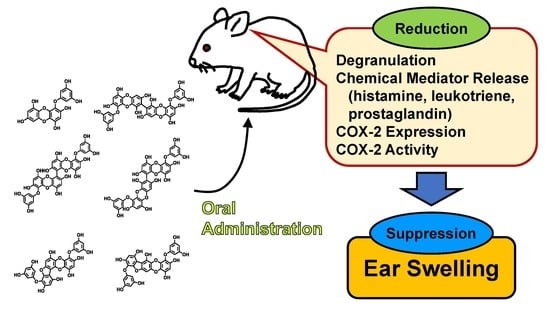
Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling
Abstract
:1. Introduction
2. Results
2.1. Suppression of Mouse Ear Swelling
2.2. Suppression of Chemical Mediator Release in RBL-2H3 Cells
2.2.1. Anti-Degranulation in RBL Cells Stimulated by Antigen-Antibody or A23187
2.2.2. Suppression of Chemical Mediator Release from RBL Cells
2.3. Inhibition of Cyclooxygenase-2 (COX-2) mRNA Expression and COX-2 Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Anti-Inflammatory Effects on Mouse Ear Swelling
4.3.1. Arachidonic Acid (AA)
4.3.2. 12-O-Tetradecanoylphorbol-13-Acetate (TPA)
4.3.3. Oxazolone (OXA)
4.4. Suppression Effect on Chemical Mediator Release
4.4.1. Cell Culture
4.4.2. Cell Stimulation and Anti-Degranulation Assay
4.4.3. Determination of Released Chemical Mediators
4.5. Suppression of COX-2 Gene Expression and Enzymatic Activity
4.5.1. Cell Stimulation
4.5.2. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.5.3. Inhibitory Effect on COX-2 Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Platts-Mills, T.A.E. The allergy epidemics: 1870–2010. J. Allergy Clin. Immunol. 2015, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M. Measures for allergic disease and medical network in Japan. Jpn. J. Allegol. 2012, 61, 913–918. [Google Scholar]
- Tachibana, H. Green tea polyphenol sensing. Proc. Jpn. Acad. Ser. B 2011, 87, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Ragan, M.; Glombitza, K. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 129–241. [Google Scholar]
- Kim, S.-K.; Himaya, S.W.A. Chapter 8: Medicinal effects of phlorotannins from marine brown algae. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 97–109. [Google Scholar]
- Barbosa, M.; Lopes, G.; Ferreres, F.; Andrade, P.B.; Pereira, D.M.; Gil-Izquierdo, Á.; Valentão, P. Phlorotannin extracts from Fucales: Marine polyphenols as bioregulators engaged in inflammation-related mediators and enzymes. Algal Res. 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Eom, S.-H.; Lee, E.-H.; Park, K.; Kwon, J.-Y.; Kim, P.-H.; Jung, W.-K.; Kim, Y.-M. Eckol from Eisenia bicyclis inhibits inflammation through the Akt/NF-κB signaling in propionibacterium acnes-induced human keratinocyte HaCat cells. J. Food Biochem. 2017, 41, e12312. [Google Scholar] [CrossRef]
- Lee, S.-H.; Eom, S.-H.; Yoon, N.-Y.; Kim, M.-M.; Li, Y.-X.; Ha, S.K.; Kim, S.-K. Fucofuroeckol-A from Eisenia bicyclis inhibits inflammation in lipopolysaccharide-induced mouse macrophages via downregulation of the MAPK/NF-κB signaling pathway. J. Chem. 2016, 2016, 6509212. [Google Scholar] [CrossRef]
- Yang, Y.-I.; Woo, J.-H.; Seo, Y.-J.; Lee, K.-T.; Lim, Y.; Choi, J.-H. Protective effect of brown alga phlorotannins against hyper-inflammatory responses in lipopolysaccharide-induced sepsis models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-K.; Lee, B.; Kwon, M.; Yoon, N.; Shin, T.; Kim, N.-G.; Choi, J.-S.; Kim, H.-R. Phlorofucofuroeckol-B suppresses inflammatory responses by down-regulating nuclear factor κB activation via Akt, ERK, and JNK in LPS-stimulated microglial cells. Int. Immunopharmacol. 2015, 28, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Isolation of a new anti-allergic phlorotannin, phlorofucofuroeckol-B, from an edible brown alga, Eisenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Anti-allergic phlorotannins from the edible brown alga, Eisenia arborea. Food Sci. Technol. Res. 2007, 13, 54–60. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Imai, K.; Kakinuma, M.; Amano, H. Radical scavenging and hyaluronidase inhibitory activities of phlorotannins from the edible brown alga Eisenia arborea. Food Sci. Technol. Res. 2008, 14, 595–598. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Okamoto, T.; Yamada, Y.; Imai, K.; Ito, T.; Kakinuma, M.; Amano, H. The inhibitory effects of components from a brown alga, Eisenia arborea, on degranulation of mast cells and eicosanoid synthesis. J. Funct. Foods 2009, 1, 387–393. [Google Scholar] [CrossRef]
