Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity
2.2. Carotenoids
2.3. Correlation between Antioxidant Activity and Carotenoid Content
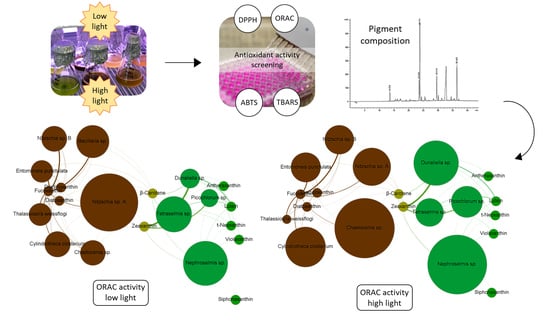
2.4. Effect of Light Intensity on Antioxidant Activity
3. Materials and Methods
3.1. Strains
3.2. Culture Conditions
3.3. Extraction
3.4. DPPH Assay
3.5. ABTS Assay
3.6. ORAC Assay
3.7. TBARS Assay
3.8. Pigments Analysis
3.9. Mass Spectrometry Analysis of Siphonaxanthin
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Račková, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free. Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef] [PubMed]
- Aklakur, M. Natural antioxidants from sea: A potential industrial perspective in aquafeed formulation. Rev. Aquac. 2016, 10, 385–399. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Zuluaga, M.; Gueguen, V.; Pavon-Djavid, G.; Letourneur, D. Carotenoids from microalgae to block oxidative stress. BioImpacts 2017, 7, 1–3. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefining 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds-a brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. Environ. Boil. Fishes 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Faramarzi, M.A.; Mohammadi, N.; Soltani, N.; Oveisi, M.R.; Nafissi-Varcheh, N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J. Appl. Phycol. 2010, 22, 43–50. [Google Scholar] [CrossRef]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; Ainane, T.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef]
- Rodríguez-García, I.; Guil-Guerrero, J.L. Evaluation of the antioxidant activity of three microalgal species for use as dietary supplements and in the preservation of foods. Food Chem. 2008, 108, 1023–1026. [Google Scholar] [CrossRef]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Mehmood, M.A.; Chang, J.-S.; Bai, F.; Zhao, X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products–A review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef]
- Barra, L.; Chandrasekaran, R.; Corato, F.; Brunet, C. The Challenge of Ecophysiological Biodiversity for Biotechnological Applications of Marine Microalgae. Mar. Drugs 2014, 12, 1641–1675. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; De Morais, A.M.M.B. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. 2015, 125, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abalde, J.; Fábregas, J.; Herrero, C. β-Carotene, vitamin C and vitamin E content of the marine microalga Dunaliella tertiolecta cultured with different nitrogen sources. Bioresour. Technol. 1991, 38, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Cezare-Gomes, E.A.; Mejia-Da-Silva, L.D.C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; De Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Adams, W.W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll cycle—A mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Physical Quenching of Singlet Oxygen and cis-trans Isomerization of Carotenoidsa. Ann. N. Y. Acad. Sci. 1993, 691, 10–19. [Google Scholar] [CrossRef]
- Patias, L.D.; Fernandes, A.S.; Petry, F.C.; Jacob-Lopes, E.; Zepka, L.Q.; Mercadante, A.Z. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 2017, 100, 260–266. [Google Scholar] [CrossRef]
- Hejazi, M.A.; Wijffels, R.H. Effect of light intensity on β-carotene production and extraction by Dunaliella salina in two-phase bioreactors. Biomol. Eng. 2003, 20, 171–175. [Google Scholar] [CrossRef]
