Amphilectene Diterpene Isonitriles and Formamido Derivatives from the Hainan Nudibranch Phyllidia Coelestis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Procedures
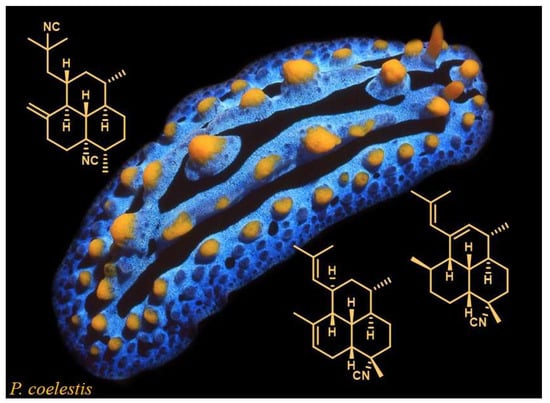
4.2. Biological Material
4.3. Extraction and Isolation Procedures
4.4. Acid Hydrolysis of Compounds 4 and 5
4.5. Feeding Deterrence Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouchet, P.; Rocroi, J.-P.; Hausdorf, B.; Kaim, A.; Kano, Y.; Nützel, A.; Parkhaev, P.; Schrödl, M.; Strong, E.E. Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 2017, 61, 1–526. [Google Scholar] [CrossRef]
- Cimino, G.; Ghiselin, M.T. Chemical defense and the evolution of opisthobranch gastropods. Proc. Calif. Acad. Sci. 2009, 60, 175–422. [Google Scholar]
- Bornancin, L.; Bonnard, I.; Mills, S.C.; Banaigs, B. Chemical mediation as a structuring element in marine gastropod predator-prey interactions. Nat. Prod. Rep. 2017, 34, 644–676. [Google Scholar] [CrossRef] [PubMed]
- Cimino, G.; Fontana, A.; Gavagnin, M. Marine opisthobranch molluscs: Chemistry and ecology in sacoglossans and dorids. Curr. Org. Chem. 1999, 3, 327–372. [Google Scholar]
- Cimino, G.; Ciavatta, M.L.; Fontana, A.; Gavagnin, M. Metabolites of marine opisthobranchs: chemistry and biological activity. In Bioactive Compounds from Natural Sources; Tringali, C., Ed.; Taylor & Francis Ltd.: London, UK, 2001; pp. 578–637. [Google Scholar]
- Dean, L.J.; Prinsep, M.R. The chemistry and chemical ecology of nudibranchs. Nat. Prod. Rep. 2017, 34, 1359–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burreson, B.J.; Scheuer, P.J.; Finer, J.; Clardy, J. Isocyanopupukeanane, a marine invertebrate allomone with a new sesquiterpene skeleton. J. Am. Chem. Soc. 1975, 97, 4763–4764. [Google Scholar] [CrossRef]
- Garson, M.J.; Simpson, J.S. Marine isocyanides and related natural products—structure, biosynthesis and ecology. Nat. Prod. Rep. 2004, 19, 164–179. [Google Scholar] [CrossRef]
- Emsermann, J.; Kauhl, U.; Opatz, T. Marine isonitriles and their related compounds. Mar. Drugs 2016, 14, 16. [Google Scholar] [CrossRef]
- Wright, A.D.; Wang, H.; Gurrath, M.; König, G.M.; Kocak, G.; Neumann, G.; Loria, P.; Foley, M.L.; Tilley, J. Inhibition of heme detoxification processes underlies the antimalarial activity of terpene isonitrile compounds from marine sponges. J. Med. Chem. 2001, 44, 873–885. [Google Scholar] [CrossRef]
- Young, R.M.; Adendorff, M.R.; Wright, A.D.; Davies-Coleman, M.T. Antiplasmodial activity: The first proof of inhibition of heme crystallization by marine isonitriles. Eur. J. Med. Chem. 2015, 93, 373–380. [Google Scholar] [CrossRef]
- Fusetani, N.; Hirota, H.; Okino, T.; Tomono, Y.; Yoshimura, E. Antifouling activity of isocyanoterpenoids and related compounds from a marine sponge and nudibranchs. J. Nat. Toxins 1996, 5, 249–259. [Google Scholar]
