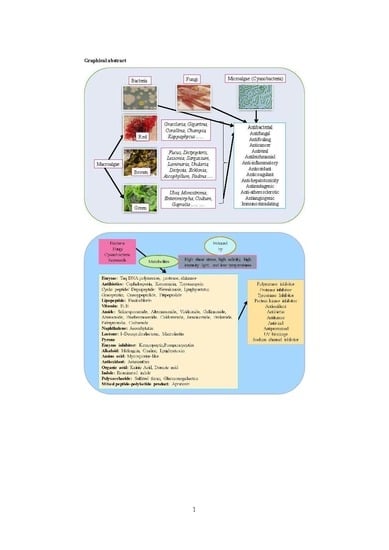
Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology
Abstract
:1. Introduction
2. Bioactive NPs from Marine Bacteria and Fungi
2.1. Antibiotic Activity
2.2. Anticancer Activity
2.3. Antidiabetic Activity
3. Metabolites with Potential Beneficial Activities from Marine Algae
3.1. Marine Microalgae: Blue-Green Algae (Cyanobacteria)
3.1.1. Antibiotic Activity
3.1.2. Antitumor Activity
3.1.3. Antifungal Activity
3.1.4. Antimalarial Activity
3.1.5. Anti-inflammatory Activity
3.2. Marine Macroalgae
3.2.1. Red Seaweeds
Antioxidant Activity
Antibiotic Activity
Antitumor and Anticoagulant Activities
3.2.2. Brown Seaweeds
Antibacterial and Antioxidant Activities
Antidepressant Activity
Anticancer Activity
Antiangiogenic and Anticoagulant Activities
Antiparasitic Activity
3.2.3. Green Seaweeds
Antioxidant and Anticancer Activities
Antibacterial and Antifouling Activities
Anticoagulant Activity
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jahromi, S.T.; Barzkar, N. Marine bacterial chitinase as sources of energy, eco-friendly agent, and industrial biocatalyst. Int. J. Biol. Macromol. 2018, 120, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Rey-Ladino, J.; Ross, A.G.; Cripps, A.W.; McManus, D.P.; Quinn, R. Natural products and the search for novel vaccine adjuvants. Vaccine 2011, 29, 6464–6471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donia, M.; Hamann, M.T. Marine natural products and their potential applications as anti-infective agents. Lancet Infect. Dis. 2003, 3, 338–348. [Google Scholar] [CrossRef]
- Proksch, P.; Edrada, R.; Ebel, R. Drugs from the seas-current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.T.; Barzkar, N. Future direction in marine bacterial agarases for industrial applications. Appl. Microbiol. Biotechnol. 2018, 102, 6847–6863. [Google Scholar] [CrossRef] [PubMed]
- McArthur, K.A.; Mitchell, S.S.; Tsueng, G.; Rheingold, A.; White, D.J.; Grodberg, J.; Lam, K.S.; Potts, B.C. Lynamicins a−e, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. J. Nat. Prod. 2008, 71, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T. Seaweeds in pharmaceutical studies and applications. In XIth International Seaweed Symposium; Bird, C.J., Ragan, M.A., Eds.; Kluwer Academic Publishers Group: Qingdao, China, 1984; Volume 116/117, pp. 29–40. [Google Scholar]
- El-Gendy, M.M.; Hawas, U.W.; Jaspars, M. Novel bioactive metabolites from a marine derived bacterium nocardia sp. Alaa 2000. J. Antibiot. 2008, 61, 379. [Google Scholar] [CrossRef] [PubMed]
- Gil-Turnes, M.S.; Hay, M.E.; Fenical, W. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 1989, 246, 116. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine fungi: A source of potential anticancer compounds. Front. Microbiol. 2018, 8, 2536. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Zhang, D.; Lee, U.; Kang, J.S.; Choi, H.D.; Son, B.W. Dehydroxychlorofusarielin b, an antibacterial polyoxygenated decalin derivative from the marine-derived fungus aspergillus sp. J. Nat. Prod. 2007, 70, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Rukachaisirikul, V.; Sukpondma, Y.; Preedanon, S.; Phongpaichit, S.; Rungjindamai, N.; Sakayaroj, J. Epoxydons and a pyrone from the marine-derived fungus nigrospora sp. Psu-f5. J. Nat. Prod. 2008, 71, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Prachyawarakorn, V.; Mahidol, C.; Sureram, S.; Sangpetsiripan, S.; Wiyakrutta, S.; Ruchirawat, S.; Kittakoop, P. Diketopiperazines and phthalides from a marine derived fungus of the order pleosporales. Planta Med. 2008, 74, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Guan, H.; Edrada, R.; Lin, W.; Wu, J.; Proksch, P. Chromones from the endophytic fungus pestalotiopsis sp. Isolated from the chinese mangrove plant rhizophora mucronata. J. Nat. Prod. 2009, 72, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds—A review. Open Access Libr. J. 2014, 1, 1. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Jang, J.-H.; Ko, W.; Kim, K.-S.; Sohn, J.H.; Kang, M.-S.; Ahn, J.S.; Kim, Y.-C.; Oh, H. Ptp1b inhibitory and anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungus penicillium sp. Jf-55. Mar. Drugs 2013, 11, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S.; Tsueng, G.; McArthur, K.A.; Mitchell, S.S.; Potts, B.C.; Xu, J. Effects of halogens on the production of salinosporamides by the obligate marine actinomycete salinispora tropica. J. Antibiot. 2007, 60, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.; Lloyd, G.K.; Miller, B.R.; Palladino, M.A.; Kiso, Y.; Hayashi, Y.; Neuteboom, S.T. Npi-2358 is a tubulin-depolymerizing agent: In-vitro evidence for activity as a tumor vascular-disrupting agent. Anti-Cancer Drugs 2006, 17, 25–31. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Sohn, J.H.; Ahn, J.S.; Oh, H. Alternaramide, a cyclic depsipeptide from the marine-derived fungus alternaria sp. Sf-5016. J. Nat. Prod. 2009, 72, 2065–2068. