bottom-up. PLoS ONE 2013, 8, e62573. [Google Scholar] [CrossRef] [PubMed]
- MarinLit, A Database of the Marine Natural Products Literature. Available online: http://pubs.rsc.org/marinlit (accessed on 10 December 2016).
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepčić, K. Bioactive alkylpyridinium compounds from marine sponges. J. Toxicol.-Toxin Rev. 2000, 19, 139–160. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1999, 16, 155–198. [Google Scholar] [CrossRef]
- Köck, M.; Muñoz, J.; Cychon, C.; Timm, C.; Schmidt, G. The Arctic sponge Haliclona viscosa as a source of a wide array of 3-alkyl pyridine alkaloids. Phytochem. Rev. 2013, 12, 391–406. [Google Scholar] [CrossRef]
- Schmidt, G.; Timm, C.; Köck, M. Haliclocyclin C, a new monomeric 3-alkyl pyridinium alkaloid from the arctic marine sponge Haliclona viscosa. Z. Naturforschung Sect. B J. Chem. Sci. 2011, 66, 745–748. [Google Scholar] [CrossRef]
- Teruya, T.; Kobayashi, K.; Suenaga, K.; Kigoshi, H. Cyclohaliclonamines A–E: Dimeric, trimeric, tetrameric, pentameric, and hexameric 3-alkyl pyridinium alkaloids from a marine sponge Haliclona sp. J. Nat. Prod. 2006, 69, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Turk, T.; Sepčić, K.; Mancini, I.; Guella, G. 3-Alkylpyridinium and 3-alkylpyridine compounds from marine sponges, their synthesis, biological activities and potential use. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 35, pp. 355–397. [Google Scholar]
- Sepčić, K.; Guella, G.; Mancini, I.; Pietra, F.; Serra, M.D.; Menestrina, G.; Tubbs, K.; Macek, P.; Turk, T. Characterization of anticholinesterase-active 3-alkylpyridinium polymers from the marine sponge Reniera sarai in aqueous solutions. J. Nat. Prod. 1997, 60, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Timm, C.; Volk, C.; Sasse, F.; Köck, M. The first cyclic monomeric 3-alkylpyridinium alkaloid from natural sources: Identification, synthesis, and biological activity. Org. Biomol. Chem. 2008, 6, 4036–4040. [Google Scholar] [CrossRef] [PubMed]
- Timm, C.; Mordhorst, T.; Köck, M. Synthesis of 3-alkyl pyridinium alkaloids from the Arctic sponge Haliclona viscosa. Mar. Drugs 2010, 8, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; Hollenbeak, K.H.; Campbell, D.C. Marine natural products—Halitoxin, toxic complex of several marine sponges of genus Haliclona. J. Org. Chem. 1978, 43, 3916–3922. [Google Scholar] [CrossRef]
- Sakai, R.; Higa, T.; Jefford, C.W.; Bernardinelli, G. Manzamine A, a novel antitumor alkaloid from a sponge. J. Am. Chem. Soc. 1986, 108, 6404–6405. [Google Scholar] [CrossRef]
- Cimino, G.; de Stefano, S.; Scognamiglio, G.; Sodano, G.; Trivellone, E. Sarains—A new class of alkaloids from the marine sponge Reniera-Sarai. Bulletin Des Sociétés Chimiques Belges 1986, 95, 783–800. [Google Scholar] [CrossRef]
- Volk, C.A.; Köck, M. Viscosamine: The first naturally occurring trimeric 3-alkyl pyridinium alkaloid. Org. Lett. 2003, 5, 3567–3569. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Larance, M.; Lamond, A.I. Multidimensional proteomics for cell biology. Nat. Rev. Mol. Cell Biol. 2015, 16, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; Choi, Y.H.; Kim, H.K. NMR-based metabolomics at work in phytochemistry. Phytochem. Rev. 2007, 6, 3–14. [Google Scholar] [CrossRef]
- Leiss, K.A.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. An overview of NMR-based metabolomics to identify secondary plant compounds involved in host plant resistance. Phytochem. Rev. 2011, 10, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Magnusdottir, M.; Brynjolfson, S.; Palsson, B.O.; Paglia, G. UPLC-UV-MS(E) analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Anal. Bioanal. Chem. 2012, 404, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Pacini, T.; Fu, W.; Gudmundsson, S.; Chiaravalle, A.E.; Brynjolfson, S.; Palsson, B.O.; Astarita, G.; Paglia, G. Multidimensional analytical approach based on UHPLC-UV-ion mobility-MS for the screening of natural pigments. Anal. Chem. 2015, 87, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jang, K.H.; Jeon, J.