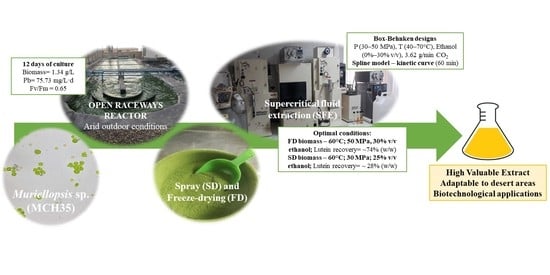
Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth Parameters and Carotenoid Profile of Muriellopsis sp. (MCH35)
2.2. Effects of Drying Processes on the Extraction Yield of Muriellopsis sp. (MCH35) by SFE
2.3. The Effect of Drying Methods on Lutein Recovery of Muriellopsis sp. (MCH35) by SFE
2.4. Global Yield and Kinetic Curve of Muriellopsis sp. (MCH35)
3. Material and Methods
3.1. Microalgal Strain and Chemicals
3.2. Microalgal Culture Conditions
3.3. Growth Measurements
3.4. Drying Treatment on Microalgal Biomass
3.5. Detecting Individual Carotenoids
3.6. Recovery of Individual Carotenoids
3.7. SFE
3.7.1. Experimental Design
3.7.2. Mathematical Modeling of Overall Extraction Curve (OEC) and Spline Linear Model
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Forján, E.; Navarro, F.; Cuaresma, M.; Vaquero, I.; Ruíz-Domínguez, M.C.; Gojkovic, Ž.; Vázquez, M.; Márquez, M.; Mogedas, B.; Bermejo, E. Microalgae: Fast-growth sustainable green factories. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1705–1755. [Google Scholar] [CrossRef]
- Tomaselli, L. The microalgal cell. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley Online Library: Hoboken, NY, USA, 2004; Volume 1, pp. 3–19. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaur, R.; Bansal, A.; Kapur, S.; Sundaram, S. Biotechnological exploitation of cyanobacteria and microalgae for bioactive compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–259. [Google Scholar]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Nutrition tid-bites: Essential fatty acids in health and chronic disease. Food Rev. Int. 1997, 13, 623–631. [Google Scholar] [CrossRef]
- Kini, S.; Divyashree, M.; Mani, M.K.; Mamatha, B.S. Algae and cyanobacteria as a source of novel bioactive compounds for biomedical applications. In Advances in Cyanobacterial Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 173–194. [Google Scholar]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, P.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles atherosclerosis study. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef] [Green Version]
- Heber, D.; Lu, Q.-Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef]
- Marticorena, P.; Gonzalez, L.; Riquelme, C.; Silva Aciares, F. Effects of beneficial bacteria on biomass, photosynthetic parameters and cell composition of the microalga Muriellopsis sp. adapted to grow in seawater. Aquac. Res. 2020. [Google Scholar] [CrossRef]
- Rocha, S.; Candia, O.; Valdebenito, F.; Espinoza-Monje, J.F.; Azócar, L. Biomass quality index: Searching for suitable biomass as an energy source in Chile. Fuel 2020, 264, 116820. [Google Scholar] [CrossRef]
- Hu, Q. Environmental Effects on Cell Composition; Wiley Online Library: Hoboken, NY, USA, 2004; Volume 1, pp. 83–93. [Google Scholar]
- Solovchenko, A.; Khozin-Goldberg, I.; Recht, L.; Boussiba, S. Stress-induced changes in optical properties, pigment and fatty acid content of Nannochloropsis sp.: Implications for non-destructive assay of total fatty acids. Mar. Biotechnol. 2011, 13, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Sevilla, J.M.; Fernández, F.A.; Grima, E.M. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Orikasa, T.; Koide, S.; Okamoto, S.; Imaizumi, T.; Muramatsu, Y.; Takeda, J.-I.; Shiina, T.; Tagawa, A. Impacts of hot air and vacuum drying on the quality attributes of kiwifruit slices. J. Food Eng. 2014, 125, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, T.; Mani, S.; Das, K.; Chinnasamy, S.; Bhatnagar, A.; Singh, R.; Singh, M. Effect of cell rupturing methods on the drying characteristics and lipid compositions of microalgae. Bioresour. Technol. 2012, 126, 131–136. [Google Scholar] [CrossRef]
