Bioactive Compounds Isolated from Marine-Derived Microbes in China: 2009–2018
Abstract
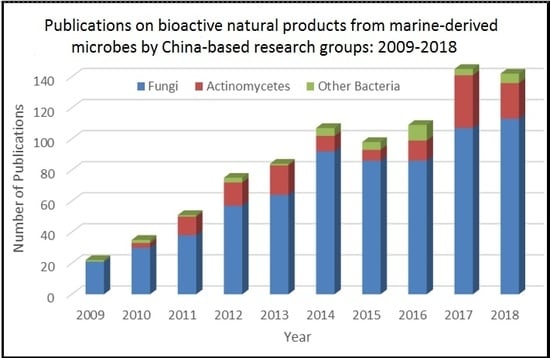
:1. Introduction
2. Polyketides
2.1. Bacterial Polyketides
2.2. Fungal Polyketides
3. Peptides
3.1. Bacterial Peptides
3.2. Fungal Peptides
4. Alkaloids
4.1. Bacterial Alkaloids
4.2. Fungal Alkaloids
5. Terpenoids, Terpenes and Meroterpenoids
6. Mixed-Structure Compounds
7. Other Compounds
8. Discussion
9. Methodology
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cragg, G.M.; Newman, D.J.; Snader, K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 3864, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef]
- Knight, V.; Sanglier, J.J.; DiTullio, D.; Braccili, S.; Bonner, P.; Waters, J.; Hughes, D.; Zhang, L. Diversifying microbial natural products for drug discovery. Appl. Microbiol. Biotechnol. 2003, 62, 446–458. [Google Scholar] [CrossRef]
- Carrano, L.; Marinelli, F. The relevance of chemical dereplication in microbial natural product screening. J. Appl. Bioanal. 2015, 1, 55–67. [Google Scholar] [CrossRef]
- Handelsmanl, J.; Rondon, M.R.; Goodman, R.M.; Brady, S.F.; Clardy, J. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem. Biol. 1998, 5, 245–249. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Sekurova, O.N.; Schneider, O.; Zotchev, S.B. Minireview Novel bioactive natural products from bacteria via bioprospecting, genome mining and metabolic engineering. Microb. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Dannert, C. NextGen microbial natural products discovery. Microb. Biotechnol. 2015, 8, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhu, G.; Fan, Y.; Du, Y.; Lan, M.; Xu, Y.; Zhu, W. Natural products research in China from 2015 to 2016. Front. Chem. 2018, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.Y.; Tan, R.X. Artemisinin, a miracle of traditional Chinese medicine. Nat. Prod. Rep. 2015, 32, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B.; Warude, D.; Pushpangadan, P.; Bhatt, N. Ayurveda and traditional Chinese medicine: A comparative overview. Evidence-Based Complement. Altern. Med. 2005, 2, 465–473. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Z.; Li, X.; Wang, A.; Wu, H.; Liu, J.; Cao, S.; Liu, Q. Salviachinensines A–F, Antiproliferative phenolic derivatives from the Chinese medicinal plant Salvia chinensis. J. Nat. Prod. 2018, 81, 2531–2538. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, Y.; Zhou, H.; Zhou, J.; Zhang, H.; Zhang, M.; Zeng, H.; Yao, G. Grayanane diterpenoid glucosides from the leaves of Rhododendron micranthum and their bioactivities evaluation. J. Nat. Prod. 2018, 81, 2673–2681. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, T.; Zhu, W. New marine natural products of microbial origin from 2010 to 2013. Chinese J. Org. Chem. 2013, 33, 1195–1234. [Google Scholar] [CrossRef]
- Li, Z. Advances in marine microbial symbionts in the China Sea and related pharmaceutical metabolites. Mar. Drugs 2009, 8, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.Q.; Wang, J.F.; Hao, Y.Y.; Wang, Y. Recent advances in the discovery and development of marine microbial natural products. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, Z.G.; Song, B.B.; Chen, C.H.; Xiao, W.W.; Hu, B.; Wang, J.W.; Jiang, X.B.; Zhu, Y.H.; Wang, H.J. Advances in the study of the structures and bioactivities of metabolites isolated from mangrove-derived fungi in the South China Sea. Mar. Drugs 2013, 11, 3601–3616. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Bai, L.; Deng, Z. Toward steadfast growth of antibiotic research in China: From natural products to engineered biosynthesis. Biotechnol. Adv. 2012, 30, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 4, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Khosla, C. Structures and mechanisms of polyketide synthases. J. Org. Chem. 2009, 74, 6416–6420. [Google Scholar] [CrossRef]