- Gall, E.; Lelchat, F.; Hupel, M.; Jégou, C.; Stiger-Pouvreau, V. Extraction and purification of phlorotannins from brown algae. In Natural Products from Marine Algae; Stengel, D.B., Connan, S., Eds.; Springer: New York, NY, USA, 2015; Volume 1308, pp. 131–143. [Google Scholar]
- Kim, J.; Yoon, M.; Yang, H.; Jo, J.; Han, D.; Jeon, Y.-J.; Cho, S. Enrichment and purification of marine polyphenol phlorotannins using macroporous adsorption resins. Food Chem. 2014, 162, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Ko, J.-Y.; Oh, J.-Y.; Kim, C.-Y.; Lee, H.-J.; Kim, J.; Jeon, Y.-J. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014, 158, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Tanaka, R.; Katsuzaki, H.; Imai, K.; Matsushita, T. The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J. Funct. Foods 2013, 5, 2019–2023. [Google Scholar] [CrossRef]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Miyata, M. Anti-inflammatory effects of 6,6′-bieckol and 6,8′-bieckol from Eisenia arborea on mouse ear swelling. Food Sci. Technol. Res. 2017, 23, 475–480. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Wang, J.H.; Liu, X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora—Implications for health. Mol. Nutr. Food Res. 2007, 51, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Kujubu, D.A.; Fletcher, B.S.; Varnum, B.C.; Lim, R.W.; Herschman, H.R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J. Biol. Chem. 1991, 266, 12866–12872. [Google Scholar] [PubMed]
- Meurer, R.; Opas, E.E.; Humes, J.L. Effects of cyclooxygenase and lipoxygenase inhibitors on inflammation associated with oxazolone-induced delayed hypersensitivity. Biochem. Pharmacol. 1988, 37, 3511–3514. [Google Scholar] [CrossRef]
- Rao, T.S.; Currie, J.L.; Shaffer, A.F.; Isakson, P.C. Comparative evaluation of arachidonic acid (AA)- and tetradecanoylphorbol acetate (TPA)-induced dermal inflammation. Inflammation 1993, 17, 723–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.-S.; Shin, T.-S.; Hua, H.; Jang, B.-C.; Choi, J.-S.; Byun, D.-S.; Utsuki, T.; Ingram, D.; Kim, H.-R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Park, J.-H.; Lee, B.; Chee, H.; Lee, K.; Oh, S.-M. Suppression of NF-κB by Dieckol Extracted from Ecklonia cava Negatively Regulates LPS Induction of Inducible Nitric Oxide Synthase Gene. Appl. Biochem. Biotechnol. 2014, 173, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Paudel, U.; Lee, Y.-H.; Kwon, T.-H.; Park, N.-H.; Yun, B.-S.; Hwang, P.-H.; Yi, H.-K. Eckols reduce dental pulp inflammation through the ERK1/2 pathway independent of COX-2 inhibition. Oral Dis. 2014, 20, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-I.; Shin, H.-C.; Kim, S.H.; Park, W.-Y.; Lee, K.-T.; Choi, J.-H. 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE2 production and inflammatory cytokine expression in macrophages: The inhibition of NFκB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; DiIulio, N.A.; Fairchild, R.L. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: Interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+T cells. J. Exp. Med. 1996, 183, 1001–1012. [Google Scholar] [PubMed]
