Phytoene Accumulation in the Novel Microalga Chlorococcum sp. Using the Pigment Synthesis Inhibitor Fluridone
Abstract
:1. Introduction
2. Results
2.1. Strain Identification & Morphology
2.2. Microplate Bioassays
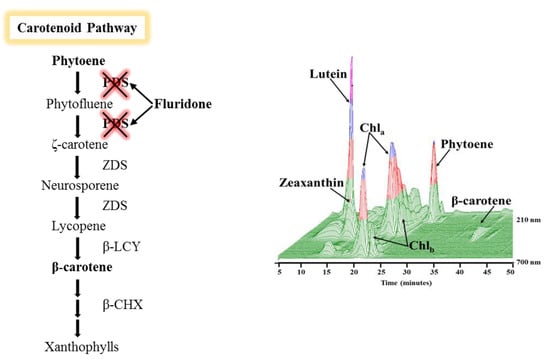
2.3. Phytoene Quantification
2.4. Fatty Acid Analysis
2.5. Intracellular Oil Body Visualization
3. Discussion
3.1. Strain Identification & Microplate Bioassays
3.2. Phytoene Quantification
3.3. FAME Analysis & Confocal Fluorescence Microscopy
4. Conclusions
5. Materials and Methods
5.1. Cultivation
5.2. Strain Identification
5.3. Microscopy
5.4. Microplate Bioassays
5.5. Scale-up Bioassays
5.6. Carotenoid Analyses & Quantification
5.7. Lipid Analysis
5.8. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DOE | Department of Energy |
| GGPP | Geranylgeranyl pyrophosphate |
| PDS | Phytoene desaturase |
| PCR | Polymerase chain reaction |
| HPLC | High pressure liquid chromatography |
| MTBE | Methyl tert-butyl ether |
| SEM | Standard error of the means |
| FAME | Fatty acid methyl ester |
| FA | Fatty acid |
| DIC | Differential interference contrast |
| SEM | Scanning electron microscopy |
| ANOVA | Analysis of variance |
References
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K. Marine Cosmeceuticals: Trends and Prospects; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.; Mesquita, J. Ultrastructural Study of Haematococcus lacustris (Girod.) Rostafinski (Volvocales). Cytologia 1984, 49, 215–228. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1. [Google Scholar] [CrossRef]
- Paiva, S.A.; Russell, R.M. β-Carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef]
- Salguero, A.; de la Morena, B.; Vigara, J.; Vega, J.M.; Vilchez, C.; León, R. Carotenoids as protective response against oxidative damage in Dunaliella bardawil. Biomol. Eng. 2003, 20, 249–253. [Google Scholar] [CrossRef]
- Haegele, A.D.; Gillette, C.; O’Neill, C.; Wolfe, P.; Heimendinger, J.; Sedlacek, S.; Thompson, H.J. Plasma xanthophyll carotenoids correlate inversely with indices of oxidative DNA damage and lipid peroxidation. Cancer Epidemiol. Prev. Biomark. 2000, 9, 421–425. [Google Scholar]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Fuller, B.; Smith, D.; Howerton, A.; Kern, D. Anti-inflammatory effects of CoQ10 and colorless carotenoids. J. Cosmet. Dermatol. 2006, 5, 30–38. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. TRENDS Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Bartley, G.E.; Scolnik, P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell 1995, 7, 1027–1038. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Benítez-González, A.; Stinco, C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, T. Functions of carotenoids. In The Biochemistry of the Carotenoids; Springer: Berlin, Germany, 1980; pp. 77–95. [Google Scholar]
- Grung, M.; Liaaen-Jensen, S. Algal carotenoids 52; secondary carotenoids of algae 3; carotenoids in a natural bloom of Euglena sanguinea. Biochem. Syst. Ecol. 1993, 21, 757–763. [Google Scholar] [CrossRef]
- Katz, A.; Jimenez, C.; Pick, U. Isolation and characterization of a protein associated with carotene globules in the alga Dunaliella bardawil. Plant Physiol. 1995, 108, 1657–1664. [Google Scholar] [CrossRef]
- Pick, U.; Zarka, A.; Boussiba, S.; Davidi, L. A hypothesis about the origin of carotenoid lipid droplets in the green algae Dunaliella and Haematococcus. Planta 2019, 249, 31–47. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Zarka, A.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S.J.J. Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga haematococcus pluvialis (Chlorophyceae) 1. J. Phycol. 2005, 41, 819–826. [Google Scholar] [CrossRef]