- Lamers, P.P.; Van De Laak, C.C.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Avron, M.; Young, D.H.; Kauss, H. On the Factors Which Determine Massive β-Carotene Accumulation in the Halotolerant Alga Dunaliella bardawil. Plant Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Motuhi, S.-E.; Mehiri, M.; Payri, C.E.; La Barre, S.; Bach, S. Marine Natural Products from New Caledonia—A Review. Mar. Drugs 2016, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- Laurent, D.; Pietra, F. Natural-Product Diversity of the New Caledonian Marine Ecosystem Compared to Other Ecosystems: A Pharmacologically Oriented View. Chem. Biodivers. 2004, 1, 539–594. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Gordon, M.H. The Mechanism of Antioxidant Action in Vitro. In Food Antioxidants; Hudson, B.J.F., Ed.; Springer: Dordrecht, The Netherlands, 1990; pp. 1–18. ISBN 978-94-010-6824-6. [Google Scholar]
- Assunção, M.F.G.; Amaral, R.; Martins, C.B.; Ferreira, J.D.; Ressurreição, S.; Santos, S.D.; Varejão, J.M.T.B.; Santos, L.M.A. Screening microalgae as potential sources of antioxidants. J. Appl. Phycol. 2016, 1–13. [Google Scholar] [CrossRef]
- The Problems of Using One-Dimensional Methods to Evaluate Multifunctional Food and Biological Antioxidants—Frankel—2000—Journal of the Science of Food and Agriculture—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/1097-0010%28200010%2980%3A13%3C1925%3A%3AAID-JSFA714%3E3.0.CO%3B2-4 (accessed on 9 July 2019).
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Quantitative analysis, in vitro assessment of bioavailability and antioxidant activity of food carotenoids—A review. J. Food Compos. Anal. 2010, 23, 726–740. [Google Scholar] [CrossRef]
- Serive, B.; Nicolau, E.; Bérard, J.-B.; Kaas, R.; Pasquet, V.; Picot, L.; Cadoret, J.-P. Community analysis of pigment patterns from 37 microalgae strains reveals new carotenoids and porphyrins characteristic of distinct strains and taxonomic groups. PLoS ONE 2017, 12, e0171872. [Google Scholar] [CrossRef]
- Yoshii, Y.; Takaichi, S.; Maoka, T.; Suda, S.; Sekiguchi, H.; Nakayama, T.; Inouye, I. Variation of Siphonaxanthin Series Among the Genus Nephroselmis (prasinophyceae, Chlorophyta), Including a Novel Primary Methoxy Carotenoid1. J. Phycol. 2005, 41, 827–834. [Google Scholar] [CrossRef]
- Dambeck, M.; Sandmann, G. Antioxidative Activities of Algal Keto Carotenoids Acting as Antioxidative Protectants in the Chloroplast. Photochem. Photobiol. 2014, 90, 814–819. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Ohkubo, T.; Tanaka, Y.; Noda, K.; Sugawara, T.; Hirata, T. Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 2010, 17, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Li, Z.-S.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The Green Algal Carotenoid Siphonaxanthin Inhibits Adipogenesis in 3T3-L1 Preadipocytes and the Accumulation of Lipids in White Adipose Tissue of KK-Ay Mice. J. Nutr. 2015, jn.114.200931. [Google Scholar] [CrossRef] [Green Version]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; John Wiley & Sons: Somerset, NJ, USA, 2008; ISBN 978-1-4051-7249-3. [Google Scholar]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J. Appl. Phycol. 2016, 28, 849–860. [Google Scholar] [CrossRef]
- Petrushkina, M.; Gusev, E.; Sorokin, B.; Zotko, N.; Mamaeva, A.; Filimonova, A.; Kulikovskiy, M.; Maltsev, Y.; Yampolsky, I.; Guglya, E.; et al. Fucoxanthin production by heterokont microalgae. Algal Res. 2017, 24, 387–393. [Google Scholar] [CrossRef]
- Heo, J.; Shin, D.-S.; Cho, K.; Cho, D.-H.; Lee, Y.J.; Kim, H.-S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Xie Phototrophic Cultivation of a Thermo-Tolerant Desmodesmus sp. for Lutein Production: Effects of Nitrate Concentration, Light Intensity and Fed-Batch Operation—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0960852413009838 (accessed on 5 December 2018).