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xu, Q.Z.; Shen, Y.; Liu, X.; Jiao, B.; Zhang, W.; Ni, K. Macrolactin s, a novel macrolactin antibiotic from marine bacillus sp. Nat. Prod. Res. 2008, 22, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, Y.; Arai, M.; Tomoda, H.; Omura, S. Fungerin, a fungal alkaloid, arrests the cell cycle in m phase by inhibition of microtubule polymerization. J. antIbiot. 2004, 57, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Eksioglu, E.A.; Liu, C.; Paul, V.J.; Luesch, H. Grassystatins a−c from marine cyanobacteria, potent cathepsin e inhibitors that reduce antigen presentation. J. Med. Chem. 2009, 52, 5732–5747. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide a, a cyclic depsipeptide from a palmyra atoll collection of the marine cyanobacterium lyngbya majuscula. J. Nat. Prod. 2009, 73, 393–398. [Google Scholar] [CrossRef]
- Medina, R.A.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Huang, N.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H.; McPhail, K.L. Coibamide a, a potent antiproliferative cyclic depsipeptide from the panamanian marine cyanobacterium leptolyngbya sp. J. Am. Chem. Soc. 2008, 130, 6324–6325. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Pan, Z.; Wu, C.; Wang, W.; Fang, L.; Su, W. Efficient synthesis and stereochemical revision of coibamide a. J. Am. Chem. Soc. 2015, 137, 13488–13491. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.V.; Sorrels, C.M.; Gerwick, W.H. Cloning and biochemical characterization of the hectochlorin biosynthetic gene cluster from the marine cyanobacterium lyngbya majuscula. J. Nat. Prod. 2007, 70, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Ross, C.; Paul, V.J.; Luesch, H. Pompanopeptins a and b, new cyclic peptides from the marine cyanobacterium lyngbya confervoides. Tetrahedron 2008, 64, 4081–4089. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharmac. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Kim, S.-K.; Karadeniz, F. Anti-hiv activity of extracts and compounds from marine algae. Adv. Food Nutr. Res. 2011, 64, 255–265. [Google Scholar] [PubMed]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, C.K.; Rhie, K.T.; Lee, H.K. Antiviral effects of sulfated exopolysaccharide from the marine microalga gyrodinium impudicum strain kg03. Mar. Biotechnol. 2004, 6, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nuhu, A.A. Spirulina (arthrospira): An important source of nutritional and medicinal compounds. J. Mar. Biol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; He, H.; Schulz, S.; Liu, X.; Fusetani, N.; Xiong, H.; Xiao, X.; Qian, P.-Y. Potent antifouling compounds produced by marine streptomyces. Bioresour. Technol. 2010, 101, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Goh, B.P.L.; Tripathi, A.; Lim, M.G.; Dickinson, G.H.; Lee, S.S.C.; Teo, S.L.M. Natural antifoulants from the marine cyanobacterium lyngbya majuscula. Biofouling 2010, 26, 685–695. [Google Scholar] [CrossRef]
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactins—Ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef]
- Taori, K.; Matthew, S.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatins 5–7, potent elastase inhibitors from floridian marine cyanobacteria, lyngbya spp. J. Nat. Prod. 2007, 70, 1593–1600. [Google Scholar] [CrossRef]
- Volk, R.-B.; Furkert, F.H. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol. Res. 2006, 161, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Sharma, D. Antibacterial activity of bloom forming cyanobacteria against clinically isolated human pathogenic microbes. J. Algal Biomass Utilization 2013, 4, 83–89. [Google Scholar]
- Martins, R.F.; Ramos, M.F.; Herfindal, L.; Sousa, J.A.; Skærven, K.; Vasconcelos, V.M. Antimicrobial and cytotoxic assessment of marine cyanobacteria-synechocystis and synechococcus. Mar. Drugs 2008, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Orjala, J.; Sticher, O. A novel extracellular diterpenoid with antibacterial activity from the cyanobacterium nostoc commune. J. Nat. Prod. 1999, 62, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Gekwick, W.H.; Reyes, S.; Alvarado, B. Two malyngamides from the caribbean cyanobacterium lyngbya majuscula. Phytochemistry 1987, 26, 1701–1704. [Google Scholar] [CrossRef]
- Raveh, A.; Carmeli, S. Antimicrobial ambiguines from the cyanobacterium fischerella sp. Collected in israel. J. Nat. Prod. 2007, 70, 196–201. [Google Scholar] [CrossRef]
- Ishida, K.; Murakami, M. Kasumigamide, an antialgal peptide from the cyanobacterium microcystis aeruginosa. J. Org. Chem. 2000, 65, 5898–5900. [Google Scholar] [CrossRef]