- Gao, C.H.; Tian, X.P.; Qi, S.H.; Luo, X.M.; Wang, P.; Zhang, S.J. Antibacterial and antilarval compounds from marine gorgonian-associated bacterium Bacillus amyloliquefaciens SCSIO 00856. J. Antibiot. (Tokyo) 2010, 63, 191–193. [Google Scholar] [CrossRef]
- Wu, C.; Tan, Y.; Gan, M.; Wang, Y.; Guan, Y.; Hu, X.; Zhou, H.; Shang, X.; You, X.; Yang, Z.; et al. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using PCR-based screening. J. Nat. Prod. 2013, 76, 2153–2157. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.; Qin, F.; Sun, C.; Liang, H.; Wei, X.; Wong, N.K.; Ye, L.; Zhang, Y.; Shao, M.; et al. Neoabyssomicins A–C, polycyclic macrolactones from the deep-sea derived Streptomyces koyangensis SCSIO 5802. Tetrahedron 2017, 73, 5366–5372. [Google Scholar] [CrossRef]
- Huang, H.; Song, Y.; Li, X.; Wang, X.; Ling, C.; Qin, X.; Zhou, Z.; Li, Q.; Wei, X.; Ju, J. Abyssomicin monomers and dimers from the marine-derived Streptomyces koyangensis SCSIO 5802. J. Nat. Prod. 2018, 81, 1892–1898. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, L.; Pei, X.; Deng, F.; Hu, D.; Chen, G.; Wang, C.; Hong, K.; Yao, X.; Gao, H. Chalcomycins from marine-derived Streptomyces sp. and their antimicrobial activities. Mar. Drugs 2017, 15, 153. [Google Scholar] [CrossRef]
- Nong, X.H.; Zhang, X.Y.; Xu, X.Y.; Wang, J.; Qi, S.H. Nahuoic Acids B–E, Polyhydroxy polyketides from the marine-derived Streptomyces sp. SCSGAA 0027. J. Nat. Prod. 2016, 79, 141–148. [Google Scholar] [CrossRef]
- Gong, T.; Zhen, X.; Li, X.L.; Chen, J.J.; Chen, T.J.; Yang, J.L.; Zhu, P. Tetrocarcin Q, a new spirotetronate with a unique glycosyl group from a marine-derived actinomycete Micromonospora carbonacea LS276. Mar. Drugs 2018, 16, 74. [Google Scholar] [CrossRef]
- Gan, M.; Liu, B.; Tan, Y.; Wang, Q.; Zhou, H.; He, H.; Ping, Y.; Yang, Z.; Wang, Y.; Xiao, C. Saccharothrixones A–D, tetracenomycin-type polyketides from the marine-derived actinomycete Saccharothrix sp. 10-10. J. Nat. Prod. 2015, 78, 2260–2265. [Google Scholar] [CrossRef]
- Akhter, N.; Liu, Y.; Auckloo, B.N.; Shi, Y.; Wang, K.; Chen, J.; Wu, X.; Wu, B. Stress-driven discovery of new angucycline-type antibiotics from a marine Streptomyces pratensis NA-ZhouS1. Mar. Drugs 2018, 16, 331. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, J.; Wang, L.; Wang, Y.; Kong, F.; Zhu, W. New α-glucosidase inhibitors from marine algae-derived Streptomyces sp. OUCMDZ-3434. Sci. Rep. 2016, 6, 20004. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Lu, X.; Chen, H.; Liu, L.; Yu, Z.; Chen, Y. Hexaricins, pradimicin-like polyketides from a marine sediment-derived Streptosporangium sp. and their antioxidant effects. J. Nat. Prod. 2018, 81, 2069–2074. [Google Scholar] [CrossRef]
- Anjum, K.; Sadiq, I.; Chen, L.; Kaleem, S.; Li, X.C.; Zhang, Z.; Lian, X.Y. Novel antifungal janthinopolyenemycins A and B from a co-culture of marine-associated Janthinobacterium spp. ZZ145 and ZZ148. Tetrahedron Lett. 2018, 59, 3490–3494. [Google Scholar] [CrossRef]