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Angeles Vargas, M.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Bonnie, T.Y.P.; Choo, Y.M. Oxidation and Thermal Degradation of Carotenoids. J. Oil Palm Res. 1999, 11, 62–78. [Google Scholar]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Zhang, Y.; Diono, W.; Kanda, H.; Yamaguchi, R.; Takemura, R.; Fukaya, T.; Goto, M. Improved Carotenoid Processing with Sustainable Solvents Utilizing Z-Isomerization-Induced Alteration in Physicochemical Properties: A Review and Future Directions. Molecules 2019, 24, 2149. [Google Scholar] [CrossRef] [Green Version]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Riceevans, C. Polyphenolic Flavanols as Scavengers of Aqueous Phase Radicals and as Chain-Breaking Antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Cho, E.J.; Yokozawa, T.; Rhyu, D.Y.; Kim, H.Y.; Shibahara, N.; Park, J.C. The Inhibitory Effects of 12 Medicinal Plants and Their Component Compounds on Lipid Peroxidation. Am. J. Chin. Med. 2003, 31, 907–917. [Google Scholar] [CrossRef] [Green Version]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Cequier-Sánchez, E.; Rodríguez, C.; Ravelo, A.G.; Zárate, R. Dichloromethane as a solvent for lipid extraction and assessment of lipid classes and fatty acids from samples of different natures. J. Agric. Food Chem. 2008, 56, 4297–4303. [Google Scholar] [CrossRef]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Yoshida, K.; Terao, J.; Suzuki, T.; Takama, K. Inhibitory effect of phosphatidylserine on iron-dependent lipid peroxidation. Biochem. Biophys. Res. Commun. 1991, 179, 1077–1081. [Google Scholar] [CrossRef]
- Grima, E.M.; Camacho, F.; Pérez, J.; Sánchez, J. Biochemical productivity and fatty acid profiles of Isochrysis galbana Parke and Tetraselmis sp. as a function of incident light intensity. Process. Biochem. 1994, 29, 119–126. [Google Scholar] [CrossRef]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Guiheneuf, F.; Mimouni, V.; Ulmann, L.; Tremblin, G. Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture. J. Exp. Mar. Boil. Ecol. 2009, 369, 136–143. [Google Scholar] [CrossRef]
- Mitra, M.; Patidar, S.K.; Mishra, S. Integrated process of two stage cultivation of Nannochloropsis sp. for nutraceutically valuable eicosapentaenoic acid along with biodiesel. Bioresour. Technol. 2015, 193, 363–369. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- Boelen, P.; Van Dijk, R.; Damsté, J.S.S.; Rijpstra, W.I.C.; Buma, A.G. On the potential application of polar and temperate marine microalgae for EPA and DHA production. AMB Express 2013, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Kong, Z.; Chen, S.; Ran, Z.; Ye, M.; Xu, J.; Zhou, C.; Liao, K.; Cao, J.; Yan, X. The comparative study for physiological and biochemical mechanisms of Thalassiosira pseudonana and Chaetoceros calcitrans in response to different light intensities. Algal Res. 2017, 27, 89–98. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nishio, N.; Nagai, S. Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 1992, 74, 61–63. [Google Scholar] [CrossRef]
- Wang, B.; Zarka, A.; Trebst, A.; Boussiba, S. Astaxanthin Accumulation in Haematococcus Pluvialis (chlorophyceae) as an Active Photoprotective Process under High Irradiance1. J. Phycol. 2003, 39, 1116–1124. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin Accumulation in the Green Alga Haematococcus pluvialis1. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Walne, P.R. Experiments in the Large-Scale Culture of the Larvae of Ostrea Edulis L.; H.M.S.O.: London, UK, 1966. [Google Scholar]
- Kenny, O.; Brunton, N.P.; Smyth, T.J. In Vitro Protocols for Measuring the Antioxidant Capacity of Algal Extracts. In Natural Products from Marine Algae: Methods and Protocols; Stengel, D.B., Connan, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; pp. 375–402. ISBN 978-1-4939-2684-8. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Watanabe, J.; Oki, T.; Takebayashi, J.; Yada, H.; Wagaki, M.; Takano-Ishikawa, Y.; Yasui, A. Improvement and Interlaboratory Validation of the Lipophilic Oxygen Radical Absorbance Capacity: Determination of Antioxidant Capacities of Lipophilic Antioxidant Solutions and Food Extracts. Anal. Sci. 2016, 32, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Bin Ibrahim, M.; Bin Hazali, N.; Rasad, M.S.B.A.; Ghani, R.A.; Wahab, R.A.; Arief, S.J.; Yahya, M.N.A. Antioxidant activity and phenolic profile of various morphological parts of underutilised Baccaurea angulata fruit. Food Chem. 2015, 172, 778–787. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Welschmeyer, N.; Roy, S.; Llewellyn, C.; Egeland, E.S.; Johnsen, G. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]


| DPPH | ABTS | ORAC | TBARS | ||
|---|---|---|---|---|---|
| (IC50 in µg of dry extract·mL−1) | (IC50 in µg of dry extract·mL−1) | (µg Trolox equivalent·mg−1 of dry extract) | (IC50 in µg of dry extract·mL−1) | ||
| Nephroselmis sp. | LL | 695.80 ± 57.28 hi | 558.16 ± 70.02 j | 138.82 ± 0.88 f | 63.39 ± 5.04 h |
| HL | 395.93 ± 70.98 f | 311.08 ± 26.80 f | 188.32 ± 0.51 b | 31.40 ± 2.13 e | |
| Tetraselmis sp. | LL | 753.99 ± 81.35 jk | 193.17 ± 11.18 e | 110.48 ± 0.71 i | 15.43 ± 2.47 c |