-E.; Yang, W.-Y.; Sim, C.J.; Oh, K.-B.; Shin, J. Cyclic Bis-1,3-dialkylpyridiniums from the Sponge Haliclona sp. Mar. Drugs 2012, 10, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.H.H.L.; Seleghim, M.H.R.; Timm, C.; Grube, A.; Köck, M.; Nascimento, G.G.F.; Martins, A.C.T.; Silva, E.G.O.; de Souza, A.O.; Minarini, P.R.R.; et al. Antimicrobial and antimycobacterial activity of cyclostellettamine alkaloids from sponge Pachychalina sp. Mar. Drugs 2006, 4, 1–8. [Google Scholar] [CrossRef]
- Castro-Perez, J.; Roddy, T.P.; Nibbering, N.M.; Shah, V.; McLaren, D.G.; Previs, S.; Attygalle, A.B.; Herath, K.; Chen, Z.; Wang, S.P.; et al. Localization of fatty acyl and double bond positions in phosphatidylcholines using a dual stage CID fragmentation coupled with ion mobility mass spectrometry. J. Am. Soc. Mass Spectrom. 2011, 22, 1552–1567. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Kliman, M.; Claude, E.; Geromanos, S.; Astarita, G. Applications of ion-mobility mass spectrometry for lipid analysis. Anal. Bioanal. Chem. 2015, 407, 4995–5007. [Google Scholar] [CrossRef]
- Grube, A.; Timm, C.; Köck, M. Synthesis and mass spectrometric analysis of cyclostellettamines H, I, K and L. Eur. J. Org. Chem. 2006, 2006, 1285–1295. [Google Scholar] [CrossRef]
- Paleari, L.; Trombino, S.; Falugi, C.; Gallus, L.; Carlone, S.; Angelini, C.; Sepčić, K.; Turk, T.; Faimali, M.; Noonan, D.M.; et al. Marine sponge-derived polymeric alkylpyridinium salts as a novel tumor chemotherapeutic targeting the cholinergic system in lung tumors. Int. J. Oncol. 2006, 29, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Kubota, T.; Tsuda, M.; Mikami, Y.; Kobayashi, J. Pyrinodemins B-D, potent cytotoxic bis-pyridine alkaloids from marine sponge Amphimedon sp. Chem. Pharm. Bull. 2000, 48, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N.; Asai, N.; Matsunaga, S.; Honda, K.; Yasumuro, K. Cyclostellettamines A-F, pyridine alkaloids which inhibit binding of methyl quinuclidinyl benzilate (QNB) to muscarinic acetylcholine receptors, from the marine sponge, Stelletta maxima. Tetrahedron Lett. 1994, 35, 3967–3970. [Google Scholar] [CrossRef]
- Paglia, G.; Williams, J.P.; Menikarachchi, L.; Thompson, J.W.; Tyldesley-Worster, R.; Halldorsson, S.; Rolfsson, O.; Moseley, A.; Grant, D.; Langridge, J.; et al. Ion mobility derived collision cross sections to support metabolomics applications. Anal. Chem. 2014, 86, 3985–3993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paglia, G.; Angel, P.; Williams, J.P.; Richardson, K.; Olivos, H.J.; Thompson, J.W.; Menikarachchi, L.; Lai, S.; Walsh, C.; Moseley, A.; et al. Ion-Mobility-Derived Collision Cross Section as an Additional Measure for Lipid Fingerprinting and Identification. Anal. Chem. 2015, 87, 1137–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacristán-Soriano, O.; Banaigs, B.; Becerro, M.A. Temporal Trends in the Secondary Metabolite Production of the Sponge Aplysina aerophoba. Mar. Drugs 2012, 10, 677–693. [Google Scholar] [CrossRef] [PubMed]
- De Weerdt, W.H. A systematic revision of the North-Eastern Atlantic shallow-water Haplosclerida (Porifera, Demospongiae), Part II: Chalinidae. Beaufortia 1986, 36, 81–165. [Google Scholar]
- Lundbeck, W. Porifera. (Part I.) Homorrhaphidae and Heterorrhaphidae. In The Danish Ingolf-Expedition. 6(1). (Bianco Luno: Copenhagen); Forgotten Books: London, UK, 1902; pp. 1–108. [Google Scholar]
- Van Soest, R. Haliclona (Rhizoniera) rosea (Bowerbank, 1866). Van Soest, R.W.M., Boury-Esnault, N., Hooper, J.N.A., Rützler, K., de Voogd, N.J., Alvarez de Glasby, B., Hajdu, E., Pisera, A.B., Manconi, R., Schoenberg, C., et al., Eds.; 2016. Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=166637 (accessed on 11 January 2017).