- Sun, P.; Xu, D.X.; Mándi, A.; Kurtán, T.; Li, T.J.; Schulz, B.; Zhang, W. Structure, absolute configuration, and conformational study of 12-membered macrolides from the fungus Dendrodochium sp. associated with the sea cucumber Holothuria nobilis Selenka. J. Org. Chem. 2013, 78, 7030–7047. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhang, Z.H.; Zhong, Y.; Huang, J.J.; Li, X.X.; Jiang, J.Y.; Deng, Y.Y.; Zhang, L.H.; He, F. Sumalactones A-D, four new curvularin-type macrolides from a marine deep sea fungus: Penicillium sumatrense. RSC Adv. 2017, 7, 40015–40019. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Huang, J.; Proksch, P.; Zhu, K.; Lin, W. Hansforesters A–M, polyesters from the sponge-associated fungus Hansfordia sinuosae with antibacterial activities. RSC Adv. 2018, 8, 39756–39768. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Liu, C.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Clindanones A and B and cladosporols F and G, polyketides from the deep-sea derived fungus: Cladosporium cladosporioides HDN14-342. RSC Adv. 2016, 6, 76498–76504. [Google Scholar] [CrossRef]
- Bao, J.; Sun, Y.L.; Zhang, X.Y.; Han, Z.; Gao, H.C.; He, F.; Qian, P.Y.; Qi, S.H. Antifouling and antibacterial polyketides from marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. J. Antibiot. (Tokyo) 2013, 66, 219–223. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Lin, S.T.; Kumaravel, K.; Zhou, H.; Wang, S.Y.; Liu, Y.H. Polyketide-derived metabolites from the sponge-derived fungus Aspergillus sp. F40. Phytochem. Lett. 2018, 27, 74–77. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, P.; Li, J.; Chen, G.; Chen, Y.; Liu, H.; She, Z. Anti-inflammatory polyketides from the mangrove-derived fungus Ascomycota sp. SK2YWS-L. Tetrahedron 2018, 74, 746–751. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Liu, W.; Liu, Y.; Huang, X.; She, Z. Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2. Mar. Drugs 2016, 14, 1–7. [Google Scholar] [CrossRef]
- Zhu, G.; Kong, F.; Wang, Y.; Fu, P.; Zhu, W. Cladodionen, a cytotoxic hybrid polyketide from the marine-derived Cladosporium sp. OUCMDZ-1635. Mar. Drugs 2018, 16, 71. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.Y.; Lan, R.F.; Du, L.; Wang, B.S.; Zhou, T.; Li, Y.P.; Zhang, Q.Q.; Ying, M.G.; Zheng, Q.H.; et al. Dicitrinone D, an antimitotic polyketide isolated from the marine-derived fungus Penicillium citrinum. Tetrahedron 2017, 73, 5900–5911. [Google Scholar] [CrossRef]
- Chen, X.W.; Li, C.W.; Cui, C.B.; Hua, W.; Zhu, T.J.; Gu, Q.Q. Nine new and five known polyketides derived from a deep sea-sourced Aspergillus sp. 16-02-1. Mar. Drugs 2014, 12, 3116–3137. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.M.; Meng, L.H.; Wang, B.G. Polyketides from the marine mangrove-derived fungus Aspergillus ochraceus MA-15 and their activity against aquatic pathogenic bacteria. Phytochem. Lett. 2015, 12, 232–236. [Google Scholar] [CrossRef]
- Fang, F.; Zhao, J.; Ding, L.; Huang, C.; Naman, C.B.; He, S.; Wu, B.; Zhu, P.; Luo, Q.; Gerwick, W.H.; et al. 5-Hydroxycyclopenicillone, a new β-amyloid fibrillization inhibitor from a sponge-derived fungus Trichoderma sp. HPQJ-34. Mar. Drugs 2017, 15, 260. [Google Scholar] [CrossRef]