- Luesch, H.; Harrigan, G.; Goetz, G.; Horgen, F. The cyanobacterial origin of potent anticancer agents originally isolated from sea hares. Curr. Med. Chem. 2002, 9, 1791–1806. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; He, S.; Yan, X. New peptides isolated from marine cyanobacteria, an overview over the past decade. Mar. Drugs 2017, 15, 132. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Menakha, M. Pharmaceutical applications of cyanobacteria—A review. J. Acute Med. 2015, 5, 15–23. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Ohtani, I.; Patterson, G.M.; Moore, R.E.; Corbett, T.H.; Valeriote, F.A.; Demchik, L. Total structures of cryptophycins, potent antitumor depsipeptides from the blue-green alga nostoc sp. Strain gsv 224. J. Am. Chem. Soc. 1994, 116, 4729–4737. [Google Scholar] [CrossRef]
- Panda, D.; DeLuca, K.; Williams, D.; Jordan, M.A.; Wilson, L. Antiproliferative mechanism of action of cryptophycin-52: Kinetic stabilization of microtubule dynamics by high-affinity binding to microtubule ends. Proc. Natl. Acad. Sci. USA 1998, 95, 9313–9318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sueyoshi, K.; Kaneda, M.; Sumimoto, S.; Oishi, S.; Fujii, N.; Suenaga, K.; Teruya, T. Odoamide, a cytotoxic cyclodepsipeptide from the marine cyanobacterium okeania sp. Tetrahedron 2016, 72, 5472–5478. [Google Scholar] [CrossRef]
- Leão, P.N.; Costa, M.; Ramos, V.; Pereira, A.R.; Fernandes, V.C.; Domingues, V.F.; Gerwick, W.H.; Vasconcelos, V.M.; Martins, R. Antitumor activity of hierridin b, a cyanobacterial secondary metabolite found in both filamentous and unicellular marine strains. PLoS ONE 2013, 8, e69562. [Google Scholar]
- Abed, R.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Shishido, T.K.; Humisto, A.; Jokela, J.; Liu, L.; Wahlsten, M.; Tamrakar, A.; Fewer, D.P.; Permi, P.; Andreote, A.P.; Fiore, M.F. Antifungal compounds from cyanobacteria. Mar. Drugs 2015, 13, 2124–2140. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Molinski, T.F. Majusculoic acid, a brominated cyclopropyl fatty acid from a marine cyanobacterial mat assemblage. J. Nat. Prod. 2005, 68, 604–606. [Google Scholar] [CrossRef]
- Sivakumar, J.; Santhanam, P. Antipathogenic activity of spirulina powder. Recent Res. Sci. Technol. 2011, 3, 158–161. [Google Scholar]
- Clark, B.R.; Engene, N.; Teasdale, M.E.; Rowley, D.C.; Matainaho, T.; Valeriote, F.A.; Gerwick, W.H. Natural products chemistry and taxonomy of the marine cyanobacterium blennothrix cantharidosmum. J. Nat. Prod. 2008, 71, 1530–1537. [Google Scholar] [CrossRef]
- Vining, O.B.; Medina, R.A.; Mitchell, E.A.; Videau, P.; Li, D.; Serrill, J.D.; Kelly, J.X.; Gerwick, W.H.; Proteau, P.J.; Ishmael, J.E. Depsipeptide companeramides from a panamanian marine cyanobacterium associated with the coibamide producer. J. Nat. Prod. 2015, 78, 413–420. [Google Scholar] [CrossRef]
- Chai, Q.-Y.; Yang, Z.; Lin, H.-W.; Han, B.-N. Alkynyl-containing peptides of marine origin: A review. Mar. Drugs 2016, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Plavšić, M.; Terzic, S.; Ahel, M.; Van Den Berg, C. Folic acid in coastal waters of the adriatic sea. Mar. Freshw. Res. 2003, 53, 1245–1252. [Google Scholar] [CrossRef]
- Villa, F.A.; Lieske, K.; Gerwick, L. Selective myd88-dependent pathway inhibition by the cyanobacterial natural product malyngamide f acetate. Eur. J. Pharmacol. 2010, 629, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Motuhi, S.-E.; Mehiri, M.; Payri, C.; La Barre, S.; Bach, S. Marine natural products from new caledonia—A review. Mar. Drugs 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Munro, M.H.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Lee, S.W.; Park, J.G.; Baek, K.H. Antioxidant and antibacterial properties of essential oil extracted from an edible seaweed undaria pinnatifida. J. Food Biochem. 2017, 41, e12278. [Google Scholar] [CrossRef]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the terrestrial: The potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef]
- Taylor, F. Pharmacodynamic basis of herbal medicine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 699. [Google Scholar]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Lee, J.-B.; Ohta, Y.; Hayashi, K.; Hayashi, T. Immunostimulating effects of a sulfated galactan from codium fragile. Carbohydr. Res. 2010, 345, 1452–1454. [Google Scholar] [CrossRef]
- De Souza, É.T.; Pereira de Lira, D.; Cavalcanti de Queiroz, A.; Costa da Silva, D.J.; Bezerra de Aquino, A.; Campessato Mella, E.A.; Prates Lorenzo, V.; De Miranda, G.E.C.; de Araújo-Júnior, J.X.; de Oliveira Chaves, M.C. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Potent anti-inflammatory activity of pheophytin a derived from edible green alga, enteromorpha prolifera (sujiao-nori). Int. J. Immunopharmacol. 1997, 19, 355–358. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Manikumar, G.; Taylor, H.; Hughes, T.J.; Gaetano, K.; Gerwick, W.H.; McPhail, A.T.; McPhail, D.R. Plant antimutagenic agents 7. Structure and antimutagenic properties of cymobarbatol and 4-isocymbarbatol, new cymopols from green alga (cymopolia barbata). J. Nat. Prod. 1989, 52, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Fischel, J.; Lemee, R.; Formento, P.; Caldani, C.; Moll, J.; Pesando, D.; Meinesz, A.; Grelier, P.; Pietra, P.; Guerriero, A. Cell growth inhibitory effects of caulerpenyne, a sesquiterpenoid from the marine algae caulerpa taxifolia. Anticancer Res. 1995, 15, 2155–2160. [Google Scholar] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mrna expression in white adipose tissue of diabetic/obese kk-ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Koizumi, S.; Hayashi, K.; Hayashi, T. Structure of rhamnan sulfate from the green alga monostroma nitidum and its anti-herpetic effect. Carbohydr. Polym. 2010, 81, 572–577. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, X.; Conte, M.M.; Khalil, Z.; Capon, R.J. Spiralisones a–d: Acylphloroglucinol hemiketals from an australian marine brown alga, zonaria spiralis. Org. Biomol. Chem. 2012, 10, 9671–9676. [Google Scholar] [CrossRef]
- Choi, B.W.; Ryu, G.; Park, S.H.; Kim, E.S.; Shin, J.; Roh, S.S.; Shin, H.C.; Lee, B.H. Anticholinesterase activity of plastoquinones from sargassum sagamianum: Lead compounds for alzheimer’s disease therapy. Phytother. Res. 2007, 21, 423–426. [Google Scholar] [CrossRef]
- Rahelivao, M.; Gruner, M.; Andriamanantoanina, H.; Andriamihaja, B.; Bauer, I.; Knölker, H.-J. Red algae (rhodophyta) from the coast of madagascar: Preliminary bioactivity studies and isolation of natural products. Mar. Drugs 2015, 13, 4197–4216. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Previously undescribed antioxidative azocinyl morpholinone alkaloid from red seaweed gracilaria opuntia with anti-cyclooxygenase and lipoxygenase properties. Nat. Prod. Res. 2018, 32, 1150–1160. [Google Scholar] [CrossRef]
- Ren, D.; Wood, T.K. (5z)-4-bromo-5-(bromomethylene)-3-butyl-2 (5h)-furanone reduces corrosion from desulfotomaculum orientis. Environ. Microbiol. 2004, 6, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Hamman, M.T.; Otto, C.S.; Scheuer, P.J.; Dunbar, D.C. Kahalalides: Bioactive peptides from a marine mollusk elysia rufescens and its algal diet bryopsis sp. J. Org. Chem. 1998, 63, 4856. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Houck, J.; Bhayana, J.; Lee, T. The inhibition of pepsin and peptic ulcers. Gastroenterology 1960, 39, 196. [Google Scholar] [CrossRef]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Pushpamali, W.A.; Nikapitiya, C.; De Zoysa, M.; Whang, I.; Kim, S.J.; Lee, J. Isolation and purification of an anticoagulant from fermented red seaweed lomentaria catenata. Carbohydr. Polym. 2008, 73, 274–279. [Google Scholar] [CrossRef]
- Deepa, S.; Bhuvana, B.; Hemamalini, S.; Janet, C.; Kumar, S. Therapeutic potential and pharmacological significance of the marine algae gracilaria corticata. Pharm. Biol. Eval. 2017, 4, 68–72. [Google Scholar]
- Yasoda, H.N.; Chi, Z.; Zhu, K. Probiotics and sea cucumber farming. SPC Bechedemer Inf. Bull. 2006, 24, 45. [Google Scholar]
- Kurihara, H.; Kagawa, Y.; Konno, R.; Kim, S.M.; Takahashi, K. Lipoxygenase inhibitors derived from marine macroalgae. Bioorg. Med. Chem. Lett. 2014, 24, 1383–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witvrouw, M.; Este, J.; Mateu, M.; Reymen, D.; Andrei, G.; Snoeck, R.; Ikeda, S.; Pauwels, R.; Bianchini, N.V.; Desmyter, J. Activity of a sulfated polysaccharide extracted from the red seaweed aghardhiella tenera against human immunodeficiency virus and other enveloped viruses. Antivir. Chem. Chemother. 1994, 5, 297–303. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Pujol, C.A.; Carlucci, M.J.; Chattopadhyay, K.; Damonte, E.B.; Ray, B. Anti-herpetic activity of a sulfated xylomannan from scinaia hatei. Phytochemistry 2008, 69, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral activities of sulfated polysaccharides isolated from sphaerococcus coronopifolius (rhodophytha, gigartinales) and boergeseniella thuyoides (rhodophyta, ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef] [PubMed]
- Serkedjieva, J. Antiviral activity of the red marine alga ceramium rubrum. Phytother. Res. 2004, 18, 480–483. [Google Scholar] [CrossRef]
- Heo, S.-J.; Cha, S.-H.; Lee, K.-W.; Jeon, Y.-J. Antioxidant activities of red algae from jeju island. Algae 2006, 21, 149–156. [Google Scholar] [CrossRef]
- Nogueira, C.C.R.; de Palmer Paixão, I.C.N.; Teixeira, V.L. Antioxidant activity of natural products isolated from red seaweeds. Nat. Prod. Commun. 2014, 9, 1934578X1400900737. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Tseng, C.-N.; Yang, J.-I.; Huang, H.-W.; Fang, Y.; Tang, J.-Y.; Chang, F.-R.; Chang, H.-W. Antiproliferation and induction of apoptosis in ca9-22 oral cancer cells by ethanolic extract of gracilaria tenuistipitata. Molecules 2012, 17, 10916–10927. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Yang, J.-I.; Lee, J.-C.; Tseng, C.-N.; Chan, Y.-C.; Hseu, Y.-C.; Tang, J.-Y.; Chuang, L.-Y.; Huang, H.-W.; Chang, F.-R. Anti-proliferative effect of methanolic extract of gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement. Alternat. Med. 2012, 12, 142. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Structure and antibacterial activity of brominated diterpenes from the red alga sphaerococcus coronopifolius. Chem. biodivers. 2010, 7, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, E. Algae as promising organisms for environment and health. Plant Signal. Behav. 2011, 6, 1338–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotokawa, T.; Noda, T.; Pi, S.; Hirama, M. A three-step synthesis of halomon. Angew. Chem. Int. Ed. 2000, 39, 3430–3432. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; France, D.; Cornell-Kennon, S.; Gerwick, W.H. DNA methyl transferase inhibiting halogenated monoterpenes from the madagascar red marine alga portieria h ornemannii. J. Nat. Prod. 2006, 69, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Groth, I.; Grünewald, N.; Alban, S. Pharmacological profiles of animal-and nonanimal-derived sulfated polysaccharides–comparison of unfractionated heparin, the semisynthetic glucan sulfate ps3, and the sulfated polysaccharide fraction isolated from delesseria sanguinea. Glycobiology 2008, 19, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Farias, W.R.; Valente, A.-P.; Pereira, M.S.; Mourão, P.A. Structure and anticoagulant activity of sulfated galactans isolation of a unique sulfated galactan from the red algaebotryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J. Biol. Chem. 2000, 275, 29299–29307. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Pujol, C.A.; Zúñiga, E.A. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed schizymenia binderi (gigartinales, rhodophyta). Carbohydr. Res. 2005, 340, 2392–2402. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1R–49R. [Google Scholar] [CrossRef]
- Haugan, J.A. Algal carotenoids 54. Carotenoids of brown algae (phaeophyceae). Biochem. Syst. Ecol. 1994, 22, 31–41. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Piper, R. Extraordinary animals: An Encyclopedia of Curious and Unusual Animals; Greenwood Publishing Group: London, UK, 2007; p. 320. [Google Scholar]
- Zhang, H.; Pang, Z.; Han, C. Undaria pinnatifida (wakame): A seaweed with pharmacological properties. Sci. Int. 2014, 2, 32–36. [Google Scholar] [CrossRef]
- Khan, M.N.A.; Cho, J.-Y.; Lee, M.-C.; Kang, J.-Y.; Park, N.G.; Fujii, H.; Hong, Y.-K. Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed undaria pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-K.; Jung, U.; Roh, C. Fucoidan from marine brown algae inhibits lipid accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.D.; Blunden, G.; Tasdemir, D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (phaeophyceae) from british and irish waters. Phytother. Res. 2010, 24, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Lutay, N.; Nilsson, I.; Wadström, T.; Ljungh, Å. Effect of heparin, fucoidan and other polysaccharides on adhesion of enterohepatic helicobacter species to murine macrophages. Appl. Biochem. Biotechnol. 2011, 164, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from sargassum sp. And fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Salgado, L.T.; Tomazetto, R.; Cinelli, L.P.; Farina, M.; Amado Filho, G.M. The influence of brown algae alginates on phenolic compounds capability of ultraviolet radiation absorption in vitro. Braz. J. Oceanogr. 2007, 55, 145–154. [Google Scholar] [CrossRef]
- Kang, M.-C.; Wijesinghe, W.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H. Dieckol isolated from brown seaweed ecklonia cava attenuates type іі diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Moubayed, N.M.; Al Houri, H.J.; Al Khulaifi, M.M.; Al Farraj, D.A. Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from saudi arabia (red sea and arabian gulf). Saudi J. Biol. Sci. 2017, 24, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.; Aderogba, M.A.; Amoo, S.O.; Stirk, W.A.; Van Staden, J. Potential antiradical and alpha-glucosidase inhibitors from ecklonia maxima (osbeck) papenfuss. Food Chem. 2013, 141, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Puspita, M.; Deniel, M.; Widowati, I.; Radjasa, O.; Douzenel, P.; Bedoux, G.; Bourgougnon, N. Antioxidant and antibacterial activity of solid-liquid and enzyme-assisted extraction of phenolic compound from three species of tropical sargassum. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bali, Indonesia, 2017; p. 012057. [Google Scholar]
- Khan, A.; Uddin, N.; Khaliq, S.; Nawaz, S.; Rasheed, M.; Dar, A.; Hanif, M.; Siddiqui, P.J.A. Brown seaweeds administration generate psychotherapeutic response associated with brain norepinephrine modulation in rats. J. Pharm. Phytother. 2017, 9, 11–18. [Google Scholar] [Green Version]
- Grosso, C.; Andrade, P.; Valentao, P.; Mouga, T.; Jäger, A. Seaweeds: New source of mao-a inhibiting compounds. Planta Med. 2011, 77, PM69. [Google Scholar] [CrossRef]
- Jacobs, R.S.; Culver, P.; Langdon, R.; O’Brien, T.; White, S. Some pharmacological observations on marine natural products. Tetrahedron 1985, 41, 981–984. [Google Scholar] [CrossRef]
