Fatty Acid Composition and Thermotropic Behavior of Glycolipids and Other Membrane Lipids of Ulva lactuca (Chlorophyta) Inhabiting Different Climatic Zones
Abstract
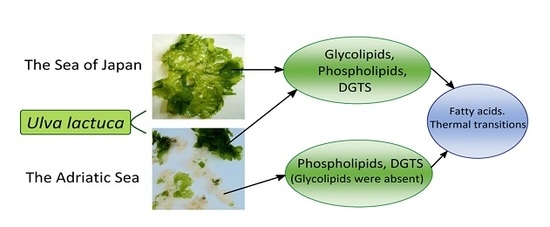
:1. Introduction
2. Results and Discussion
2.1. The Fatty Acid Composition of Phospholipids
2.2. The Fatty Acid Composition of Betaine Lipid 1,2-diacylglycero-O-4’-(N,N,N-tri-methyl)-homoserine (DGTS)
2.3. The Fatty Acid Composition of Glycolipids
2.4. Thermotropic Behavior and Molecular Species of Polar Lipids
3. Materials and Methods
3.1. Plants
3.2. Extraction and Isolation of Lipids
3.3. Analysis of the FattyAcid Composition
3.4. Calorimetry
3.5. Analysis of the Molecular Species Composition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahlstein, I.; Daniel, J.S.; Solomon, S. Pace of shifts in climate regions increases with global temperature. Nature Clim. Chang. 2013, 3, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Beney, L.; Gervais, P. Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl. Microbiol. Biotechnol. 2001, 57, 34–42. [Google Scholar] [PubMed]
- Török, Z.; Tsvetkova, N.M.; Balogh, G.; Horváth, I.; Nagy, E.; Pénzes, Z.; Hargitai, J.; Bensaude, O.; Csermely, P.; Crowe, J.H.; et al. Heat shock protein coinducers with no effect on protein denaturation specifically modulate the membrane lipid phase. Proc. Natl. Acad. Sci. USA 2003, 100, 3131–3136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinensky, M. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous adaptation and the regulation of membrane lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef] [PubMed]
- Sanina, N.M.; Goncharova, S.N.; Kostetsky, E.Y. Seasonal change of fatty acid composition and thermotropic behavior of polar lipids marine macrophytes. Phytochemistry 2008, 69, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Tchernov, D.; Gorbunov, M.Y.; de Vargas, C.; Narayan Yadav, S.; Milligan, A.; Haggblom, M.; Falkowski, P. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA 2004, 101, 13531–13535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, H.C.C.; Asaeda, T. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J. Plant Interact. 2017, 12, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Kostetsky, E.Y.; Sanina, N.M.; Mazeika, A.N.; Tsybulsky, A.V.; Vorobyeva, N.S.; Shnyrov, V.L. Tubular immunostimulating complex based on cucumarioside A2-2 and monogalactosyldiacylglycerol from marine macrophytes. J. Nanobiotechnol. 2011, 9, 9–35. [Google Scholar] [CrossRef]