- Xing, P.Y.; Liu, R.; Zhang, D.C.; SUN, C.M. Pumilacidin-like lipopeptides derived from marine bacterium Bacillus sp. Strain 176 suppress the motility of Vibrio alginolyticus. Appl. Environ. Microbiol. 2017, 83, e00450-17. [Google Scholar]
- Wang, Q.; Zhang, Y.; Wang, M.; Tan, Y.; Hu, X.; He, H.; Xiao, C.; You, X.; Wang, Y.; Gan, M. Neo-actinomycins A and B, natural actinomycins bearing the 5H-oxazolo[4,5-b]phenoxazine chromophore, from the marine-derived Streptomyces sp. IMB094. Sci. Rep. 2017, 7, 4–11. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W.; Jiang, H.L.; Zhou, J.; Chen, X.M.; Lian, Y.Y.; Jiang, H.; Lin, F. Rakicidins G–I, cyclic depsipeptides from marine Micromonospora chalcea FIM 02-523. Tetrahedron 2018, 74, 4151–4154. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Zhang, H.; Sun, J.; Zheng, L.; Liu, H.; Wang, J.; Shen, A.; Geng, M.; Guo, Y. Chromopeptide A, a highly cytotoxic depsipeptide from the marine sediment-derived bacterium Chromobacterium sp. HS-13-94. Acta Pharm. Sin. B 2015, 5, 62–66. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, Z.; Huang, J.; Liu, D.; Wu, C.; Guo, P.; Lin, W. Versicotides D–F, new cyclopeptides with lipid-lowering activities. RSC Adv. 2017, 7, 49235–49243. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Li, N.; Li, F.; Zhu, T.; Gu, Q.; Guo, P.; Li, D. Saroclides A and B, cyclic depsipeptides from the mangrove-derived fungus Sarocladium kiliense HDN11-112. J. Nat. Prod. 2018, 81, 1050–1054. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, X.Y.; Nong, X.H.; Wang, J.; Huang, Z.H.; Qi, S.H. Eight linear peptides from the deep-sea-derived fungus Simplicillium obclavatum EIODSF 020. Tetrahedron 2016, 72, 3092–3097. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Chen, Y.; Huang, H.; Zhang, W.; Ju, J. Cyclic heptapeptides, cordyheptapeptides C–E, from the marine-derived fungus Acremonium persicinum SCSIO 115 and their cytotoxic activities. J. Nat. Prod. 2012, 75, 1215–1219. [Google Scholar] [CrossRef]
- Yang, X.W.; Zhang, G.Y.; Ying, J.X.; Yang, B.; Zhou, X.F.; Steinmetz, A.; Liu, Y.H.; Wang, N. Isolation, characterization, and bioactivity evaluation of 3-((6-methylpyrazin-2-yl)methyl)-1H-indole, a new alkaloid from a deep-sea-derived actinomycete Serinicoccus profundi sp. nov. Mar. Drugs 2013, 11, 33–39. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Chai, W.; Lian, X.Y.; Zhang, Z. A unique indolizinium alkaloid streptopertusacin A and bioactive bafilomycins from marine-derived Streptomyces sp. HZP-2216E. Phytochemistry 2017, 144, 119–126. [Google Scholar] [CrossRef]
- Wang, P.; Kong, F.; Wei, J.; Wang, Y.; Wang, W.; Hong, K.; Zhu, W. Alkaloids from the mangrove-derived actinomycete Jishengella endophytica 161111. Mar. Drugs 2014, 12, 477–490. [Google Scholar] [CrossRef]
- Che, Q.; Qiao, L.; Han, X.; Liu, Y.; Wang, W.; Gu, Q.; Zhu, T.; Li, D. Anthranosides A–C, anthranilate derivatives from a sponge-derived Streptomyces sp. CMN-62. Org. Lett. 2018, 20, 5466–5469. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Y.; He, Z.; Yang, T.; Ma, J.; Tian, X.; Li, Y.; Huang, C.; Chen, X.; Li, W.; et al. Antimalarial β-carboline and indolactam alkaloids from Marinactinospora thermotolerans, a deep sea isolate. J. Nat. Prod. 2011, 74, 2122–2127. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Shao, Z.; Ren, J.; Liu, D.; Proksch, P.; Lin, W. Indole-based alkaloids from deep-sea bacterium Shewanella piezotolerans with antitumor activities. J. Antibiot. (Tokyo) 2014, 67, 395–399. [Google Scholar] [CrossRef]