- Jin, E.-S.; Lee, C.-G.; Polle, J.E. Secondary carotenoid accumulation in Haematococcus (Chlorophyceae): Biosynthesis, regulation, and biotechnology. J. Microbiol. Biotechnol. 2006, 16, 821–831. [Google Scholar]
- Von Oppen-Bezalel, L. Colorless carotenoids, phytoene and phytofluene for the skin: For prevention of aging/photo-aging from the inside and out. SÖFW-Journal 2007, 133, 38–40. [Google Scholar]
- Von Oppen-Bezalel, L.; Fishbein, D.; Havas, F.; Ben-Chitrit, O.; Khaiat, A.J.G.D. The photoprotective effects of a food supplement tomato powder rich in phytoene and phytofluene, the colorless carotenoids, a preliminary study. Glob. Dermatol. 2015, 2, 178–182. [Google Scholar]
- Engelmann, N.J.; Clinton, S.K.; Erdman, J.W., Jr. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv. Nutr. 2011, 2, 51–61. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Gressel, J.; Avron, M.J. Massive accumulation of phytoene induced by norflurazon in dunaliella bardawil (Chlorophyceae) prevents recovery from photoinhibition 1. J. Phycol. 1987, 23, 176–181. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Lers, A.; Avron, M.J.P.P. Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef]
- Soudant, E.; Bezalel, L.; Schickler, H.; Paltiel, J.; Ben-Amotz, A.; Shaish, A.; Perry, I. Carotenoid Preparation. U.S. Patent No. 6,383,474, 7 May 2002. [Google Scholar]
- Shaish, A.; Avron, M.; Ben-Amotz, A. Effect of inhibitors on the formation of stereoisomers in the biosynthesis of β-carotene in Dunaliella bardawil. Plant Cell Physiol. 1990, 31, 689–696. [Google Scholar]
- Harker, M.; Young, A.J. Inhibition of astaxanthin synthesis in the green alga, Haematococcus pluvialis. Eur. J. Phycol. 1995, 30, 179–187. [Google Scholar] [CrossRef]
- Tjahjono, A.E.; Kakizono, T.; Hayama, Y.; Nishio, N.; Nagai, S. Isolation of resistant mutants against carotenoid biosynthesis inhibitors for a green alga Haematococcus pluvialis, and their hybrid formation by protoplast fusion for breeding of higher astaxanthin producers. J. Ferment. Bioeng. 1994, 77, 352–357. [Google Scholar] [CrossRef]
- Chamovitz, D.; Sandmann, G.; Hirschberg, J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J. Biol. Chem. 1993, 268, 17348–17353. [Google Scholar]
- Chalifour, A.; Arts, M.T.; Kainz, M.J.; Juneau, P. Combined effect of temperature and bleaching herbicides on photosynthesis, pigment and fatty acid composition of Chlamydomonas reinhardtii. Eur. J. Phycol. 2014, 49, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine carotenoids: Biological functions and commercial applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G.; Fraser, P.D. Differential inhibition of phytoene desaturases from diverse origins and analysis of resistant cyanobacterial mutants. Z. Naturforsch. C 1993, 48, 307–311. [Google Scholar] [CrossRef]
- Sharon-Gojman, R.; Maimon, E.; Leu, S.; Zarka, A.; Boussiba, S. Advanced methods for genetic engineering of Haematococcus pluvialis (Chlorophyceae, Volvocales). Algal Res. 2015, 10, 8–15. [Google Scholar] [CrossRef]
- Suarez, J.V.; Banks, S.; Thomas, P.G.; Day, A. A new F131V mutation in Chlamydomonas phytoene desaturase locates a cluster of norflurazon resistance mutations near the FAD-binding site in 3D protein models. PLoS ONE 2014, 9, e99894. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, J.; Sandmann, G. Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl. Environ. Microbiol. 2006, 72, 7477–7484. [Google Scholar] [CrossRef] [PubMed]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Floyd, G.L. Variation in the ultrastructure of the biflagellate motile cells of six unicellular genera of the Chlamydomonadales and Chlorococcales (Chlorophyceae), with emphasis on the flagellar apparatus. Am. J. Bot. 1989, 76, 307–317. [Google Scholar] [CrossRef]