- Sanina, N.M.; Kostetsky, E.Y.; Shnyrov, V.L.; Tsybulsky, A.V.; Novikova, O.D.; Portniagina, O.Y.; Vorobieva, N.S.; Mazeika, A.N.; Bogdanov, M.V. The influence of monogalactosyldiacylglycerols from different marine macrophytes on immunogenicity and conformation of protein antigen of tubular immunostimulating complex. Biochimie 2012, 94, 1048–1056. [Google Scholar] [CrossRef]
- Sanina, N.; Davydova, L.; Chopenko, N.; Kostetsky, E.; Shnyrov, V. Modulation of immunogenicity and conformation of HA1 subunit of influenza A virus H1/N1 hemagglutinin in tubular immunostimulating complexes. Int. J. Mol. Sci. 2017, 18, E1895. [Google Scholar] [CrossRef] [PubMed]
- Sanina, N.M.; Goncharova, S.N.; Kostetsky, E.Y. Fatty acid composition of individual polar lipid classes from marine macrophytes. Phytochemistry 2004, 65, 721–730. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rozentsvet, O.A. Diacylgliceryltrimethylhomoserines and phospholipids of some marine macrophytes. Phytochemistry 1989, 28, 3341–3343. [Google Scholar] [CrossRef]
- Van Ginneken, V.; Gittenberger, A.; Rensing, M.; de Vries, E.; Peeters, E.T.H.M.; Verheij, E. Seaweed competition: Ulva sp. has the potential to produce the betaine lipid diacylglyceryl-O-4’-(N,N,N-trimethyl) homoserine (DGTS) in order to replace phosphatidylcholine (PC) under phosphate-limiting conditions in the P-limited Dutch Wadden Sea and outcompete an Aggressive non-indigenous Gracilaria vermiculophylla red drift algae out of this Unique Unesco world heritage coastal area. Ocean & Fish Open Access J. 2017, 2, 555596. [Google Scholar] [CrossRef]
- Kostetsky, E.Y.; Goncharova, S.N.; Sanina, N.M.; Shnyrov, V.L. Season influence on lipid composition of marine macrophytes. Bot. Mar. 2004, 47, 134–139. [Google Scholar] [CrossRef]
- Kalita, T.L.; Tytlianov, E.A. Effect of temperature and illumination on growth and reproduction of the green alga Ulva fenestrata. Russ. J. Mar. Biol. 2003, 29, 316–322. [Google Scholar] [CrossRef]
- Kobayashi, K.; Endo, K.; Wada, H. Roles of lipids in photosynthesis. In Lipids in Plant and Algae Development; Subcell, B., Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Basel, Switzerland, 2016; Volume 86, pp. 21–50. ISBN 978-3-319-25979-6. [Google Scholar]