- Cheng, Z.; Lou, L.; Liu, D.; Li, X.; Proksch, P.; Yin, S.; Lin, W. Versiquinazolines A–K, fumiquinazoline-type alkaloids from the gorgonian-derived fungus Aspergillus versicolor LZD-14-1. J. Nat. Prod. 2016, 79, 2941–2952. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, D.; Cheng, W.; Proksch, P.; Lin, W. Versiquinazolines L–Q, new polycyclic alkaloids from the marine-derived fungus Aspergillus versicolor. RSC Adv. 2018, 8, 31427–31439. [Google Scholar] [CrossRef]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Li, G.; Zhu, W. New quinazolinone alkaloids within rare amino acid residue from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Org. Lett. 2011, 13, 1130–1133. [Google Scholar] [CrossRef]
- Song, F.; Ren, B.; Yu, K.; Chen, C.; Guo, H.; Yang, N.; Gao, H.; Liu, X.; Liu, M.; Tong, Y.; et al. Quinazolin-4-one coupled with pyrrolidin-2-iminium alkaloids from marine-derived fungus Penicillium aurantiogriseum. Mar. Drugs 2012, 10, 1297–1306. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Yu, R.; Feng, Y.; Wang, L.; Che, Q.; Gu, Q.; Li, D.; Li, J.; Zhu, T. Chetracins E and F, cytotoxic epipolythiodioxopiperazines from the marine-derived fungus: Acrostalagmus luteoalbus HDN13-530. RSC Adv. 2018, 8, 53–58. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.; Li, X.M.; Zhu, L.W.; Wang, B.G. Indolediketopiperazine alkaloids from Eurotium cristatum EN-220, an endophytic fungus isolated from the marine alga Sargassum thunbergii. Mar. Drugs 2017, 15, 24. [Google Scholar] [CrossRef]
- Zhang, P.; Mandi, A.; Li, X.M.; Du, F.Y.; Wang, J.N.; Li, X.; Kurtan, T.; Wang, B.G. Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org. Lett. 2014, 16, 4834–4837. [Google Scholar] [CrossRef]
- Zhong, W.M.; Wang, J.F.; Shi, X.F.; Wei, X.Y.; Chen, Y.C.; Zeng, Q.; Xiang, Y.; Chen, X.Y.; Tian, X.P.; Xiao, Z.H.; et al. Eurotiumins A–E, five new alkaloids from the marine-derived fungus Eurotium sp. SCSIO F452. Mar. Drugs 2018, 16, 136. [Google Scholar] [CrossRef]
- Li, H.; Sun, W.; Deng, M.; Zhou, Q.; Wang, J.; Liu, J.; Chen, C.; Qi, C.; Luo, Z.; Xue, Y.; et al. Asperversiamides, linearly fused prenylated indole alkaloids from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2018, 83, 8483–8492. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Nong, X.; Wei, X.; Qi, S. Oxindole alkaloids from the fungus Penicillium commune DFFSCS026 isolated from deep-sea-derived sediments. Tetrahedron 2015, 71, 610–615. [Google Scholar] [CrossRef]
- Zhou, R.; Liao, X.; Li, H.; Li, J.; Feng, P.; Zhao, B.; Xu, S. Isolation and synthesis of misszrtine A: A novel indole alkaloid from marine sponge-associated Aspergillus sp. SCSIO XWS03F03. Front. Chem. 2018, 6, 212. [Google Scholar] [CrossRef]
- Wu, C.J.; Li, C.W.; Gao, H.; Huang, X.J.; Cui, C.B. Penicimutamides D–E: Two new prenylated indole alkaloids from a mutant of the marine-derived: Penicillium purpurogenum G59. RSC Adv. 2017, 7, 24718–24722. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Feng, H.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Campyridones A–D, pyridone alkaloids from a mangrove endophytic fungus Campylocarpon sp. HDN13-307. Tetrahedron 2016, 72, 5679–5683. [Google Scholar] [CrossRef]
- Cui, H.; Yu, J.; Chen, S.; Ding, M.; Huang, X.; Yuan, J.; She, Z. Alkaloids from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. Bioorganic Med. Chem. Lett. 2017, 27, 803–807. [Google Scholar] [CrossRef]