- Nagano, T.; Wu, W.; Tsumura, K.; Yonemoto-Yano, H.; Kamada, T.; Haruma, K. The inhibitory effect of soybean and soybean isoflavone diets on 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Biosci. Biotechnol. Biochem. 2016, 80, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Yamazaki, K.; Sano, M. Preventive effects of black tea theaflavins against mouse type IV allergy. J. Sci. Food Agric. 2010, 90, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar]
- Yang, Y.-I.; Jung, S.-H.; Lee, K.-T.; Choi, J.-H. 8,8′-Bieckol, isolated from edible brown algae, exerts its anti-inflammatory effects through inhibition of NF-κB signaling and ROS production in LPS-stimulated macrophages. Int. Immunopharmacol. 2014, 23, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Yamada, K.; Shoji, K.; Mori, M.; Sugano, M. Effect of tea polyphenols on histamine release from rat basophilic leukemia (RBL-2H3) cells: The structure–inhibitory activity relationship. Allergy 1997, 52, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Takano-Ishikawa, Y.; Goto, M.; Yamaki, K. Structure–activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytomedicine 2006, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.; Park, E.-J.; Kim, D.-S.; Jeon, Y.-J.; Shin, T.-K.; Park, J.-W.; Woo, H.-C.; Lee, K.-W.; Jee, Y.-H. Anti-inflammatory effects of enzymatic extract from Ecklonia cava on TPA-induced ear skin edema. Food Sci. Biotechnol. 2008, 17, 745–750. [Google Scholar]
- Sugiura, Y.; Matsuda, K.; Okamoto, T.; Kakinuma, M.; Amano, H. Anti-allergic effects of the brown alga Eisenia arborea on Brown Norway rats. Fish. Sci. 2008, 74, 180–186. [Google Scholar] [CrossRef]
- Dominique, T.; Jean-Louis, B.; Barbara, B.; Tara, D.; Susan, F.-T.; Marina, H.; Ildico, H.-E.K.; Inge, M.; Androniki, N.; Monika, N.-B.; et al. Safety of Ecklonia cava phlorotannins as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e05003. [Google Scholar]
- Hwang, H.; Terada, M.; Shin, H.-C. Single dose oral toxicity and 4-weeks repeated oral toxicity studies of Ecklonia cava extract. Seikatsu Eisei 2008, 52, 282–289. [Google Scholar]
- Evans, J.T.; Hauschka, T.S.; Mittelman, A. Differential susceptibility of four mouse strains to induction of multiple large-bowel neoplasms by 1,2-dimethylhydrazine2. JNCI J. Natl. Cancer Inst. 1974, 52, 999–1000. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Nam, J.H.; Cho, J.Y.; Kim, K.S.; Hwang, D.Y. Annual tendency of research papers used ICR mice as experimental animals in biomedical research fields. Lab. Anim. Res. 2017, 33, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.M.; Spires, D.A.; Bedord, C.J.; Wagner, B.; Ballaron, S.J.; de Young, L.M. The mouse ear inflammatory response to topical arachidonic acid. J. Investig. Dermatol. 1984, 82, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yoshino, K.; Maeda-Yamamoto, M.; Miyase, T.; Sano, M. Inhibitory effects of tea catechins and O-methylated derivatives of (−)-epigallocatechin-3-O-gallate on mouse type IV allergy. J. Agric. Food Chem. 2000, 48, 5649–5653. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B.; Lewis, R.A.; Seldin, D.; Austen, K.F. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J. Immunol. 1981, 126, 1290–1294. [Google Scholar] [PubMed]
- Matsui, T.; Ito, C.; Itoigawa, M.; Okada, T.; Furukawa, H. Effect of natsudaidain isolated from Citrus plants on TNF-α and cyclooxygenase-2 expression in RBL-2H3 cells. J. Pharm. Pharmacol. 2009, 61, 109–114. [Google Scholar] [CrossRef] [PubMed]



| Sensitizer | Test Groups | Control | Eckol | 6,6′-Bieckol | 6,8′-Bieckol |
| AA | Ear swelling (mm) | 0.193 ± 0.026 | 0.169 ± 0.011 | 0.112 ** ± 0.049 | 0.116 ** ± 0.036 |
| Suppression (%) | — | 12.7 ± 5.4 | 41.9 # ± 25.4 | 39.8 # ± 18.7 | |