- Wang, Z.; Benning, C. Chloroplast lipid synthesis and lipid trafficking through ER–plastid membrane contact sites. Biochem. Soc. Trans. 2012, 40, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hölzl, G.; vom Dorp, K.; Peisker, H.; Melzer, M.; Frentzen, M.; Dörmann, P. Identification and characterization of a plastidial phosphatidylglycerophosphate phosphatase in Arabidopsis thaliana. Plant J. 2017, 89, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Hansen, H.S.; Jorgesen, M.H.; Michaelsen, K.F. The essentiality of long chains n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Bagga, D.; Wang, L.; Farias-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Bergenthal, D.; Kokate, C.K.; Mangold, H.K. Biologically active ether lipids: incorporation of long-chain precursors into 1(3),2-diacylglycero-3(1)-O-4’-(N,N,N-trimethyl)homoserines and other lipids of Chlorella fusca. J. Lipid Mediat. 1989, 1, 37–48. [Google Scholar] [PubMed]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and glycolipids in plants and algae. In Lipids in Plant and Algae Development; Subcell, B., Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Basel, Switzerland, 2016; Volume 86, pp. 51–84. ISBN 978-3-319-25979-6. [Google Scholar]
- Gombos, Z.; Wada, H.; Murata, N. The recovery of photosynthesis from low-temperature photoinhibition is accelerated by the unsaturation of membrane lipids: a mechanism of chilling tolerance. Proc. Natl. Acad. Sci. USA 1994, 91, 8787–8791. [Google Scholar] [CrossRef]
- Vigh, L.; Nakamoto, H.; Landry, J.; Gomez-Munos, A.; Harwood, J.L.; Horvath, I. Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann. N. Y. Acad. Sci. 2007, 1113, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Li, S. Calorimetric and molecular mechanics studies of the thermotropic phase behavior of membrane phospholipids. Biochim. Biophys. Acta 1999, 1422, 273–307. [Google Scholar] [CrossRef]
- Raison, J.K.; Wright, L.C. Thermal phase-transitions in the polar lipids of plant membranes—Their induction by desaturated phospholipids and their possible relation to chilling injury. Biochim. Biophys. Acta 1983, 731, 69–78. [Google Scholar] [CrossRef]
- Welti, R.; Glaser, M. Lipid domains in model and biological membranes. Chem. Phys. Lipids 1994, 73, 121–137. [Google Scholar] [CrossRef]
- Brown, M.F. Curvature forces in membrane lipid−protein interactions. Biochemistry 2012, 51, 9782–9795. [Google Scholar] [CrossRef]
- Garab, G.; Ughy, B.; Goss, R. Role of MGDG and non-bilayer lipid phases in the structure and dynamics of chloroplast thylakoid membranes. In Lipids in Plant and Algae Development; Subcell, B., Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Basel, Switzerland, 2016; Volume 86, pp. 127–157. ISBN 978-3-319-25979-6. [Google Scholar]