- Shao, C.L.; Wang, C.Y.; Gu, Y.C.; Wei, M.Y.; Pan, J.H.; Deng, D.S.; She, Z.G.; Lin, Y.C. Penicinoline, a new pyrrolyl 4-quinolinone alkaloid with an unprecedented ring system from an endophytic fungus Penicillium sp. Bioorganic Med. Chem. Lett. 2010, 20, 3284–3286. [Google Scholar] [CrossRef]
- Ji, N.Y.; Liu, X.H.; Miao, F.P.; Qiao, M.F. Aspeverin, a new alkaloid from an algicolous strain of Aspergillus versicolor. Org. Lett. 2013, 15, 2327–2329. [Google Scholar] [CrossRef]
- Zhang, Z.; Min, X.; Huang, J.; Zhong, Y.; Wu, Y.; Li, X.; Deng, Y.; Jiang, Z.; Shao, Z.; Zhang, L.; et al. Cytoglobosins H and I, new antiproliferative cytochalasans from deep-sea-derived fungus Chaetomium globosum. Mar. Drugs 2016, 14, 233. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, D.; Liang, F.; Wu, Z.; She, Z.; Li, C. Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semi-synthesis. Fitoterapia 2017, 123, 23–28. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Zhou, T.; Zhao, Y.Y.; Chen, L.; Gong, M.W.; Xia, Q.W.; Ying, M.G.; Zheng, Q.H.; Zhang, Q.Q. Antitumor effects and related mechanisms of penicitrinine A, a novel alkaloid with a unique spiro skeleton from the marine fungus Penicillium citrinum. Mar. Drugs 2015, 13, 4733–4753. [Google Scholar] [CrossRef]
- He, J.B.; Ji, Y.N.; Hu, D.B.; Zhang, S.; Yan, H.; Liu, X.C.; Luo, H.R.; Zhu, H.J. Structure and absolute configuration of penicilliumine, a new alkaloid from Penicillium commune 366606. Tetrahedron Lett. 2014, 55, 2684–2686. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Punctaporonins H–M: Caryophyllene-Type Sesquiterpenoids from the Sponge-Associated Fungus Hansfordia sinuosae. Mar. Drugs 2014, 3904–3916. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Shao, C.L.; Yang, R.Y.; Zheng, C.J.; Chen, Y.Y.; Fu, X.M.; Qian, P.Y.; She, Z.G.; De Voogd, N.J.; et al. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef]
- Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z.; et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. MedChemComm 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, P.; Liao, G.; Zeng, Y.; Cai, C.; Kong, F.; Guo, Z.; Proksch, P.; Dai, H.; Mei, W. New eudesmane-type sesquiterpenoids from the mangrove-derived endophytic fungus Penicillium sp. J-54. Mar. Drugs 2018, 16, 108. [Google Scholar] [CrossRef]
- Li, H.J.; Xie, Y.L.; Xie, Z.L.; Chen, Y.; Lam, C.K.; Lan, W.J. Chondrosterins A–E, Triquinane-type sesquiterpenoids from soft coral-associated fungus Chondrostereum sp. Mar. Drug. 2012, 10, 627–638. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Cheng, Z.; Proksch, P.; Lin, W. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus: Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017, 7, 7259–7267. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Trichocarotins A–H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg. Chem. 2018, 81, 319–325. [Google Scholar] [CrossRef]
- Fang, W.; Wang, J.; Wang, J.; Shi, L.; Li, K.; Lin, X.; Min, Y.; Yang, B.; Tang, L.; Liu, Y.; et al. Cytotoxic and antibacterial eremophilane sesquiterpenes from the marine-derived fungus Cochliobolus lunatus SCSIO41401. J. Nat. Prod. 2018, 81, 1405–1410. [Google Scholar] [CrossRef]