- Wright, A.D.; McCluskey, A.; Robertson, M.J.; MacGregor, K.A.; Gordon, C.P.; Guenther, J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela Hooperi. Org. Biomol. Chem. 2011, 9, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Schnermann, M.J.; Shenvi, R.A. Syntheses and biological studies of marine terpenoids derived from inorganic cyanide. Nat. Prod. Rep. 2015, 32, 543–577. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Gavagnin, M.; Haber, M.; Guo, Y.-W.; Fontana, A.; Manzo, E.; Genta-Jouve, G.; Tsoukatou, M.; Rudman, W.B.; Cimino, G.; et al. Packaging and delivery of chemical weapons: A defensive Trojan horse stratagem in chromodorid nudibranchs. PLoS ONE 2013, 8, e62075. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Ciavatta, M.L.; Wang, J.-R.; Cirillo, I.; Mathieu, V.; Kiss, R.; Mollo, E.; Guo, Y.-W.; Gavagnin, M. Extending the record of bis-γ-pyrone polypropionates from marine pulmonate mollusks. J. Nat. Prod. 2013, 76, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- He, W.-F.; Li, Y.; Feng, M.-T.; Gavagnin, M.; Mollo, E.; Mao, S.-C.; Guo, Y.-W. New isoquinolinequinone alkaloids from the South China Sea nudibranch Jorunna funebris and its possible sponge-prey Xestospongia sp. Fitoterapia 2014, 96, 109–114. [Google Scholar] [CrossRef]
- Carbone, M.; Ciavatta, M.L.; Mathieu, V.; Ingels, A.; Kiss, R.; Pascale, P.; Mollo, E.; Ungur, N.; Guo, Y.-W.; Gavagnin, M. Marine terpenoid diacylguanidines: structure, synthesis and biological evaluation of naturally occurring actinofide and synthetic analogs. J. Nat. Prod. 2017, 80, 1339–1346. [Google Scholar] [CrossRef]
- Zhou, Z.-F.; Li, X.-L.; Yao, L.-G.; Li, J.; Gavagnin, M.; Guo, Y.-W. Marine bis-γ-pyrone polypropionates of onchidione family and their effects on the XBP1 gene expression. Bioorg. Med. Chem. Lett. 2018, 28, 1093–1096. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, W.-T.; Li, S.-W.; Ye, J.-Y.; Huan, X.-J.; Gavagnin, M.; Yao, L.-G.; Wang, H.; Miao, Z.-H.; Li, X.-W.; et al. Cytotoxic nitrogenous terpenoids from two South China Sea nudibranchs Phyllidiella pustulosa, Phyllidia coelestis, and their sponge-prey Acanthella cavernosa. Mar. Drugs 2019, 17, 56. [Google Scholar] [CrossRef]
- Wratten, S.J.; Faulkner, D.J.; Hirotsu, K.; Clardy, J. Diterpenoid isocyanides from the marine sponge Hymeniacidon amphilecta. Tetrahedron Lett. 1978, 19, 4345–4348. [Google Scholar] [CrossRef]
- Hirota, H.; Tomono, Y.; Fusetani, N. Terpenoids with antifouling activity against barnacle larvae from the marine sponge Acanthella cavernosa. Tetrahedron 1996, 57, 2359–2368. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Fontana, A.; Puliti, R.; Scognamiglio, G.; Cimino, G. Structures and absolute stereochemistry of isocyanide and isothiocyanate amphilectenes from the Caribbean sponge Cribochalina sp. Tetrahedron 1999, 55, 12629–12636. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Gavagnin, M.; Manzo, E.; Puliti, R.; Mattia, C.A.; Mazzarella, L.; Cimino, G.; Simpson, J.S.; Garson, M.J. Structural and stereochemical revision of isocyanide and isothiocyanate amphilectenes from the Caribbean marine sponge Cribochalina sp. Tetrahedron 2005, 61, 8049–8053. [Google Scholar] [CrossRef]
- Molinski, T.F.; Faulkner, D.J.; Van Duyne, G.D.; Clardy, J. Three new diterpene isonitriles from a Palauan sponge of the genus Halichondria. J. Org. Chem. 1987, 52, 3334–3337. [Google Scholar] [CrossRef]
- Lamoral-Theys, D.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Kiss, R.; Costantino, V. Evaluation of the antiproliferative activity of diterpene isonitriles from the sponge Pseudoaxinella flava in apoptosis-sensitive and apoptosis-resistant cancer cell lines. J. Nat. Prod. 2011, 74, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D.; Angerhofer, C.K. Novel potent antimalarial diterpene isocyanates, isothiocyanates, and isonitriles from the tropical marine sponge Cymbastela hooperi. J. Org. Chem. 1996, 61, 3259–3267. [Google Scholar] [CrossRef]