- Oliveira, E.G.; Duarte, J.H.; Moraes, K.; Crexi, V.T.; Pinto, L.A. Optimisation of Spirulina platensis convective drying: Evaluation of phycocyanin loss and lipid oxidation. Int. J. Food Sci. Technol. 2010, 45, 1572–1578. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Effect of drying and extraction conditions on the recovery of bioactive compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chang, J.-S.; Lee, D.-J. Dewatering and drying methods for microalgae. Dry. Technol. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Grima, E.M.; Fernández, F.A.; Medina, A.R. 10 Downstream Processing of Cell-mass and Products. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley Online Library: Hoboken, NY, USA, 2004; Volume 215. [Google Scholar]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Riveros, K.; Sepulveda, C.; Bazaes, J.; Marticorena, P.; Riquelme, C.; Acién, G. Overall development of a bioprocess for the outdoor production of Nannochloropsis gaditana for aquaculture. Aquac. Res. 2018, 49, 165–176. [Google Scholar] [CrossRef]
- San Pedro, A.; González-López, C.; Acién, F.; Molina-Grima, E. Outdoor pilot production of Nannochloropsis gaditana: Influence of culture parameters and lipid production rates in raceway ponds. Algal Res. 2015, 8, 205–213. [Google Scholar] [CrossRef]
- González-Garcinuño, Á.; Tabernero, A.; Sánchez-Álvarez, J.M.; del Valle, E.M.M.; Galán, M.A. Effect of nitrogen source on growth and lipid accumulation in Scenedesmus abundans and Chlorella ellipsoidea. Bioresour. Technol. 2014, 173, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.; Acien, F.G.; Gómez, C.; Jiménez-Ruiz, N.; Riquelme, C.; Molina-Grima, E. Utilization of centrate for the production of the marine microalgae Nannochloropsis gaditana. Algal Res. 2015, 9, 107–116. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Camacho-Rodríguez, J.; González-Céspedes, A.; Cerón-García, M.; Fernández-Sevilla, J.; Acién-Fernández, F.; Molina-Grima, E. A quantitative study of eicosapentaenoic acid (EPA) production by Nannochloropsis gaditana for aquaculture as a function of dilution rate, temperature and average irradiance. Appl. Microbiol. Biotechnol. 2014, 98, 2429–2440. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Morales-Amaral, M.d.M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res. 2015, 9, 297–305. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp.(Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Eeckhout, M.; Ruyssen, T.; Foubert, I. Influence of drying and storage on lipid and carotenoid stability of the microalga Phaeodactylum tricornutum. J. Agric. Food Chem. 2011, 59, 11063–11069. [Google Scholar] [CrossRef]