- Nishibori, N.; Itoh, M.; Kashiwagi, M.; Arimochi, H.; Morita, K. In vitro cytotoxic effect of ethanol extract prepared from sporophyll of undaria pinnatifida on human colorectal cancer cells. Phytother. Res. 2012, 26, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Díaz-Marrero, A.R.; Cueto, M.; Darias, J. On the relative stereochemistry of atomaric acid and related compounds. Tetrahedron 2003, 59, 2059–2062. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Kim, S.-K. Fucoidan, a sulfated polysaccharides from brown algae as therapeutic target for cancer. In Handbook of Anticancer Drugs from Marine Origin; Springer: Cham, Switzerland, 2015; pp. 145–164. [Google Scholar]
- Hu, X.; Jiang, X.; Hwang, H.; Liu, S.; Guan, H. Antitumour activities of alginate-derived oligosaccharides and their sulphated substitution derivatives. Eur. J. Phycol. 2004, 39, 67–71. [Google Scholar] [CrossRef]
- Ponnan, A.; Ramu, K.; Marudhamuthu, M.; Marimuthu, R.; Siva, K.; Kadarkarai, M. Antibacterial, antioxidant and anticancer properties of turbinaria conoides (j. Agardh) kuetz. Clin. Phytosci. 2017, 3, 5. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.-Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Mori, H.; Nakayama, Y.; Takahashi, M. Anti-plasmin inhibitor. Vi.: Structure of phlorofucofuroeckol a, a novel phlorotannin with both dibenzo-1, 4-dioxin and dibenzofuran elements, from ecklonia kurome okamura. Chem. Pharmaceut. Bull. 1990, 38, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, J.; Chang, A.K.; Liu, B.; Yang, L.; Li, Q.; Wang, P.; Zou, X. Fucoidan extract derived from undaria pinnatifida inhibits angiogenesis by human umbilical vein endothelial cells. Phytomedicine 2012, 19, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Xue, C.; Zhao, X.; Mori, M.; Sugawara, T.; Hirata, T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int. J. Mol. Med. 2005, 15, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, K.-W.; Cho, K.-S.; Hahn, O.-J.; Lee, K.-H.; Lee, B.-Y.; Ko, J.-J.; Chung, K.-H. Biological effects of fucoidan isolated from fucus vesiculosus on thrombosis and vascular cells. Korean J. Hematol. 2010, 45, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Yoon, K.-D.; Min, S.-Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.-G.; Huh, H.; Kim, J. Inhibition of hiv-1 reverse transcriptase and protease by phlorotannins from the brown alga ecklonia cava. Biol. Pharmaceut. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, K.; Medeiros, V.; Queiroz, L.; Abreu, L.; Rocha, H.; Ferreira, C.; Juca, M.; Aoyama, H.; Leite, E. Inhibition of reverse transcriptase activity of hiv by polysaccharides of brown algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Béress, A.; Wassermann, O.; Bruhn, T.; Béress, L.; Kraiselburd, E.N.; Gonazalez, L.V.; de Motta, G.E.; Chavez, P.J. A new procedure for the isolation of anti-hiv compounds (polysaccharides and polyphenols) from the marine alga fucus vesiculosus. J. Nat. Prod. 1996, 59, 552. [Google Scholar] [CrossRef]
- Dos Santos, V.A.; Leite, K.M.; da Costa Siqueira, M.; Regasini, L.O.; Martinez, I.; Nogueira, C.T.; Galuppo, M.K.; Stolf, B.S.; Pereira, A.M.S.; Cicarelli, R. Antiprotozoal activity of quinonemethide triterpenes from maytenus ilicifolia (celastraceae). Molecules 2013, 18, 1053–1062. [Google Scholar] [CrossRef]
- Nara, T.; Kamei, Y.; Tsubouchi, A.; Annoura, T.; Hirota, K.; Iizumi, K.; Dohmoto, Y.; Ono, T.; Aoki, T. Inhibitory action of marine algae extracts on the trypanosoma cruzi dihydroorotate dehydrogenase activity and on the protozoan growth in mammalian cells. Parasitol. Int. 2005, 54, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.; Calegari-Silva, T.C.; Lopes, U.G.; Teixeira, V.L.; de Palmer Paixão, I.C.; Cirne-Santos, C.; Bou-Habib, D.C.; Saraiva, E.M. Dolabelladienetriol, a compound from dictyota pfaffii algae, inhibits the infection by leishmania amazonensis. PLoS Negl. Trop. Dis. 2012, 6, e1787. [Google Scholar] [CrossRef] [PubMed]
- Gallé, J.-B.; Attioua, B.; Kaiser, M.; Rusig, A.-M.; Lobstein, A.; Vonthron-Sénécheau, C. Eleganolone, a diterpene from the french marine alga bifurcaria bifurcata inhibits growth of the human pathogens trypanosoma brucei and plasmodium falciparum. Mar. Drugs 2013, 11, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Lee, H.-S.; Kang, I.-J.; Won, M.-H.; You, S. Antioxidant properties of extract and fractions from enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Huang, L.; Liu, X.; Liu, D.; Zhang, Q.; Liu, S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from ulva pertusa (chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Hickey, R.M. Extraction and characterization of bioactive carbohydrates with health benefits from marine resources: Macro-and microalgae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds; Springer: Boston, MA, USA, 2012; pp. 159–172. [Google Scholar]