- Georgiev, G.A.; Ivanova, S.; Jordanova, A.; Tsanova, A.; Getov, V.; Dimitrov, M.; Lalchev, Z. Interaction of monogalactosyldiacylglycerol with cytochrome b6f complex in surface films. Biochem. Biophys. Res. Commun. 2012, 419, 648–651. [Google Scholar] [CrossRef]
- Joyard, J.; Teyssier, E.; Miège, C.; Seigneurin-Berny, D.; Maréchal, E.; Block, M.; Dorne, A.-J.; Rolland, N.; Ajlani, G.; Douce, R. The biochemical machinery of plastid envelope membranes. Plant Physiol. 1998, 118, 715–723. [Google Scholar] [CrossRef]
- Arao, T.; Yamada, M. Positional distribution of fatty acids in galactolipids of algae. Phytochemistry 1989, 28, 805–810. [Google Scholar] [CrossRef]
- Keough, K.M.W.; Giffin, B.; Kariel, N. The influence of unsaturation on the phase transition temperatures of a series of heteroacid phosphatidylcholines containing twenty-carbon chains. Biochim. Biophys. Acta 1987, 902, 1–10. [Google Scholar] [CrossRef]
- Kostetsky, E.Y.; Sanina, N.M.; Naumenko, N.V. The influence of fatty acid composition on the profile of the phase transition thermogram of phosphatidylcholine from holothurians Cucumaria fraudatrix. Zh. Evol. Biochem. Physiol. 1992, 28, 426–433. [Google Scholar]
- Demé, B.; Cataye, C.; Block, M.A.; Maréchal, E.; Jouhet, J. Contribution of galactoglycerolipids to the 3-dimensional architecture of thylakoids. FASEB J. 2014, 28, 3373–3383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, J.R.; Staehelin, A.L. Three-dimensional architecture of grana and stroma thylakoids of higher plants as determined by electron tomography. Plant Physiol. 2011, 155, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, H.; Sharpe, R.M.; Herbstova, M.; Yarbrough, R.; Edwards, G.E. Differential mobility of pigment-protein complexes in granal and agranal thylakoid membranes of C3 and C4 plants. Plant Physiol. 2013, 161, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, K.L. Manual of Algae of Far Eastern Seas of the USSR. Green Algae; Nauka: Leningrad, Russia, 1979; pp. 46–110. [Google Scholar]
- Bligh, E.G.; Dyer, W.I. A rapid method of total lipid extraction and purification. Canad. J. Biochem. Physiol. 1959, 37, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Vaskovsky, V.E.; Terekhova, T.A. HPTLC of phospholipids mixtures containing phosphatidylglycerol. J. High Resolut. Chromatogr. 1979, 2, 671–672. [Google Scholar] [CrossRef]
- Vaskovsky, V.E.; Khotimchenko, S.V. HPTLC of polar lipids of algae and other plants. J. Chromatogr. 1982, 5, 635–636. [Google Scholar] [CrossRef]