- Jin, E.; Polle, J.E.; Lee, H.K.; Hyun, S.M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Blanco, A.M.; Moreno, J.; Del Campo, J.A.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007, 73, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; Chianese, S.; Musmarra, D. Enhancing biomass and lutein production from Scenedesmus almeriensis: Effect of carbon dioxide concentration and culture medium reuse. Front. Plant Sci. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Schalch, W.; Cohn, W.; Barker, F.M.; Köpcke, W.; Mellerio, J.; Bird, A.C.; Robson, A.G.; Fitzke, F.F.; van Kuijk, F.J. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin–the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch. Biochem. Biophys. 2007, 458, 128–135. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Jung, J.H.; Kim, J.Y.; Heo, J.; Cho, D.H.; Kim, H.S.; An, S.; An, I.S.; Bae, S. The Protective Effect of Violaxanthin from Nannochloropsis oceanica against Ultraviolet B-Induced Damage in Normal Human Dermal Fibroblasts. Photochem. Photobiol. 2019, 95, 595–604. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Gross, M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: Astaxanthin, a carotenoid without vitamin a activity, enhances in vitro immunoglobulin production in response to at-dependent stimulant and antigen. Nutr. Cancer 1995, 23, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, F.; Altimari, P.; Pagnanelli, F. Sequential extraction of lutein and β-carotene from wet microalgae biomass. J. Chem. Technol. Biotechnol. 2020, 95, 3024–3033. [Google Scholar] [CrossRef]
- Corzzini, S.C.; Barros, H.D.; Grimaldi, R.; Cabral, F.A. Extraction of edible avocado oil using supercritical CO2 and a CO2/ethanol mixture as solvents. J. Food Eng. 2017, 194, 40–45. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.; Welti-Chanes, J. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehariya, S.; Iovine, A.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D. Supercritical fluid extraction of lutein from Scenedesmus almeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Irzyniec, Z.; Klimczak, J.; Michalowski, S. Freeze-drying of the black currant juice. Dry. Technol. 1995, 13, 417–424. [Google Scholar] [CrossRef]
- Yen, H.-W.; Chiang, W.-C.; Sun, C.-H. Supercritical fluid extraction of lutein from Scenedesmus cultured in an autotrophical photobioreactor. J. Taiwan Inst. Chem. Eng. 2012, 43, 53–57. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, S.; Shi, X. Supercritical fluid extraction and determination of lutein in heterotrophically cultivated Chlorella pyrenoidosa. J. Food Process Eng. 2007, 30, 174–185. [Google Scholar] [CrossRef]
- Smyth, T.J.; Zytner, R.; Stiver, W. Influence of water on the supercritical fluid extraction of naphthalene from soil. J. Hazard. Mater. 1999, 67, 183–196. [Google Scholar] [CrossRef]
- Nagy, B.; Simándi, B. Effects of particle size distribution, moisture content, and initial oil content on the supercritical fluid extraction of paprika. J. Supercrit. Fluids 2008, 46, 293–298. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.-A.A.; Badens, E. Effects of high water content and drying pre-treatment on supercritical CO2 extraction from Dunaliella salina microalgae: Experiments and modelling. J. Supercrit. Fluids 2016, 116, 271–280. [Google Scholar] [CrossRef]
- Crampon, C.; Mouahid, A.; Toudji, S.-A.A.; Lépine, O.; Badens, E. Influence of pretreatment on supercritical CO2 extraction from Nannochloropsis oculata. J. Supercrit. Fluids 2013, 79, 337–344. [Google Scholar] [CrossRef]
- Ivanovic, J.; Ristic, M.; Skala, D. Supercritical CO2 extraction of Helichrysum italicum: Influence of CO2 density and moisture content of plant material. J. Supercrit. Fluids 2011, 57, 129–136. [Google Scholar] [CrossRef]