- Pengzhan, Y.; Quanbin, Z.; Ning, L.; Zuhong, X.; Yanmei, W.; Zhi’en, L. Polysaccharides from ulva pertusa (chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. phYcol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Tabarsa, M.; Lee, S.-J.; You, S. Structural analysis of immunostimulating sulfated polysaccharides from ulva pertusa. Carbohydr. Res. 2012, 361, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Celikler, S.; Tas, S.; Vatan, O.; Ziyanok-Ayvalik, S.; Yildiz, G.; Bilaloglu, R. Anti-hyperglycemic and antigenotoxic potential of ulva rigida ethanolic extract in the experimental diabetes mellitus. Food Chem. Toxicol. 2009, 47, 1837–1840. [Google Scholar] [CrossRef] [PubMed]
- Sunilson, J.A.J.; Suraj, R.; Anandarajagopal, K.; Rejitha, G.; Vignesh, M.; Promwichit, P. Preliminary phytochemical analysis, elemental determination and antibacterial screening of codium decorticatum—A marine green algae. Int. J. Biol. Chem. 2009, 3, 84–89. [Google Scholar]
- Khan, M.N.; Choi, J.S.; Lee, M.C.; Kim, E.; Nam, T.J.; Fujii, H.; Hong, Y.K. Anti-inflammatory activities of methanol extracts from various seaweed species. J. Environ. Biol. 2008, 29, 465–469. [Google Scholar] [PubMed]
- Lee, J.-B.; Hayashi, K.; Maeda, M.; Hayashi, T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004, 70, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hirayama, M.; Morimoto, K.; Yamamoto, N.; Okuyama, S.; Hori, K. High mannose-binding lectin with preference for the cluster of α1–2-mannose from the green alga boodlea coacta is a potent entry inhibitor of hiv-1 and influenza viruses. J. Biol. Chem. 2011, 286, 19446–19458. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga ulva lactuca as activators of the cytoprotective nrf2–are pathway. Free Rad. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathy, S.; Sumathi, P.; Madhushree, M. Antimutagenic assay of carotenoids from green algae chlorococcum humicola using salmonella typhimurium ta98, ta100 and ta102. Asian Pacif. J. Trop. Dis. 2011, 1, 308–316. [Google Scholar] [CrossRef]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (hl-60) cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Matsubara, K.; Ohkubo, T.; Tanaka, Y.; Noda, K.; Sugawara, T.; Hirata, T. Anti-angiogenic effect of siphonaxanthin from green alga, codium fragile. Phytomedicine 2010, 17, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Selvin, J.; Lipton, A.P. Biopotentials of ulva fasciata and hypnea musciformis collected from the peninsular coast of india. J. Mar. Sci. Technol. 2004, 12, 1–6. [Google Scholar]
- Yuvaraj, N.; Kanmani, P.; Satishkumar, R.; Paari, K.; Pattukumar, V.; Arul, V. Extraction, purification and partial characterization of cladophora glomerata against multidrug resistant human pathogen acinetobacter baumannii and fish pathogens. World J. Fish Mar. Sci. 2011, 3, 51–57. [Google Scholar]
- Smyrniotopoulos, V.; Abatis, D.; Tziveleka, L.-A.; Tsitsimpikou, C.; Roussis, V.; Loukis, A.; Vagias, C. Acetylene sesquiterpenoid esters from the green alga caulerpa prolifera. J. Nat. Prod. 2003, 66, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Deacon-Smith, R.; Lee-Potter, J.; Rogers, D. Anticoagulant activity in extracts of british marine algae. Bot. Mar. 1985, 28, 333–338. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsuura, Y.; Hori, K.; Miyazawa, K. An anticoagulant proteoglycan from the marine green alga, codium pugniformis. J. Appl. Phycol. 2000, 12, 9–14. [Google Scholar] [CrossRef]
- Maeda, M.; Uehara, T.; Harada, N.; Sekiguchi, M.; Hiraoka, A. Heparinoid-active sulphated polysaccharides frommonostroma nitidum and their distribution in the chlorophyta. Phytochemistry 1991, 30, 3611–3614. [Google Scholar] [CrossRef]
- Synytsya, A.; Choi, D.J.; Pohl, R.; Na, Y.S.; Capek, P.; Lattová, E.; Taubner, T.; Choi, J.W.; Lee, C.W.; Park, J.K. Structural features and anti-coagulant activity of the sulphated polysaccharide sps-cf from a green alga capsosiphon fulvescens. Mar. Biotechnol. 2015, 17, 718–735. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Watanabe, Y.; Naganuma, T.; Ogawa, T.; Muramoto, K. Lectins of marine origin and their clinical applications. In Antitumor Potential and Other Emerging Medicinal Properties of Natural Compounds; Springer: New York, NY, USA, 2013; pp. 33–54. [Google Scholar]




| Marine Microorganisms (Bacteria, Fungi, and Cyanobacteria) | |||||
|---|---|---|---|---|---|
| Compound | Chemical Structure | Source/Species | Biological Activity | Mechanism of Action | References |
| Salinosporamide A |  | Actinomycete/Salinispora tropica | Treatment of multiple myeloma (anticancer); antimalarial | Inhibits proteasome activity by covalently modifying the threonine residue of the active site of the 20S proteasome | [20] |
| Plinabulin |  | Fungi/Aspergillus sp. | Treatment of solid tumors and lymphomas | Depolymerizes microtubules in A549 human lung carcinoma cells | [21] |
| Alternaramide |  | Ascomycete fungi/Alternaria sp. | Anti-inflammatory | Inhibits inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-кB and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264.7 and BV2 cells | [22] |