- Meng, L.H.; Li, X.M.; Liu, Y.; Wang, B.G. Penicibilaenes A and B, sesquiterpenes with a tricyclo[6.3.1.01,5]dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org. Lett. 2014, 16, 6052–6055. [Google Scholar] [CrossRef]
- Yang, X.W.; Peng, K.; Liu, Z.; Zhang, G.Y.; Li, J.; Wang, N.; Steinmetz, A.; Liu, Y. Strepsesquitriol, a rearranged zizaane-type sesquiterpenoid from the deep-sea-derived actinomycete Streptomyces sp. SCSIO 10355. J. Nat. Prod. 2013, 2360–2363. [Google Scholar] [CrossRef]
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559. [Google Scholar] [CrossRef]
- Reveglia, P.; Cimmino, A.; Masi, M.; Nocera, P.; Berova, N.; Ellestad, G.; Evidente, A. Pimarane diterpenes: Natural source, stereochemical configuration, and biological activity. Chirality 2018, 1115–1134. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.L.; Tao, M.; Chen, Y.; Dan, F.; Zhang, W. Scopararanes C–G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella Scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Chen, Y.; Li, S.; Tan, G.; Sun, Z.; Pan, Q.; Ye, W.; Li, H.; Zhang, W. Cytotoxic pimarane-type diterpenes from the marine sediment-derived fungus Eutypella sp. FS46. Nat. Prod. Res. 2017, 31, 404–410. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.; Li, X.M.; Xu, G.M.; Zhang, P.; Meng, L.H.; Wang, B.G. Tetranorlabdane diterpenoids from the deep sea sediment-derived fungus Aspergillus wentii SD-310. Planta Med. 2016, 82, 877–881. [Google Scholar] [CrossRef]
- Li, X.; Li, X.D.; Li, X.M.; Xu, G.M.; Liu, Y.; Wang, B.G. Wentinoids A–F, six new isopimarane diterpenoids from Aspergillus wentii SD-310, a deep-sea sediment derived fungus. RSC Adv. 2017, 7, 4387–4394. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.; Li, X.M.; Xu, G.M.; Liu, Y.; Wang, B.G. 20-nor-isopimarane epimers produced by Aspergillus wentii SD-310, a fungal strain obtained from deep sea sediment. Mar. Drugs 2018, 16, 440. [Google Scholar] [CrossRef]
- Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16-5c. Org. Lett. 2013, 15, 721–723. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, H.; Shao, C.; Xia, X.; Ma, L.; Huang, X.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org. Lett. 2013, 15, 2522–2525. [Google Scholar] [CrossRef]
- Guo, C.J.; Knox, B.P.; Chiang, Y.M.; Lo, H.C.; Sanchez, J.F.; Lee, K.H.; Oakley, B.R.; Bruno, K.S.; Wang, C.C.C. Molecular genetic characterization of a cluster in A. terreus for biosynthesis of the meroterpenoid terretonin. Org. Lett. 2012, 14, 5684–5687. [Google Scholar] [CrossRef]
- Shi, Z.; Miao, F.; Fang, S.; Liu, X.; Yin, X.; Ji, N. Sesteralterin and tricycloalterfurenes A–D: terpenes with rarely occurring frameworks from the marine-alga-epiphytic fungus Alternaria alternata k21-1. J. Nat. Prod. 2017, 80, 2524–2529. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, B.; Liu, D.; Gao, S.; Proksch, P. Brasilianoids A–F, New meroterpenoids from the sponge-associated fungus Penicillium brasilianum. Front. Chem. 2018, 6, 314. [Google Scholar] [CrossRef]
- Chen, S.; Ding, M.; Liu, W.; Huang, X.; Liu, Z.; Lu, Y.; Liu, H.; She, Z. Anti-inflammatory meroterpenoids from the mangrove endophytic fungus Talaromyces amestolkiae YX1. Phytochemistry 2018, 146, 8–15. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, X.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Austalides S–U, new meroterpenoids from the sponge-derived fungus Aspergillus aureolatus HDN14-107. Mar. Drugs 2016, 14, 131. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Jia, C.; Lang, J.; Niaz, S.I.; Li, J.; Yuan, J.; Yu, J.; Chen, S.; Liu, L. Antiviral and anti-inflammatory meroterpenoids: Stachybonoids A–F from the crinoid-derived fungus Stachybotrys chartarum 952. RSC Adv. 2017, 7, 49910–49916. [Google Scholar] [CrossRef]