- Meireles, M.A.A. Extraction of bioactive compounds from Latin American plants. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 243–274. [Google Scholar]
- Jesus, S.P.; Calheiros, M.N.; Hense, H.; Meireles, M.A.A. A simplified model to describe the kinetic behavior of supercritical fluid extraction from a rice bran oil byproduct. Food Public Health 2013, 3, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Mezzomo, N.; Martínez, J.; Ferreira, S.R. Supercritical fluid extraction of peach (Prunus persica) almond oil: Kinetics, mathematical modeling and scale-up. J. Supercrit. Fluids 2009, 51, 10–16. [Google Scholar] [CrossRef]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenéz, D.; Muñoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 104824. [Google Scholar] [CrossRef]
- Sanzo, G.D.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [Green Version]
- Del Valle, J.M. Extraction of natural compounds using supercritical CO2: Going from the laboratory to the industrial application. J. Supercrit. Fluids 2015, 96, 180–199. [Google Scholar] [CrossRef]
- Cerón-García, M.C.; González-López, C.V.; Camacho-Rodríguez, J.; López-Rosales, L.; García-Camacho, F.; Molina-Grima, E. Maximizing carotenoid extraction from microalgae used as food additives and determined by liquid chromatography (HPLC). Food Chem. 2018, 257, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Fernández Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E.; Cerón García, M.d.C. Extracción de carotenoides mediante el uso de mezclas ternarias. ES200703145A, 13 November 2016. [Google Scholar]
- Ruiz-Domínguez, M.C.; Cerezal, P.; Salinas, F.; Medina, E.; Renato-Castro, G. Application of Box-Behnken Design and Desirability Function for Green Prospection of Bioactive Compounds from Isochrysis galbana. Appl. Sci. Basel 2020, 10, 2789. [Google Scholar] [CrossRef] [Green Version]
- Gilbert-López, B.; Mendiola, J.A.; Fontecha, J.; van den Broek, L.A.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream processing of Isochrysis galbana: A step towards microalgal biorefinery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef] [Green Version]







| Biomass | Carotenoids Content (mg/g Biomass) * | ||||
|---|---|---|---|---|---|
| Lutein | Zeaxanthin | Violaxanthin | Astaxanthin | β-carotene | |
| Spray-dried (SD) | 3.45 ± 0.20 b | 0.60 ± 0.04 b | 0.15 ± 0.10 a | 0.45 ± 0.15 a | 0.45 ± 0.04 b |
| Freeze-dried (FD) | 4.20 ± 0.30 a | 0.75 ± 0.06 a | 0.30 ± 0.20 a | 0.30 ± 0.02 b | 0.60 ± 0.05 a |
| Biomass | Run | T | P | Ethanol | Yield | Lutein Content | Lutein Recovery |
| (°C) | (MPa) | (%, v/v) | (%, w/w) | (mg/g Extract) | (%, w/w) | ||
| Spray-dried | 1 | 40 | 30 | 15 | 1.52 ± 0.08 | 45.47 ± 1.45 | 20.05 ± 0.48 |
| 2 | 70 | 30 | 15 | 1.84 ± 0.09 | 43.95 ± 0.10 | 23.38 ± 0.13 | |
| 3 | 40 | 50 | 15 | 1.63 ± 0.08 | 34.27 ± 0.36 | 16.23 ± 0.04 | |
| 4 | 70 | 50 | 15 | 2.17 ± 0.02 | 34.70 ± 0.21 | 21.79 ± 0.92 | |
| 5 | 40 | 40 | 0 | 0.11 ± 0.01 | 2.60 ± 0.03 | 0.09 ± 0.3 × 10−3 | |
| 6 | 70 | 40 | 0 | 0.42 ± 0.02 | 6.28 ± 0.06 | 0.76 ± 1.2 × 10−3 | |
| 7 | 40 | 40 | 30 | 1.87 ± 0.09 | 27.69 ± 0.31 | 15.04 ± 0.05 | |
| 8 | 70 | 40 | 30 | 2.31 ± 0.08 | 23.69 ± 0.34 | 15.86 ± 0.14 | |
| 9 | 55 | 30 | 0 | 0.32 ± 0.10 | 1.27 ± 0.02 | 0.12 ± 0.03 | |
| 10 | 55 | 50 | 0 | 0.44 ± 0.02 | 7.80 ± 10−3 | 0.99 ± 0.01 | |