| Macrolactin S |  | Bacterium/Bacillus sp. | Antibacterial | FabG inhibition agent | [23] |
| Oxaline |  | Ascomycete fungi/Penicillium sp. | Antitumor | Inhibits cell proliferation and induces cell cycle arrest at the G2/M phase in Jurkat cells | [24] |
| Grassystatin C |  | Tropical cyanobacteria/Okeania lorea | Cathepsin inhibition | Potent cathepsin E inhibitor that reduces antigen presentation | [25] |
| Palmyramide A |  | Filamentous cyanobacteria/Moorea producens | Antitumor | Sodium channel blocking activity in neuro-2a cells and cytotoxic activity in H-460 human lung carcinoma cells | [26] |
| Coibamide A |  | Pantropical cyanobacteria/Caldora penicillata | Antitumor cytotoxicity | Inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts | [27,28] |
| Hectochlorin |  | Cyanobacterium/Lyngbya majusculea JHB | Cytotoxin, antifungal | Inhibits the growth of human cell lines by hyper-polymerization of actin | [29] |
| Pompanopeptin A |  | Cyanobacterium/Lyngbya confervoides | Trypsin inhibitor | Inhibits trypsin with an IC50 value of 2.4 μM; selectivity is conferred by the arginine residue | [30] |
| Natural Compound | Chemical Structure | Species | Biological Activity | Mechanism of action | References |
|---|---|---|---|---|---|
| Sulfated galactan |  | Green alga/Codium fragile | Immunostimulating effects via activation of macrophages | Stimulates the production of nitric oxide by inducing iNOS at mRNA and protein levels and induces the expression of several cytokine mRNA, such as IL-1β, IL-6, IL-10, and TNF-α | [71] |
| Caulerpin |  | Green alga/Caulerpa racemosa | Anti-inflammatory and antinociceptive | Inhibits capsaicin-induced ear edema model and significantly reduces the number of recruited cells | [72] |
| Pheophytin A |  | Green alga/Enteromorpha prolifera | Anti-inflammatory | Exhibits significant suppression of TPA-induced inflammatory reaction, such as edema formation in BALB/c mouse ear | [73] |
| Cymopols |  | Green alga/Cymopolia barbata | Antimutagenic | Inhibits the mutagenicity of 2-aminoanthracene in T-98 strain. Behaves as a metabolic activator | [74] |
| Caulerpenyne |  | Green alga/Caulerpa taxifolia | Anticancer | Shows cytotoxicity in cultured cell lines, such as KB cells and hamster fibroblasts | [75,76] |
| Fucoxanthin |  | Brown algae | Antidiabetic and antiobesity | Suppresses McP-1, promotes adrb3 and gluT4 expression, and induces uncoupling protein 1 expression in white adipose tissue (WAT) mitochondria, leading to oxidation of fatty acids and heat production in WAT | [77] |
| Dieckol |  | Brown alga/Ecklonia cava | Anti-inflammatory and neuroprotective agent | Suppresses LPS-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2) and the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in murine BV2 microglia | [78] |
| Spiralisone A |  | Brown alga/Zonaria spiralis | Kinase inhibitor and antibacterial | Shows inhibitory activity against neurodegenerative diseases targeting CDK5/p25, CK1δ, and GSK3β kinases. Inhibits the Gram-positive bacteria Bacillus subtilis (ATCC 6051 and 6633) | [79] |
| Sargaquinoic acid |  | Brown alga/Sargassum sagamianum | AChE inhibitor | Inhibits acetylcholinesterase activity | [80] |
| Phorbasterone B |  | Red seaweed | Antimicrobial | Exhibits antimicrobial activity against Bacillus cereus, Staphylococcus aureus, Streptococcus pneumoniae, and Candida albicans | [81] |
| Azocinyl- morpholinone |  | Red seaweed/Gracilaria opuntia | Antioxidant, anti-inflammatory by inhibiting cyclooxygenase and lipoxygenase | Azocinyl morpholinone significantly mitigated the carrageenan-induced paw edema | [82] |
| (5Z)-4-bromo-5-(bromo-methylene)-3-butyl-2(5H)-furanone |  | Red seaweed/Delisea pulchra | Antifouling agent | Inhibits microbial-induced corrosion related to Gram-positive bacteria | [83] |
| Kahalalide A |  | Red seaweed/Bryopsis sp. | Antibacterial agent | Shows in vitro activity against Mycobacterium tuberculosis | [84,85] |
| Kahalalide F |  | Red seaweed/Bryopsis sp. | Antibacterial and anti-HIV agent | Shows antibacterial activity against M. tuberculosis and proposed for the treatment of lung cancer | [84,85] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drugs 2019, 17, 464. https://doi.org/10.3390/md17080464
Barzkar N, Jahromi ST, Poorsaheli HB, Vianello F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Marine Drugs. 2019; 17(8):464. https://doi.org/10.3390/md17080464
Chicago/Turabian StyleBarzkar, Noora, Saeid Tamadoni Jahromi, Hadi Bolooki Poorsaheli, and Fabio Vianello. 2019. "Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology" Marine Drugs 17, no. 8: 464. https://doi.org/10.3390/md17080464
APA StyleBarzkar, N., Jahromi, S. T., Poorsaheli, H. B., & Vianello, F. (2019). Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Marine Drugs, 17(8), 464. https://doi.org/10.3390/md17080464