- Liu, N.; Shang, F.; Xi, L.J.; Huang, Y. Tetroazolemycins A and B, two new oxazole-thiazole siderophores from deep-sea Streptomyces olivaceus FXJ8.012. Mar. Drugs 2013, 11, 1524–1533. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, R.; Xu, J.; Tian, Y.; Xu, J.; Liu, Y. Two new altenusin/thiazole hybrids and a new benzothiazole derivative from the marine sponge-derived fungus Alternaria sp. SCSIOS02F49. Molecules 2018, 23, 2844. [Google Scholar] [CrossRef]
- Chen, H.; Huang, M.; Li, X.; Liu, L.; Chen, B.; Wang, J.; Lin, Y. Phochrodines A–D, first naturally occurring new chromenopyridines from mangrove entophytic fungus Phomopsis sp. 33#. Fitoterapia 2018, 124, 103–107. [Google Scholar]
- Sun, Y.; Liu, J.; Li, L.; Gong, C.; Wang, S.; Yang, F.; Hua, H.; Lin, H. New butenolide derivatives from the marine sponge-derived fungus Aspergillus terreus. Bioorganic Med. Chem. Lett. 2018, 28, 315–318. [Google Scholar] [CrossRef]
- Ding, L.; Li, T.; Liao, X.; He, S.; Xu, S. Asperitaconic acids A–C, antibacterial itaconic acid derivatives produced by a marine-derived fungus of the genus Aspergillus. J. Antibiot. (Tokyo) 2018, 71, 902–904. [Google Scholar] [CrossRef]
- Zhang, S.; Gui, C.; Shao, M.; Kumar, P.S.; Huang, H.; Ju, J. Antimicrobial tunicamycin derivatives from the deep sea-derived Streptomyces xinghaiensis SCSIO S15077. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Huang, D.; Shao, Z.Z.; Yu, Y.; Cai, M.M.; Zheng, L.Y.; Li, G.Y.; Yu, Z.N.; Yi, X.F.; Zhang, J.B.; Hao, F.H. Identification, characteristics and mechanism of 1-deoxy-N-acetylglucosamine from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Mar. Drugs 2018, 16, 52. [Google Scholar] [CrossRef]
- Chen, M.; Wang, K.; Liu, M.; She, Z.; Wang, C. Bioactive Steroid Derivatives and Butyrolactone Derivatives from a Gorgonian-Derived Aspergillus sp. Fungus. 2015, 12, 1398–1406. [Google Scholar]
- Chen, L.; Zhang, Q.Y.; Jia, M.; Ming, Q.L.; Yue, W.; Rahman, K.; Qin, L.P.; Han, T. Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit. Rev. Microbiol. 2016, 42, 454–473. [Google Scholar] [CrossRef]
- Ancheeva, E.; Daletos, G.; Proksch, P. Lead compounds from mangrove-associated microorganisms. Mar. Drugs 2018, 16, 319. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Wu, W.; Liu, X.; Zaleta-Pinet, D.A.; Clark, B.R. Bioactive Compounds Isolated from Marine-Derived Microbes in China: 2009–2018. Mar. Drugs 2019, 17, 339. https://doi.org/10.3390/md17060339
Sun W, Wu W, Liu X, Zaleta-Pinet DA, Clark BR. Bioactive Compounds Isolated from Marine-Derived Microbes in China: 2009–2018. Marine Drugs. 2019; 17(6):339. https://doi.org/10.3390/md17060339
Chicago/Turabian StyleSun, Weiwei, Wenhui Wu, Xueling Liu, Diana A. Zaleta-Pinet, and Benjamin R. Clark. 2019. "Bioactive Compounds Isolated from Marine-Derived Microbes in China: 2009–2018" Marine Drugs 17, no. 6: 339. https://doi.org/10.3390/md17060339
APA StyleSun, W., Wu, W., Liu, X., Zaleta-Pinet, D. A., & Clark, B. R. (2019). Bioactive Compounds Isolated from Marine-Derived Microbes in China: 2009–2018. Marine Drugs, 17(6), 339. https://doi.org/10.3390/md17060339