| 11 | 55 | 30 | 30 | 7.84 ± 0.27 | 13.30 ± 0.11 | 30.23 ± 0.45 | |
| 12 | 55 | 50 | 30 | 1.72 ± 0.06 | 43.76 ± 0.41 | 21.76 ± 0.30 | |
| 13 | 55 | 40 | 15 | 1.67 ± 0.03 | 31.21 ± 0.12 | 15.12 ± 0.52 | |
| 14 | 55 | 40 | 15 | 1.70 ± 0.09 | 47.14 ± 0.75 | 23.25 ± 0.18 | |
| 15 | 55 | 40 | 15 | 1.57 ± 0.03 | 38.68 ± 0.10 | 17.65 ± 0.63 | |
| Biomass | Run | T | P | Ethanol | Yield | Lutein Content | Lutein Recovery |
| (°C) | (MPa) | (%, v/v) | (%, w/w) | (mg/g Extract) | (%, w/w) | ||
| Freeze-dried | 16 | 40 | 30 | 15 | 4.79 ± 0.24 | 25.96 ± 1.18 | 29.62 ± 0.71 |
| 17 | 70 | 30 | 15 | 4.55 ± 0.16 | 46.00 ± 0.26 | 49.84 ± 1.54 | |
| 18 | 40 | 50 | 15 | 1.62 ± 0.08 | 60.69 ± 2.06 | 23.39 ± 0.29 | |
| 19 | 70 | 50 | 15 | 2.32 ± 0.05 | 53.69 ± 2.41 | 29.64 ± 0.20 | |
| 20 | 40 | 40 | 0 | 2.07 ± 0.10 | 13.42 ± 0.31 | 6.62 ± 0.01 | |
| 21 | 70 | 40 | 0 | 1.15 ± 0.05 | 8.03 ± 0.39 | 2.20 ± 0.04 | |
| 22 | 40 | 40 | 30 | 2.69 ± 0.13 | 39.82 ± 1.29 | 25.52 ± 0.28 | |
| 23 | 70 | 40 | 30 | 3.77 ± 0.13 | 42.25 ± 2.55 | 37.90 ± 0.91 | |
| 24 | 55 | 30 | 0 | 0.76 ± 0.03 | 4.72 ± 0.30 | 0.86 ± 3.0 × 10−3 | |
| 25 | 55 | 50 | 0 | 1.18 ± 0.04 | 7.91 ± 1.00 | 2.22 ± 0.20 | |
| 26 | 55 | 30 | 30 | 6.05 ± 0.30 | 40.98 ± 4.49 | 59.04 ± 2.21 | |
| 27 | 55 | 50 | 30 | 5.74 ± 0.29 | 54.48 ± 0.83 | 74.52 ± 0.47 | |
| 28 | 55 | 40 | 15 | 3.91 ± 0.16 | 38.14 ± 1.82 | 35.55 ± 0.58 | |
| 29 | 55 | 40 | 15 | 3.33 ± 0.07 | 41.33 ± 0.33 | 32.72 ± 1.43 | |
| 30 | 55 | 40 | 15 | 3.07 ± 0.12 | 37.58 ± 1.09 | 27.47 ± 0.07 |
| Parameters | Stages of the General Extraction Curve | |||||
|---|---|---|---|---|---|---|
| Spray-Drying | Freeze-Drying | |||||
| CER | FER | DC | CER | FER | DC | |
| Time (min.) | 12.54 | 29.01 | 150.0 | 11.24 | 44.11 | 160.0 |
| Accumulated extract (%) | 1.66 | 2.01 | 2.54 | 3.25 | 4.15 | 5.10 |
| Recovery (%) | 65.85 | 13.78 | 20.37 | 64.72 | 17.98 | 17.30 |
| Total Recovery (%) | 65.85 | 79.63 | 100.0 | 64.72 | 82.70 | 100.0 |
| M (g/min) | 0.0026 | 4.6 × 10−4 | 9.1 × 10−5 | 0.0057 | 5.3 × 10−4 | 1.7 × 10−4 |
| Y (mg extract/g biomass) | 15.91 | 3.79 | 5.50 | 30.76 | 9.45 | 9.53 |
| Y (g extract/g (CO2 85%+ ethanol 15%) | 1.5 × 10−3 | 2.4 × 10−4 | 5.4 × 10−5 | 3.5 × 10−3 | 3.2 × 10−4 | 1.0 × 10−4 |
| R2 | 0.9273 | 1.0000 | 1.0000 | 0.9451 | 1.0000 | 1.0000 |
| Drying Methods | bo | a1 | a2 | a3 | ||
| Spray-Drying | 0.7098 | 0.0758 | −0.0547 | −0.0167 | ||
| Freeze-Drying | 1.3251 | 0.1711 | −0.1437 | −0.0192 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Domínguez, M.C.; Marticorena, P.; Sepúlveda, C.; Salinas, F.; Cerezal, P.; Riquelme, C. Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile. Mar. Drugs 2020, 18, 528. https://doi.org/10.3390/md18110528
Ruiz-Domínguez MC, Marticorena P, Sepúlveda C, Salinas F, Cerezal P, Riquelme C. Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile. Marine Drugs. 2020; 18(11):528. https://doi.org/10.3390/md18110528
Chicago/Turabian StyleRuiz-Domínguez, Mari Carmen, Paola Marticorena, Claudia Sepúlveda, Francisca Salinas, Pedro Cerezal, and Carlos Riquelme. 2020. "Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile" Marine Drugs 18, no. 11: 528. https://doi.org/10.3390/md18110528
APA StyleRuiz-Domínguez, M. C., Marticorena, P., Sepúlveda, C., Salinas, F., Cerezal, P., & Riquelme, C. (2020). Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile. Marine Drugs, 18(11), 528. https://doi.org/10.3390/md18110528