| HL | >1000 | 341.38 ± 28.86 g | 89.16 ± 1.51 o | 22.77 ± 4.54 d | |
| Dunaliella sp. | LL | 823.98 ± 77.14 kl | 430.69 ± 31.48 h | 59.51 ± 1.47 s | 58.20 ± 8.35 gh |
| HL | 892.18 ± 67.60 m | 794.54 ± 64.60 m | 141.53 ± 0.79 e | 68.24 ± 5.65 i | |
| Picochlorum sp. | LL | >1000 | 981.96 ± 40.66 o | 55.17 ± 0.68 t | 42.10 ± 5.87 f |
| HL | 671.50 ± 61.75 h | 463.90 ± 17.30 i | 98.64 ± 0.80 l | 87.76 ± 8.36 k | |
| Schizochlamydella sp. | LL | >1000 | >1000 | n.d. | 55.41 ± 3.72 g |
| HL | >1000 | >1000 | n.d. | 43.51 ± 8.88 f | |
| Nitzschia sp. A | LL | 497.27 ± 79.37 g | 462.96 ± 17.88 i | 179.75 ± 0.78 c | 24.63 ± 6.07 d |
| HL | >1000 | >1000 | 119.76 ± 1.49 h | 98.77 ± 7.73 l | |
| Nitzschia sp. B | LL | >1000 | >1000 | 78.95 ± 1.54 p | 190.91 ± 24.36 p |
| HL | >1000 | >1000 | 92.02 ± 1.52 n | 202.28 ± 27.86 p | |
| Thalassiosira weissflogi | LL | 939.31 ± 104.41 n | 620.26 ± 54.67 k | 69.99 ± 1.49 q | 114.58 ± 6.69 m |
| HL | >1000 | >1000 | 27.71 ± 0.95 u | 164.44 ± 5.35 o | |
| Entomoneis punctulata | LL | >1000 | >1000 | 68.09 ± 1.58 r | 147.34 ± 17.47 n |
| HL | 839.30 ± 84.45 lm | >1000 | 94.20 ± 1.45 m | 473.56 ± 66.26 q | |
| Cylindrotheca closterium | LL | 890.75 ± 72.49 mn | 615.65 ± 27.05 k | 105.48 ± 1.58 j | 79.67 ± 11.87 j |
| HL | 710.60 ± 61.83 hij | 654.79 ± 21.27 l | 127.14 ± 1.29 g | 103.48 ± 15.18 l | |
| Chaetoceros sp. | LL | 484.47 ± 87.98 g | 441.03 ± 17.20 h | 170.00 ± 0.57 d | 77.97 ± 6.16 j |
| HL | 773.52 ± 68.35 k | 791.40 ± 49.81 m | 190.3 ± 0.78 a | 116.08 ± 17.32 m | |
| Bacillaria sp. | LL | 749.55 ± 87.70 ij | 895.81 ± 44.93 n | 102.19 ± 1.45 k | 60.14 ± 8.54 gh |
| Trolox | 4.71 ± 0.53 a | 6.36 ± 1.33 a | - | 0.24 ± 0.06 a | |
| α-Tocopherol | 6.20 ± 0.33 b | 10.78 ± 0.26 b | - | 1.30 ± 0.16 b | |
| Ascorbic acid | 8.73 ± 1.63 c | 6.08 ± 0.75 a | - | - | |
| β-Carotene | 257.33 ± 20.89 e | 37.04 ± 2.56 c | - | >200 | |
| Astaxanthin | 228.59 ± 41.71 d | 98.54 ± 6.58 d | - | >200 |
| Lut | t-Neo | Siph | Zea | β-Car | Fuco | Cis-Fuco | Dt | Total Carotenoids | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorophyta | Nephroselmis sp. | LL | 4.70 | n.d. | 4.11 | 13.60 | 5.40 | n.d. | n.d. | n.d. | 27.81 |
| HL | 13.50 | n.d. | 6.89 | 39.30 | 7.20 | n.d. | n.d. | n.d. | 66.89 | ||
| Tetraselmis sp. | LL | 9.51 | 1.43 | n.d. | 1.91 | 4.42 | n.d. | n.d. | n.d. | 17.27 | |
| HL | 7.04 | 1.38 | n.d. | 1.76 | 3.01 | n.d. | n.d. | n.d. | 13.19 | ||
| Dunaliella sp. | LL | 3.36 | 0.29 | n.d. | 0.53 | 1.90 | n.d. | n.d. | n.d. | 6.08 | |
| HL | 4.83 | 0.15 | n.d. | 1.55 | 2.00 | n.d. | n.d. | n.d. | 8.53 | ||
| Picochlorum sp. | LL | 7.07 | n.d. | n.d. | 2.32 | 0.89 | n.d. | n.d. | n.d. | 10.28 | |