- Linden, A.; König, G.M.; Wright, A.D. Four diterpene isonitriles from the sponge Cymbastela hooperi. Acta Cryst. Sect. C 1996, 52, 2601–2607. [Google Scholar] [CrossRef]
- Mitome, H.; Shirato, N.; Miyaoka, H.; Yamada, Y.; van Soest, R.W.M. Terpene isocyanides, isocyanates, and isothiocyanates from the Okinawan marine sponge Stylissa sp. J. Nat. Prod. 2004, 67, 833–837. [Google Scholar] [CrossRef]
- Lucas, R.; Casapullo, A.; Ciasullo, L.; Gomez-Paloma, L.; Payá, M. Cycloamphilectenes, a new type of potent marine diterpenes: Inhibition of nitric oxide production in murine macrophages. Life Sci. 2003, 72, 2543–2552. [Google Scholar] [CrossRef]
- Mollo, E.; Gavagnin, M.; Carbone, M.; Castelluccio, F.; Pozone, F.; Roussis, V.; Templado, J.; Ghiselin, M.T.; Cimino, G. Factors promoting marine invasions: A chemoecological approach. Proc. Natl. Acad. Sci. USA 2008, 105, 4582–4586. [Google Scholar] [CrossRef] [Green Version]
- Garson, M.J.; Simpson, J.S.; Flowers, A.E.; Dumdei, E.J. Cyanides and thiocyanate-derived functionality in marine organisms—structures, biosynthesis and ecology. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier Science, B.V.: Amsterdam, NL, The Netherlands, 2000; Volume 21, pp. 329–372. [Google Scholar]
- Manzo, E.; Ciavatta, M.L.; Gavagnin, M.; Mollo, E.; Guo, Y.-W.; Cimino, G. Isocyanide terpene metabolites of Phyllidiella pustulosa, a nudibranch from the South China Sea. J. Nat. Prod. 2004, 67, 1701–1704. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Avilés, E.; Rodríguez, A.D. Marine sponge Hymeniacidon sp. amphilectane metabolites potently inhibit rat brain microglia thromboxane B2 generation. Bioorg. Med. Chem. 2012, 20, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avilés, E.; Rodríguez, A.D.; Vicente, J. Two rare-class tricyclic diterpenes with antitubercular activity from the Caribbean sponge Svenzea flava. Application of vibrational circular dichroism spectroscopy for determining absolute configuration. J. Org. Chem. 2013, 78, 11294–11301. [Google Scholar] [CrossRef] [PubMed]





| H | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 1 | 2.93, m | - | 2.98, app. br s | 1.77, m | 2.91, app. t (9) |
| 2 | 1.07 eq, ddd, (13,4,1) 1.29 ax, m | 5.32, br s | 1.29, m 1.57, m | 0.68, m 1.92, m | 1.07 eq, ddd, (13, 4, 2) 1.29, m |
| 3 | 1.28, m | 1.85, m | 1.31, m | 1.19, m | 1.28, m |
| 4 | 0.95, m | 1.28, m | 1.04, m | 0.98, m | 0.95, m |
| 5 | 0.93, m 1.89, m | 0.93, m 1.30, m | 1.06, m 1.88, m | 1.10, m 1.87, m | 1.11, m 1.90, m |
| 6 | 1.80, m 2.05, m | 1.76, m 1.95, m | 1.74, m 1.90, m | 1.55, m | 1.50, m (1.60, m) |
| 8 | 1.82, m | 1.63, m | 1.68, m | 2.02, m (1.99, m) | 1.75, m (2.44, m) |
| 9 | 2.14, m 2.27, m | 1.56, m | 1.60, m 1.97, m | 1.52, m | 1.95, m |
| 10 | 5.44, m | 1.73, m | 2.05, m 2.43, m | 2.19, m 2.36, m | 5.40, br d (7) |
| 11 | - | 1.94, m | - | - | |
| 12 | 2.01, m | 1.99, m | 1.99, m | 1.53, m | 2.03, m |
| 13 | 1.70, m | 1.70, m | 1.87, m | 1.24, m | 1.80, m (1.92, m) |
| 14 | 5.46, m | 5.44, s | 5.48, br d (8) | 2.65, m 1.44, m | 5.48, br d (9) |
| 16 | 1.72, br s | 1.76, br s | 1.72, br s | 4.63, br s 4.73, br s | 1.72,br s |
| 17 | 1.65, br s | 1.81, br s | 1.61, br s | 1.71, br s | 1.64, br s |