- Kostetsky, E.Y.; Velansky, P.V.; Sanina, N.M. Phase transition of phospholipids as criterion for assessing the capacity for thermal adaptation in fish. Russ. J. Mar. Biol. 2013, 39, 136–143. [Google Scholar] [CrossRef]


| Fatty Acids | Phosphatidylglycerol | Phosphatidylethanolamine | ||||
|---|---|---|---|---|---|---|
| The Sea of Japan | The Adriatic Sea | The Sea of Japan | The Adriatic Sea | |||
| Green Thalli | Bleached Thalli | Green Thalli | Bleached Thalli | |||
| 16:0 | 35.5 ± 1.2 | 45.2 ± 0.8 | 56.2 ± 1.4 | 22.0 ± 0.6 | 29.7 ± 0.7 | 56.2 ± 1.3 |
| 16:1 (n-7) | 0.9 ± 0.1 | 2.6 ± 0.2 | 2.1 ± 0.1 | 5.4 ± 0.3 | 4.4 ± 0.2 | 2.2 ± 0.1 |
| 16:3 (n-3) | 8.6 ± 0.3 | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | tr. |
| 16:4 (n-3) | 2.1 ± 0.2 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.1 | 6.7 ± 0.3 | 0.9 ± 0.1 |
| 18:0 | 0.3 ± 0.1 | 0.5 ± 0.1 | 1.5 ± 0.1 | 4.6 ± 0.3 | 2.0 ± 0.1 | 2.8 ± 0.2 |
| 18:1 (n-9) | 0.6 ± 0.1 | 2.3 ± 0.2 | 2.8 ± 0.2 | 4.2 ± 0.2 | 3.4 ± 0.2 | 4.7 ± 0.2 |
| 18:1 (n-7) | 17.0 ± 0.7 | 19.6 ± 0.5 | 16.9 ± 0.3 | 10.5 ± 0.8 | 14.3 ± 0.5 | 18.7 ± 0.3 |
| 18:2 (n-6) | 2.3 ± 0.2 | 2.5 ± 0.1 | 1.4 ± 0.1 | 2.7 ± 0.2 | 5.8 ± 0.4 | 2.1 ± 0.1 |
| 18:3 (n-3) | 28.1 ± 0.8 | 14.1 ± 0.3 | 3.9 ± 0.1 | 26.2 ± 0.5 | 9.7 ± 0.3 | 2.5 ± 0.1 |
| 18:4 (n-3) | 1.9 ± 0.1 | 6.1 ± 0.2 | 2.0 ± 0.1 | 1.4 ± 0.1 | 9.8 ± 0.2 | 1.5 ± 0.1 |
| 22:6 (n-3) | tr. | 1.3 ± 0.1 | 0.9 ± 0.1 | 2.2 ± 0.1 | 4.5 ± 0.1 | 0.3 ± 0.1 |
| SFA | 36.4 ± 1.0 | 46.5 ± 0.7 | 60.7 ± 0.5 | 30.0 ± 0.5 | 34.6 ± 0.5 | 61.5 ± 0.7 |
| MUFA | 19.3 ± 0.4 | 25.5 ± 0.6 | 28.0 ± 0.3 | 24.0 ± 0.4 | 26.6 ± 0.6 | 29.2 ± 0.4 |
| PUFA | 44.3 ± 0.3 | 28.0 ± 0.4 | 11.3 ± 0.3 | 46.0 ± 0.7 | 38.8 ± 0.4 | 9.3 ± 0.3 |
| n-3/n-6 PUFA | 16.1 ± 0.3 | 6.5 ± 0.2 | 3.1 ± 0.1 | 9.9 ± 0.2 | 5.0 ± 0.2 | 1.9 ± 0.1 |
| UI | 152 ± 2 | 119 ± 1 | 66 ± 1 | 173 ± 2 | 183 ± 2 | 58 ± 1 |
| UFA/SFA | 1.7 ± 0.1 | 1.1 ± 0.2 | 0.6 ± 0.1 | 2.3 ± 0.1 | 1.9 ± 0.1 | 0.6 ± 0.1 |
| Fatty Acids | The Sea of Japan | The Adriatic Sea | |
|---|---|---|---|
| Green Thalli | Bleached Thalli | ||
| 14:0 | 0.4 ± 0.1 | 1.2 ± 0.1 | 2.3 ± 0.1 |
| 16:0 | 23.0 ± 0.3 | 31.0 ± 0.2 | 38.3 ± 0.3 |
| 16:1 (n-9) | 0.4 ± 0.1 | 1.0 ± 0.1 | 4.3 ± 0.2 |
| 16:1 (n-7) | 1.5 ± 0.1 | 5.8 ± 2 | 5.3 ± 0.2 |
| 18:0 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| 18:1 (n-9) | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 |
| 18:1 (n-7) | 9.9 ± 0.2 | 9.8 ± 0.2 | 9.8 ± 0.2 |
| 18:2 (n-6) | 7.1 ± 0.2 | 2.2 ± 0.1 | 2.2 ± 0.1 |