| HL | 7.27 | 0.92 | n.d. | 2.37 | 2.79 | n.d. | n.d. | n.d. | 13.35 | ||
| Schizochlamydella sp. | LL | 1.58 | n.d. | n.d. | 0.18 | 0.53 | n.d. | n.d. | n.d. | 2.29 | |
| HL | 0.18 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.18 | ||
| Bacillariophyta | Nitzschia sp. A | LL | n.d. | n.d. | n.d. | n.d. | 1.40 | 22.40 | 4.50 | 0.50 | 28.80 |
| HL | n.d. | n.d. | n.d. | 1.30 | 0.90 | 32.30 | 2.50 | 1.20 | 38.20 | ||
| Nitzschia sp. B | LL | n.d. | n.d. | n.d. | n.d. | 0.20 | 10.30 | 1.10 | 0.10 | 11.70 | |
| HL | n.d. | n.d. | n.d. | n.d. | 0.20 | 7.40 | 0.30 | 0.20 | 8.10 | ||
| Thalassiosira weissflogi | LL | n.d. | n.d. | n.d. | n.d. | 1.30 | 10.76 | 1.00 | 1.40 | 14.46 | |
| HL | n.d. | n.d. | n.d. | n.d. | n.d. | 0.10 | n.d. | n.d. | 0.10 | ||
| Entomoneis punctulata | LL | n.d. | n.d. | n.d. | n.d. | n.d. | 7.00 | 0.60 | n.d. | 7.60 | |
| HL | n.d. | n.d. | n.d. | n.d. | 1.50 | 15.30 | 2.90 | 0.60 | 20.30 | ||
| Cylindrotheca closterium | LL | n.d. | n.d. | n.d. | n.d. | 0.50 | 12.60 | 1.30 | 0.70 | 15.10 | |
| HL | n.d. | n.d. | n.d. | n.d. | 0.40 | 12.10 | 0.90 | 1.30 | 14.70 | ||
| Chaetoceros sp. | LL | n.d. | n.d. | n.d. | n.d. | 1.30 | 19.30 | 2.20 | 4.00 | 26.80 | |
| HL | n.d. | n.d. | n.d. | n.d. | n.d. | 12.40 | 1.20 | 2.40 | 16.00 | ||
| Bacillaria sp. | LL | n.d. | n.d. | n.d. | n.d. | 1.50 | 16.30 | 3.40 | 0.70 | 21.90 |
| Lut | t-Neo | Siph | Zea | β-Car | Viola | Anthe | Fuco | Cis-Fuco | Dt | Dd | Total Carotenoids | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorophyta | Nephroselmis sp. | LL | 0.48 | 0.21 | 0.14 | 0.68 | 0.65 | 0.33 | 0.11 | n.d. | n.d. | n.d. | n.d. | 2.60 |
| HL | 0.31 | 0.11 | 0.05 | 0.73 | 0.36 | 0.14 | 0.09 | n.d. | n.d. | n.d. | n.d. | 1.79 | ||
| Tetraselmis sp. | LL | 1.02 | 0.39 | n.d. | 0.59 | 0.92 | 0.47 | 0.08 | n.d. | n.d. | n.d. | n.d. | 3.47 | |
| HL | 1.63 | 0.40 | n.d. | 2.84 | 1.97 | 0.23 | 0.12 | n.d. | n.d. | n.d. | n.d. | 7.19 | ||
| Dunaliella sp. | LL | 2.57 | 0.44 | n.d. | 9.21 | 1.12 | 0.27 | 0.35 | n.d. | n.d. | n.d. | n.d. | 13.96 | |
| HL | 3.74 | 0.57 | n.d. | 11.67 | 1.87 | 0.35 | 0.59 | n.d. | n.d. | n.d. | n.d. | 18.79 | ||
| Picochlorum sp. | LL | 1.26 | 0.23 | n.d. | 4.51 | 0.13 | 0.02 | 0.05 | n.d. | n.d. | n.d. | n.d. | 6.20 | |
| HL | 0.54 | 0.08 | n.d. | 2.03 | 0.05 | 0.01 | 0.04 | n.d. | n.d. | n.d. | n.d. | 2.75 | ||
| Schizochlamydella sp. | LL | 0.08 | 0.01 | n.d. | 0.31 | 0.02 | 0.01 | 0.01 | n.d. | n.d. | n.d. | n.d. | 0.44 | |
| HL | 0.12 | 0.02 | n.d. | 0.91 | 0.02 | 0.01 | 0.02 | n.d. | n.d. | n.d. | n.d. | 1.10 | ||