| TPA | Ear swelling (mm) | 0.194 ± 0.038 | 0.116 ** ± 0.009 | 0.174 * ± 0.016 | 0.098 ** ± 0.032 |
| Suppression (%) | — | 40.0 # ± 4.5 | 34.2 ± 25.2 | 49.4 * ± 16.4 | |
| OXA | Ear swelling (mm) | 0.223 ± 0.024 | 0.180 ± 0.020 | 0.183 ± 0.030 | 0.049 ** ± 0.044 |
| Suppression (%) | — | 19.3 ± 9.1 | 17.8 ± 13.5 | 77.8 ** ± 19.7 | |
| Sensitizer | Test groups | 8,8′-bieckol | PFF-A | PFF-B | EGCG |
| AA | Ear swelling (mm) | 0.153 ± 0.011 | 0.134 * ± 0.021 | 0.112 ** ± 0.030 | 0.168 ± 0.008 |
| Suppression (%) | 21.0 ± 5.5 | 30.5 ± 10.6 | 42.2 # ± 15.7 | 12.9 ± 4.2 | |
| TPA | Ear swelling (mm) | 0.132 * ± 0.014 | 0.132 * ± 0.007 | 0.119 ** ± 0.031 | 0.167 ± 0.008 |
| Suppression (%) | 31.7 ± 7.4 | 31.7 ± 3.8 | 38.4 # ± 16.1 | 13.8 ± 4.4 | |
| OXA | Ear swelling (mm) | 0.151 * ± 0.030 | 0.171 ± 0.019 | 0.131 ** ± 0.032 | 0.210 ± 0.001 |
| Suppression (%) | 32.3 # ± 13.6 | 23.4 ± 8.6 | 41.0 * ± 14.5 | 5.7 ± 0.1 |
| Stimulant | Samples | Eckol | 6,6′-Bieckol | 6,8′-Bieckol | 8,8′-Bieckol | |
| Antigen-antibody | Suppression ratio (%) | 10 μM | 17.3 ± 0.2 | 3.8 ± 2.3 | 5.7 ± 3.9 | 68.2 ** ± 15.8 |
| 100 μM | 76.3 ± 4.6 | 14.5 ± 4.1 | 68.2 ± 5.8 | 99.7 ** ± 11.9 | ||
| A23187 | Suppression ratio (%) | 10 μM | NA | NA | 4.2 ± 2.0 | 19.3 ** ± 2.3 |
| 100 μM | 11.5 ± 5.1 | 11.2 ± 1.8 | 35.5 ± 3.3 | 82.5 ± 2.2 | ||
| Stimulant | Samples | PFF-A | PFF-B | EGCG | ||
| Antigen-antibody | Suppression ratio (%) | 10 μM | 70.1 ** ± 6.8 | NA | 21.2 ± 10.4 | |
| 100 μM | 127.8 ** ± 5.6 | 44.9 ± 2.6 | 75.4 ± 2.1 | |||
| A23187 | Suppression ratio (%) | 10 μM | NA | NA | 5.2 ± 0.1 | |
| 100 μM | 85.2 # ± 0.7 | 34.5 ± 1.2 | 80.0 ± 2.0 | |||
| Chemical mediator | Samples | Eckol | 6,6′-Bieckol | 6,8′-Bieckol | 8,8′-Bieckol | |
| Histamine | Suppression ratio (%) | 10 μM | 15.6 ± 1.2 | NA | NA | 58.4 ± 19.5 |
| 100 μM | 85.1 ** ± 2.9 | 33.3 ± 2.8 | 29.4 ± 9.8 | 84.4 ** ± 4.5 | ||
| 200 μM | — | — | — | — | ||
| Leukotriene B4 | Suppression ratio (%) | 10 μM | 10.3 ± 2.3 | NA | 12.2 ± 0.1 | 13.4 ± 0.4 |
| 100 μM | 66.6 ± 3.4 | NA | 57.9 ± 1.6 | 62.7 ± 4.9 | ||
| 200 μM | — | 19.2 ± 3.0 | — | — | ||
| Prostaglandin E2 | Suppression ratio (%) | 10 μM | 10.3 ± 0.7 | 4.3 ± 1.6 | 6.9 ± 5.3 | 12.7 ± 0.3 |
| 100 μM | 60.7 ± 0.9 | 15.3 ± 2.7 | 36.6 ± 2.7 | 63.3 ± 3.4 | ||
| Chemical mediator | Samples | PFF-A | PFF-B | EGCG | ||
| Histamine | Suppression ratio (%) | 10 μM | 23.9 ± 15.4 | NA | NA | |
| 100 μM | 94.2 ** ± 2.0 | NA | 36.1 ± 0.1 | |||
| 200 μM | — | 37.1 ± 24.7 | — | |||
| Leukotriene B4 | Suppression ratio (%) | 10 μM | 8.9 ± 0.6 | 3.4 ± 0.4 | 12.4 ± 7.1 | |
| 100 μM | 61.3 ± 5.1 | 72.5 ± 2.2 | 71.7 ± 4.3 | |||
| 200 μM | — | — | — | |||
| Prostaglandin E2 | Suppression ratio (%) | 10 μM | 14.4 ± 1.8 | 7.1 ± 2.0 | 17.4 ± 0.2 | |
| 100 μM | 82.9 ± 1.3 | 41.5 ± 11.2 | 80.3 ± 3.1 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Miyata, M. Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling. Mar. Drugs 2018, 16, 267. https://doi.org/10.3390/md16080267
Sugiura Y, Usui M, Katsuzaki H, Imai K, Kakinuma M, Amano H, Miyata M. Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling. Marine Drugs. 2018; 16(8):267. https://doi.org/10.3390/md16080267
Chicago/Turabian StyleSugiura, Yoshimasa, Masakatsu Usui, Hirotaka Katsuzaki, Kunio Imai, Makoto Kakinuma, Hideomi Amano, and Masaaki Miyata. 2018. "Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling" Marine Drugs 16, no. 8: 267. https://doi.org/10.3390/md16080267
APA StyleSugiura, Y., Usui, M., Katsuzaki, H., Imai, K., Kakinuma, M., Amano, H., & Miyata, M. (2018). Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling. Marine Drugs, 16(8), 267. https://doi.org/10.3390/md16080267