| 18:3 (n-6) | 4.5 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 |
| 18:3 (n-3) | 9.7 ± 0.1 | 6.6 ± 0.2 | 6.6 ± 0.2 |
| 18:4 (n-3) | 28.2 ± 0.1 | 21.2 ± 0.2 | 21.2 ± 0.2 |
| 20:5 (n-3) | 0.6 ± 0.1 | 3.0 ± 0.1 | 3.0 ± 0.1 |
| 22:5 (n-3) | 7.1 ± 0.2 | 8.6 ± 0.2 | 8.6 ± 0.2 |
| SFA | 24.2 ± 0.2 | 34.2 ± 0.2 | 42.6 ± 0.2 |
| MUFA | 15.6 ± 0.1 | 18.0 ± 0.2 | 25.3 ± 0.2 |
| PUFA | 60.2 ± 0.3 | 47.8 ± 0.1 | 32.1 ± 0.2 |
| n-3/n-6 PUFA | 3.8 ± 0.1 | 5.8 ± 0.1 | 2.9 ± 0.1 |
| UI | 203 ± 2 | 195 ± 1 | 127 ± 1 |
| UFA/SFA | 3.1 ± 0.1 | 1.9 ± 0.1 | 1.3 ± 0.1 |
| Fatty Acids | MGDG | DGDG | SQDG | |||
|---|---|---|---|---|---|---|
| The Sea of Japan | The Adriatic Sea | The Sea of Japan | The Adriatic Sea | The Sea of Japan | The Adriatic Sea | |
| 16:0 | 2.8 ± 0.1 | 6.4 ± 0.3 | 22.2 ± 0.3 | 28.8 ± 0.3 | 56.7 ± 0.4 | 53.4 ± 0.2 |
| 16:1 (n-7) | 0.8 ± 0.1 | 2.4 ± 0.2 | 0.7 ± 0.1 | 3.9 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.1 |
| 16:2 (n-6) | n.d. | n.d. | 7.8 ± 0.2 | 2.8 ± 0.1 | n.d. | n.d. |
| 16:3 (n-3) | 3.0 ± 0.2 | 1.5 ± 0.1 | 8.3 ± 0.2 | 2.8 ± 0.1 | n.d. | tr. |
| 16:4 (n-3) | 37.8 ± 0.4 | 32.3 ± 0.4 | 5.6 ± 0.1 | 1.5 ± 0.1 | n.d. | 0.3 ± 0.1 |
| 18:1 (n-9) | 1.0 ± 0.1 | 2.3 ± 0.1 | 1.6 ± 0.1 | 4.4 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| 18:1 (n-7) | 4.7 ± 0.2 | 7.0 ± 0.5 | 5.9 ± 0.2 | 12.5 ± 0.2 | 13.4 ± 0.2 | 22.1 ± 0.3 |
| 16:0 | 2.8 ± 0.1 | 6.4 ± 0.3 | 22.2 ± 0.3 | 28.8 ± 0.3 | 56.7 ± 0.4 | 53.4 ± 0.2 |
| 18:2 (n-6) | 1.5 ± 0.1 | 1.8 ± 0.1 | 11.8 ± 0.3 | 9.4 ± 0.2 | 3.5 ± 0.1 | 1.7 ± 0.1 |
| 18:3 (n-3) | 18.5 ± 0.3 | 14.5 ± 0.1 | 30.4 ± 0.5 | 23.2 ± 0.2 | 23.6 ± 0.3 | 11.5 ± 0.2 |
| 18:4 (n-3) | 25.7 ± 0.4 | 24.4 ± 0.4 | 3.5 ± 0.2 | 3.5 ± 0.1 | 1.6 ± 0.1 | 11.2 ± 0.2 |
| 18:2 (n-6) | 1.5 ± 0.1 | 1.8 ± 0.1 | 11.8 ± 0.3 | 9.4 ± 0.2 | 3.5 ± 0.1 | 1.7 ± 0.1 |
| SFA | 3.8 ± 0.2 | 7.0 ± 0.3 | 23.6 ± 0.4 | 31.8 ± 0.3 | 56.7 ± 0.4 | 53.7 ± 0.3 |
| MUFA | 7.9 ± 0.2 | 13.2 ± 0.3 | 8.8 ± 0.2 | 21.6 ± 0.4 | 15.6 ± 0.3 | 23.0 ± 0.3 |
| PUFA | 88.3 ± 0.8 | 79.8 ± 0.9 | 67.6 ± 0.5 | 46.6 ± 0.7 | 27.7 ± 0.4 | 23.3 ± 0.3 |
| n-3/n-6 PUFA | 50.7 ± 0.3 | 35.6 ± 0.4 | 2.5 ± 0.2 | 2.7 ± 0.1 | 7.2 ± 0.2 | 13.8 ± 0.1 |
| UI | 334 ± 3 | 302 ± 2 | 203 ± 2 | 152 ± 1 | 89 ±1 | 109 ± 2 |
| UFA/SFA | 25.3 ± 0.4 | 13.3 ± 0.2 | 3.2 ± 0.1 | 2.1 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 |
| Lipids | Tmax, °C 1 | |
|---|---|---|
| DGTS | Green thalli of U. lactuca inhabiting the Sea of Japan | −21 |
| Green thalli of U. lactuca inhabiting the Adriatic Sea | −6 | |