- Kupchan, S.M.; Britton, R.W.; Ziegler, M.F.; Sigel, C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]







| Sample Name | MTS Results (% Viability of Cells) | Identification | Depth (m) |
|---|---|---|---|
| S1 | 8% | Haliclona rosea | 24 |
| S2 | 29% | Halichondria sitiens | 24 |
| S3 | 51% | Myxilla incrustans | 24 |
| S4 | 18% | Halichondria panicea | 27 |
| S5 | 31% | Haliclona rosea | 25 |
| S6 | 31% | Lissodendoryx fragilis | 24 |
| S7 | 13% | Halichondria panicea | 27 |
| S8 | 22% | Haliclona rosea | 28 |
| Pyr/THP (Moiety) | Oligomer | Compound Name | Formula | HRMS [M + H]+ (Calcd. Mass) | HRMS [M + 2H]2+ (Calcd. Mass) | MS/MS Main Fragments (F1 and F2) |
|---|---|---|---|---|---|---|
| Pyr | dimer (cyclic) | Cyclostellettamine P | C30H49N2 | 437.3896 Δppm 1 | 218.1909 | 204.1752 (C14H22N) |
| 232.2065 (C16H26N) | ||||||
| Pyr | dimer (cyclic) | Cyclostellettamine Q | C31H51N2 | 451.4052 Δppm 3 | 225.1909 | 218.1909 (C15H24N) |
| 232.2065 (C16H26N) | ||||||
| Pyr | dimer (cyclic) | Cyclostellettamine N | C29H47N2 | 423.3779 Δppm 9 | 211.1803 | 204.1752 (C14H22N) |
| 218.1909 (C15H24N) | ||||||
| Pyr | dimer (cyclic) | Cyclostellettamine G | C33H55N2 | 479.4365 Δppm 10 | 239.211 | 232.2065 (C16H26N) |
| 246.1752 (C17H28N) | ||||||
| Pyr | dimer (cyclic) | Cyclostellettamine A | C34H57N2 | 493.4522 Δppm 3 | 246.215 | 261.2331 (C17H29N) |
| 261.2331 (C17H29N) | ||||||
| THP | dimer (cyclic) | Haliclamine A | C31H53N2 | 453.4209 Δppm 8 | 227.2113 | 204.1752 (C14H22N) |
| 246.2222 (C17H28N) | ||||||
| THP | dimer (cyclic) | Haliclamine C | C30H55N2 | 443.4365 Δppm 8 | 222.2222 | 208.2065 (C14H26N) |
| 236.2378 (C16H30N) | ||||||
| THP | dimer (cyclic) | Haliclamine D | C31H57N2 | 457.4522 Δppm 6 | 229.2259 | 222.2222 (C15H28N) |
| 236.2378 (C16H30N) | ||||||
| THP | dimer (cyclic) | Haliclamine E | C29H53N2 | 429.4209 Δppm 1 | 215.2089 | 208.2065 (C14H26N) |
| 222.2222 (C15H28N) | ||||||
| THP | dimer (cyclic) | Haliclamine H | C32H59N2 | 471.4678 Δppm 4 | 236.2378 | 222.2222 (C15H28N) |
| 250.2535 (C17H32N) | ||||||
| Pyr | trimer (cyclic) | Viscosamine C | C54H90N3 | 260.2382 [M]3+ | n/a | 390.8637 (C54H91N3) |
| 389.8637 (C54H89N3) | ||||||
| Pyr | Linear | Viscosaline B2 | C38H64N3O2 | 594.4999 Δppm 4 | 246.2191/297.7537 | 253.2321 (C35H58N2) |
| 267.7429 (C36H61N3) | ||||||
| Pyr | Linear | Viscosaline C | C39H67N3O2 | 608.5155 Δppm 5 | 253.2264/ 304.7621 | 260.2376 (C36H60N2) |
| 274.7509 (C37H63N3) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Einarsdottir, E.; Magnusdottir, M.; Astarita, G.; Köck, M.; Ögmundsdottir, H.M.; Thorsteinsdottir, M.; Rapp, H.T.; Omarsdottir, S.; Paglia, G. Metabolic Profiling as a Screening Tool for Cytotoxic Compounds: Identification of 3-Alkyl Pyridine Alkaloids from Sponges Collected at a Shallow Water Hydrothermal Vent Site North of Iceland. Mar. Drugs 2017, 15, 52. https://doi.org/10.3390/md15020052
Einarsdottir E, Magnusdottir M, Astarita G, Köck M, Ögmundsdottir HM, Thorsteinsdottir M, Rapp HT, Omarsdottir S, Paglia G. Metabolic Profiling as a Screening Tool for Cytotoxic Compounds: Identification of 3-Alkyl Pyridine Alkaloids from Sponges Collected at a Shallow Water Hydrothermal Vent Site North of Iceland. Marine Drugs. 2017; 15(2):52. https://doi.org/10.3390/md15020052
Chicago/Turabian StyleEinarsdottir, Eydis, Manuela Magnusdottir, Giuseppe Astarita, Matthias Köck, Helga M. Ögmundsdottir, Margret Thorsteinsdottir, Hans Tore Rapp, Sesselja Omarsdottir, and Giuseppe Paglia. 2017. "Metabolic Profiling as a Screening Tool for Cytotoxic Compounds: Identification of 3-Alkyl Pyridine Alkaloids from Sponges Collected at a Shallow Water Hydrothermal Vent Site North of Iceland" Marine Drugs 15, no. 2: 52. https://doi.org/10.3390/md15020052
APA StyleEinarsdottir, E., Magnusdottir, M., Astarita, G., Köck, M., Ögmundsdottir, H. M., Thorsteinsdottir, M., Rapp, H. T., Omarsdottir, S., & Paglia, G. (2017). Metabolic Profiling as a Screening Tool for Cytotoxic Compounds: Identification of 3-Alkyl Pyridine Alkaloids from Sponges Collected at a Shallow Water Hydrothermal Vent Site North of Iceland. Marine Drugs, 15(2), 52. https://doi.org/10.3390/md15020052