| 18 | 0.82, d (6) | 1.05, d (6) | 0.82, d (7) | 0.89, d (6)e [0.88, d (6)]e | 0.82, d (7) |
| 19 | 1.57, s | 1.54, s | 1.58, s | 1.34, s (1.45, s) | 1.50, s (1.59, s) |
| 20 | 1.72, br s | 0.82, d (7) | 4.79, br s 4.95, br s | 4.62, br s 4.87, br s | 1.69, s |
| NHCHO | - | - | - | 8.25 d (12) [8.09, d, (2)] | 8.24, d (12) [8.03, d (2)] |
| NHCHO | - | - | - | 5.08 (5.66) | 5.46, br s [5.02, s] |
| C | 5 | 6 | 7 | 8 | 9 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| δC | Type b | δC | Type b | δC | Type b | δC | Type b | δC | Type b | |
| 1 | 32.8 | CH | 125.7 | C | 33.1 | CH | 34.0 | CH | 33.5 | CH |
| 2 | 35.5 | CH2 | 131.1 | CH | 35.6 | CH2 | 41.5 | CH2 | 35.7 | CH2 |
| 3 | 31.3 | CH | 38.2 | CH | 33.9 | CH | 38.2 | CH | 31.3 | CH |
| 4 | 37.5 | CH | 36.2 | CH | 36.5 | CH | 42.9 | CH | 37.6 | CH |
| 5 | 26.6 | CH2 | 27.6 | CH2 | 26.6 | CH2 | 23.0 | CH2 | 26.7 | CH2 |
| 6 | 33.8 | CH2 | 34.1 | CH2 | 33.9 | CH2 | 32.4 | CH2 | 33.1 (34.8) | CH2 |
| 7 | 61.8 | C | 64.0 | C | 60.9 | C | 54.0 (56.1) | C | 54.0 (56.9) | C |
| 8 | 43.8 | CH | 46.3 | CH | 46.2 | CH | 42.7 (40.4) | CH | 44.6 (40.8) | CH |
| 9 | 24.8 | CH2 | 18.1 | CH2 | 24.6 | CH2 | 19.0 | CH2 | 24.4 | CH2 |
| 10 | 121.9 | CH | 32.1 | CH2 | 36.3 | CH2 | 33.3 | CH2 | 122.1 | CH |
| 11 | 134.2 | C | 29.0 | CH | nd | - | 147.3 | C | 134.4 | C |
| 12 | 44.5 | CH | 44.2 | CH | 46.6 | CH | 49.8 | CH | 44.6 | CH |
| 13 | 34.5 | CH | 37.4 | CH | 37.5 | CH | 44.7 | CH | 35.3 (37.9) | CH |
| 14 | 127.3 | CH | 126.0 | CH | 127.9 | CH | 41.3 | CH2 | 127.8 | CH |
| 15 | 132.3 | C | 134.0 | C | 130.5 | C | 144.0 | C | 130.9 | C |
| 16 | 25.8 | CH3 | 26.7 | CH3 | 26.0 | CH3 | 20.9 | CH3 | 26.1 | CH3 |
| 17 | 17.7 | CH3 | 19.5 | CH3 | 17.8 | CH3 | 109.1 | CH2 | 17.8 | CH3 |
| 18 | 19.6 | CH3 | 19.9 | CH3 | 19.7 | CH3 | 18.4 | CH3 | 20.0 | CH3 |
| 19 | 26.4 | CH3 | 26.0 | CH3 | 26.4 | CH3 | 27.1 (23.9) | CH3 | 26.8 (23.6) | CH3 |
| 20 | 21.0 | CH3 | 17.2 | CH3 | 108.1 | CH2 | 104.6 | CH2 | 21.1 | CH3 |
| NC | nd | - | 153.4 | C | 153.1 | C | - | - | - | - |
| NHCHO | - | - | - | 162.7 (159.9) | CH | 162.8 (160.0) | CH | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, M.; Ciavatta, M.L.; Manzo, E.; Li, X.-L.; Mollo, E.; Mudianta, I.W.; Guo, Y.-W.; Gavagnin, M. Amphilectene Diterpene Isonitriles and Formamido Derivatives from the Hainan Nudibranch Phyllidia Coelestis. Mar. Drugs 2019, 17, 603. https://doi.org/10.3390/md17110603
Carbone M, Ciavatta ML, Manzo E, Li X-L, Mollo E, Mudianta IW, Guo Y-W, Gavagnin M. Amphilectene Diterpene Isonitriles and Formamido Derivatives from the Hainan Nudibranch Phyllidia Coelestis. Marine Drugs. 2019; 17(11):603. https://doi.org/10.3390/md17110603
Chicago/Turabian StyleCarbone, Marianna, Maria Letizia Ciavatta, Emiliano Manzo, Xiao-Lu Li, Ernesto Mollo, I Wayan Mudianta, Yue-Wei Guo, and Margherita Gavagnin. 2019. "Amphilectene Diterpene Isonitriles and Formamido Derivatives from the Hainan Nudibranch Phyllidia Coelestis" Marine Drugs 17, no. 11: 603. https://doi.org/10.3390/md17110603
APA StyleCarbone, M., Ciavatta, M. L., Manzo, E., Li, X. -L., Mollo, E., Mudianta, I. W., Guo, Y. -W., & Gavagnin, M. (2019). Amphilectene Diterpene Isonitriles and Formamido Derivatives from the Hainan Nudibranch Phyllidia Coelestis. Marine Drugs, 17(11), 603. https://doi.org/10.3390/md17110603