- Miller, D.H. Cell wall chemistry and ultrastructure of chlorococcum oleofaciens (Chlorophyceae) 1, 2. J. Phycol. 1978, 14, 189–194. [Google Scholar] [CrossRef]
- Lammers, P.J.; Huesemann, M.; Boeing, W.; Anderson, D.B.; Arnold, R.G.; Bai, X.; Bhole, M.; Brhanavan, Y.; Brown, L.; Brown, J. Review of the cultivation program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res. 2017, 22, 166–186. [Google Scholar] [CrossRef]
- Chekanov, K.; Vasilieva, S.; Solovchenko, A.; Lobakova, E. Reduction of photosynthetic apparatus plays a key role in survival of the microalga Haematococcus pluvialis (Chlorophyceae) at freezing temperatures. Photosynthetica 2018, 56, 1268–1277. [Google Scholar] [CrossRef]
- Bišová, K.; Zachleder, V. Cell-cycle regulation in green algae dividing by multiple fission. J. Exp. Bot. 2014, 65, 2585–2602. [Google Scholar] [CrossRef] [Green Version]
- Franz, A.K.; Danielewicz, M.A.; Wong, D.M.; Anderson, L.A.; Boothe, J.R. Phenotypic screening with oleaginous microalgae reveals modulators of lipid productivity. ACS Chem. Biol. 2013, 8, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Dankov, K.; Busheva, M.; Stefanov, D.; Apostolova, E.L. Relationship between the degree of carotenoid depletion and function of the photosynthetic apparatus. J. Photochem. Photobiol. B Biol. 2009, 96, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Trebst, A.; Depka, B. Role of carotene in the rapid turnover and assembly of photosystem II in Chlamydomonas reinhardtii. FEBS Lett. 1997, 400, 359–362. [Google Scholar] [CrossRef]
- Rise, M.; Cohen, E.; Vishkautsan, M.; Cojocaru, M.; Gottlieb, H.E.; Arad, S.M. Accumulation of secondary carotenoids in Chlorella zofingiensis. J. Plant Physiol. 1994, 144, 287–292. [Google Scholar] [CrossRef]
- Vechtel, B.; Eichenberger, W.; Ruppel, G. Lipid bodies in Eremosphaera viridis De Bary (Chlorophyceae). Plant Cell Physiol. 1992, 33, 41–48. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Uribe, L.; Guzman, I.; Rajapakse, W.; Richins, R.D.; O’connell, M.A. Carotenoid accumulation in orange-pigmented Capsicum annuum fruit, regulated at multiple levels. J. Exp. Bot. 2011, 63, 517–526. [Google Scholar] [CrossRef] [PubMed]





| Total Phytoene (μg/mg) | Total Carotenoids (μg/mg) | |||||
|---|---|---|---|---|---|---|
| Day 4 Harvest | ||||||
| Treatments (μM) | MEAN | SEM | MEAN | SEM | ||
| 304 | 33.8 | ± | 1.7 | 40.4 | ± | 11.5 |
| 152 | 33 | ± | 0.3 | 38.6 | ± | 1.1 |
| 0 | N.D. | ± | N.D. | 70.1 | ± | 7.5 |
| Day 9 Harvest | ||||||
| Treatments (μM) | MEAN | SEM | MEAN | SEM | ||
| 304 | 4.6 | ± | 0.9 | 68.3 | ± | 4.9 |
| 152 | 14.6 | ± | 0.6 | 66.1 | ± | 4.8 |
| 0 | N.D. | ± | N.D. | 145.1 | ± | 7.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laje, K.; Seger, M.; Dungan, B.; Cooke, P.; Polle, J.; Holguin, F.O. Phytoene Accumulation in the Novel Microalga Chlorococcum sp. Using the Pigment Synthesis Inhibitor Fluridone. Mar. Drugs 2019, 17, 187. https://doi.org/10.3390/md17030187
Laje K, Seger M, Dungan B, Cooke P, Polle J, Holguin FO. Phytoene Accumulation in the Novel Microalga Chlorococcum sp. Using the Pigment Synthesis Inhibitor Fluridone. Marine Drugs. 2019; 17(3):187. https://doi.org/10.3390/md17030187
Chicago/Turabian StyleLaje, Kelly, Mark Seger, Barry Dungan, Peter Cooke, Juergen Polle, and F. Omar Holguin. 2019. "Phytoene Accumulation in the Novel Microalga Chlorococcum sp. Using the Pigment Synthesis Inhibitor Fluridone" Marine Drugs 17, no. 3: 187. https://doi.org/10.3390/md17030187
APA StyleLaje, K., Seger, M., Dungan, B., Cooke, P., Polle, J., & Holguin, F. O. (2019). Phytoene Accumulation in the Novel Microalga Chlorococcum sp. Using the Pigment Synthesis Inhibitor Fluridone. Marine Drugs, 17(3), 187. https://doi.org/10.3390/md17030187