| Bleached thalli of U. lactuca inhabiting the Adriatic Sea | 15 | |
| MGDG | Green thalli of U. lactuca inhabiting the Sea of Japan | −78 |
| Green thalli of U. lactuca inhabiting the Adriatic Sea | −60 | |
| DGDG | Green thalli of U. lactuca inhabiting the Sea of Japan | 41 |
| Green thalli of U. lactuca inhabiting the Adriatic Sea | −50 | |
| SQDG | Green thalli of U. lactuca inhabiting the Sea of Japan | 50 |
| Green thalli of U. lactuca inhabiting the Adriatic Sea | 12 |
| Molecular Species | DGTS | MGDG | DGDG | SQDG | ||||
|---|---|---|---|---|---|---|---|---|
| The Sea of Japan | The Adriatic Sea | The Sea of Japan | The Adriatic Sea | The Sea of Japan | The Adriatic Sea | The Sea of Japan | The Adriatic Sea | |
| 16:0/16:0 | 0.5 ± 0.1 | 0.3 ± 0.1 | n.d. | tr. | 0.3 ± 0.1 | 1.4 ± 0.1 | 14.2 ± 0.2 | 4.3 ± 0.2 |
| 14:0/18:1 | n.d. | n.d. | tr. | 0.5 ± 0.1 | 0.9 ± 0.1 | 8.3 ± 0.2 | 2.3 ± 0.1 | 4.7 ± 0.1 |
| 16:0/16:1 | 3.1 ± 0.1 | 9.8 ± 0.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 16:0/18:1 | 1.5 ± 0.1 | 2.9 ± 0.1 | n.d. | 0.2 ± 0.1 | 5.8 ± 0.2 | 6.7 ± 0.2 | 23.5 ± 0.3 | 38.1 ± 0.2 |
| 16:0/18:2 | 6.6 ± 0.2 | 4.8 ± 0.1 | 2.3 ± 0.1 | 1.2 ± 0.1 | 19.7 ± 0.2 | 16.2 ± 0.2 | 6.7 ± 0.2 | 2.9 ± 0.1 |
| 16:0/18:3 | 15.9 ± 0.1 | 15.4 ± 0.2 | 2.3 ± 0.1 | 3.6 ± 0.2 | 16.7 ± 0.2 | 21.1 ± 0.3 | 46.7 ± 0.2 | 22.9 ± 0.2 |
| 16:0/18:4 | 13.0 ± 0.1 | 18.1 ± 0.2 | 0.5 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 2.9 ± 0.1 | 3.7 ± 0.1 | 21.5 ± 0.2 |
| 16:0/22:5 | 4.5 ± 0.2 | 5.9 ± 0.2 | n.d. | n.d. | n.d. | n.d. | tr. | tr. |
| 16:1/18:1 | 1.2 ± 0.1 | 1.8 ± 0.1 | tr. | 0.2 ± 0.1 | 0.4 ± 0.1 | 4.6 ± 0.2 | tr. | 0.3 ± 0.1 |
| 16:1/18:3 | 1.9 ± 0.1 | 0.6 ± 0.1 | 2.6 ± 0.2 | 3.5 ± 0.2 | 1.9 ± 0.1 | 6.2 ± 0.1 | n.d. | tr. |
| 16:1/16:4 | tr. | 0.2 ± 0.1 | 0.7 ± 0.1 | 3.7 ± 0.2 | tr. | tr. | n.d. | n.d. |
| 16:1/18:4 | 1.6 ± 0.1 | 3.1 ± 0.1 | n.d. | 0.8 ± 0.1 | n.d. | tr. | n.d. | tr. |
| 18:1/16:3 | n.d | 0.4 ± 0.1 | 1.9 ± 0.1 | 1.5 ± 0.1 | 2.8 ± 0.2 | 2.9 ± 0.1 | tr. | tr. |
| 18:1/18:3 | 6.9 ± 0.1 | 2.7 ± 0.1 | 0.6 ± 0.1 | 1.1 ± 0.1 | 2.3 ± 0.1 | 3.7 ± 0.2 | 0.2 ± 0.1 | n.d. |
| 18:1/16:4 | 0.9 ± 0.1 | 0.4 ± 0.1 | 7.4 ± 0.2 | 11.5 ± 0.2 | 0.6 ± 0.1 | 1.5 ± 0.1 | n.d. | tr. |
| 18:1/18:4 | 8.6 ± 0.2 | 6.2 ± 0.2 | 0.2 ± 0.1 | 0.9 ± 0.1 | 0.2 ± 0.1 | 1.2 ± 0.1 | tr. | tr. |
| 16:2/18:3 | tr. | n.d. | 4.6 ± 0.2 | 3.0 ± 0.1 | 10.4 ± 0.2 | 3.5 ± 0.2 | tr. | n.d. |
| 16:3/18:3 | 0.5 ± 0.1 | n.d. | 6.5 ± 0.2 | 4.0 ± 0.2 | 12.4 ± 0.2 | 2.1 ± 0.1 | tr. | tr. |