| Bacillaryophyta | Nitzschia sp. A | LL | n.d. | n.d. | n.d. | 0.07 | 0.07 | n.d. | n.d. | 2.35 | n.d. | 0.02 | 0.26 | 2.77 |
| HL | n.d. | n.d. | n.d. | 0.06 | 0.06 | n.d. | n.d. | 1.44 | n.d. | 0.02 | 0.26 | 1.84 | ||
| Nitzschia sp. B | LL | n.d. | n.d. | n.d. | n.d. | 0.32 | n.d. | n.d. | 6.25 | n.d. | 0.43 | 0.68 | 7.68 | |
| HL | n.d. | n.d. | n.d. | n.d. | 0.27 | n.d. | n.d. | 5.16 | n.d. | 0.37 | 0.56 | 6.36 | ||
| Thalassiosira weissflogi | LL | n.d. | n.d. | n.d. | n.d. | 0.36 | n.d. | n.d. | 3.76 | n.d. | 0.60 | 0.91 | 5.63 | |
| HL | n.d. | n.d. | n.d. | n.d. | 0.33 | n.d. | n.d. | 3.49 | n.d. | 0.55 | 0.85 | 5.22 | ||
| Entomoneis punctulata | LL | n.d. | n.d. | n.d. | n.d. | 0.39 | n.d. | n.d. | 5.23 | n.d. | 0.15 | 0.90 | 6.67 | |
| HL | n.d. | n.d. | n.d. | n.d. | 0.33 | n.d. | n.d. | 4.34 | n.d. | 0.13 | 0.77 | 5.57 | ||
| Cylindrotheca closterium | LL | n.d. | n.d. | n.d. | n.d. | 0.17 | n.d. | n.d. | 2.82 | n.d. | 0.08 | 0.83 | 3.90 | |
| HL | n.d. | n.d. | n.d. | n.d. | 0.12 | n.d. | n.d. | 1.61 | n.d. | 0.10 | 0.65 | 2.48 | ||
| Chaetoceros sp. | LL | n.d. | n.d. | n.d. | 0.07 | 0.02 | n.d. | n.d. | 1.35 | n.d. | 0.21 | 0.07 | 1.72 | |
| HL | n.d. | n.d. | n.d. | 0.08 | 0.11 | n.d. | n.d. | 0.78 | n.d. | 0.49 | 0.26 | 1.72 | ||
| Bacillaria sp. | LL | n.d. | n.d. | n.d. | n.d. | 0.30 | n.d. | n.d. | 5.36 | n.d. | 0.21 | 0.72 | 6.59 |
| ORAC Assay | TBARS Assay | |||
|---|---|---|---|---|
| Correlation Coefficient | R2 | Correlation Coefficient | R2 | |
| total carotenoids | 0.71 ** | 0.51 | −0.12 ns | - |
| total xanthophylls | 0.71 ** | 0.51 | −0.10 ns | - |
| lutein | 0.78 ** | 0.60 | −0.34 ns | - |
| zeaxanthin | 0.70 * | 0.48 | −0.18 ns | - |
| fucoxanthin | 0.60 * | 0.35 | −0.24 ns | - |
| β-Carotene | 0.36 ns | - | −0.30 ns | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulombier, N.; Nicolau, E.; Le Déan, L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Mar. Drugs 2020, 18, 122. https://doi.org/10.3390/md18020122
Coulombier N, Nicolau E, Le Déan L, Antheaume C, Jauffrais T, Lebouvier N. Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Marine Drugs. 2020; 18(2):122. https://doi.org/10.3390/md18020122
Chicago/Turabian StyleCoulombier, Noémie, Elodie Nicolau, Loïc Le Déan, Cyril Antheaume, Thierry Jauffrais, and Nicolas Lebouvier. 2020. "Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae" Marine Drugs 18, no. 2: 122. https://doi.org/10.3390/md18020122
APA StyleCoulombier, N., Nicolau, E., Le Déan, L., Antheaume, C., Jauffrais, T., & Lebouvier, N. (2020). Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Marine Drugs, 18(2), 122. https://doi.org/10.3390/md18020122