| 16:4/18:4 | 0.4 ± 0.1 | 0.2 ± 0.1 | 22.1 ± 0.2 | 27.8 ± 0.2 | 3.2 ± 0.1 | 1.1 ± 0.1 | n.d. | n.d. |
| 18:2/16:4 | 0.2 ± 0.1 | n.d. | 5.9 ± 0.2 | 3.6 ± 0.2 | 0.3 ± 0.1 | tr. | n.d. | n.d. |
| 18:3/18:3 | 1.6 ± 0.1 | 0.2 ± 0.1 | 1.6 ± 0.1 | 1.3 ± 0.1 | 3.7 ± 0.2 | 3.5 ± 0.2 | tr. | n.d. |
| 18:3/16:4 | 0.5 ± 0.1 | tr. | 30.1 ± 0.2 | 16.7 ± 0.2 | 5.4 ± 0.1 | 0.5 ± 0.1 | n.d. | n.d. |
| 18:3/18:4 | 4.3 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.1 | 2.6 ± 0.1 | 0.6 ± 0.1 | 1.2 ± 0.1 | n.d. | n.d. |
| 20:5/22:5 | 3.8 ± 0.1 | 4.8 ± 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| SFA/SFA | 0.5 ± 0.1 | 0.3 ± 0.1 | tr. | tr. | 0.9 ± 0.1 | 1.4 ± 0.1 | 14.2 ± 0.2 | 4.9 ± 0.2 |
| SFA/MUFA | 4.6 ± 0.1 | 12.7 ± 0.2 | tr. | 0.8 ± 0.1 | 6.8 ± 0.1 | 16.2 ± 0.2 | 26.2 ± 0.2 | 45.5 ± 0.2 |
| SFA/PUFA | 50.9 ± 0.2 | 54.3 ± 0.2 | 7.6 ± 0.2 | 7.6 ± 0.2 | 39.6 ± 0.2 | 44.1 ± 0.2 | 58.9 ± 0.2 | 49.0 ± 0.2 |
| MUFA/MUFA | 2.3 ± 0.1 | 2.7 ± 0.1 | tr. | 0.2 ± 0.1 | 0.6 ± 0.1 | 5.0 ± 0.1 | tr. | 0.3 ± 0.1 |
| MUFA/PUFA | 24.7 ± 0.2 | 17.9 ± 0.1 | 15.8 ± 0.3 | 24.9 ± 0.2 | 10.3 ± 0.2 | 18.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| PUFA/PUFA | 17.0 ± 0.1 | 12.1 ± 0.1 | 76.6 ± 0.2 | 66.5 ± 0.2 | 41.8 ± 0.2 | 14.8 ± 0.1 | 0.4 ± 0.1 | tr. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostetsky, E.; Chopenko, N.; Barkina, M.; Velansky, P.; Sanina, N. Fatty Acid Composition and Thermotropic Behavior of Glycolipids and Other Membrane Lipids of Ulva lactuca (Chlorophyta) Inhabiting Different Climatic Zones. Mar. Drugs 2018, 16, 494. https://doi.org/10.3390/md16120494
Kostetsky E, Chopenko N, Barkina M, Velansky P, Sanina N. Fatty Acid Composition and Thermotropic Behavior of Glycolipids and Other Membrane Lipids of Ulva lactuca (Chlorophyta) Inhabiting Different Climatic Zones. Marine Drugs. 2018; 16(12):494. https://doi.org/10.3390/md16120494
Chicago/Turabian StyleKostetsky, Eduard, Natalia Chopenko, Maria Barkina, Peter Velansky, and Nina Sanina. 2018. "Fatty Acid Composition and Thermotropic Behavior of Glycolipids and Other Membrane Lipids of Ulva lactuca (Chlorophyta) Inhabiting Different Climatic Zones" Marine Drugs 16, no. 12: 494. https://doi.org/10.3390/md16120494
APA StyleKostetsky, E., Chopenko, N., Barkina, M., Velansky, P., & Sanina, N. (2018). Fatty Acid Composition and Thermotropic Behavior of Glycolipids and Other Membrane Lipids of Ulva lactuca (Chlorophyta) Inhabiting Different Climatic Zones. Marine Drugs, 16(12), 494. https://doi.org/10.3390/md16120494