Protective Effect of Low-Molecular-Weight Fucoidan on Radiation-Induced Fibrosis Through TGF-β1/Smad Pathway-Mediated Inhibition of Collagen I Accumulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. LMF Reduced Cell Death in Irradiated Fibroblast Cells
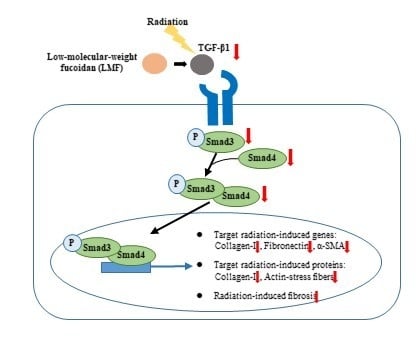
2.2. LMF Inhibited Radiation-Induced Fibrosis Through the TGF-β1/Smad Signaling Pathway
2.3. LMF Inhibited TGF-β1 Protein Activation and Extracellular Matrix Formation in Irradiated Fibroblast Cells
2.4. LMF Reduced the Contractility of Irradiated Fibroblast Cells
3. Materials and Methods
3.1. LMF Preparation
3.2. Cell Culture
3.3. Cellular Metabolic Activity after LMF Treatment
3.4. Radioprotection (Pre + Post-Treatment) and Radio-repair (Post-Treatment) Assay
3.5. Fibrosis-related mRNA expression
3.6. Western Blot Assay
3.7. Immunocytochemistry
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yarnold, J.; Brotons, M.C.V. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef]
- Small, W.; Woloschak, G.E. Radiation Toxicity: A Practical Medical Guide; Springer: New York, NY, USA, 2006; Volume 128. [Google Scholar]
- Burger, A.; Loffler, H.; Bamberg, M.; Rodemann, H.P. Molecular and cellular basis of radiation fibrosis. Int. J. Radiat. Biol. 1998, 73, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Herskind, C.; Bentzen, S.M.; Overgaard, J.; Overgaard, M.; Bamberg, M.; Rodemann, H.P. Differentiation state of skin fibroblast cultures versus risk of subcutaneous fibrosis after radiotherapy. Radiother. Oncol. 1998, 47, 263–269. [Google Scholar] [CrossRef]
- Yano, H.; Hamanaka, R.; Nakamura, M.; Sumiyoshi, H.; Matsuo, N.; Yoshioka, H. Smad, but not MAPK, pathway mediates the expression of type I collagen in radiation induced fibrosis. Biochem. Bioph. Res. Co. 2012, 418, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Falkmer, U.; Jarhult, J.; Wersall, P.; Cavallin-Stahl, E. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol. 2003, 42, 620–633. [Google Scholar] [CrossRef] [Green Version]
- Sasse, A.D.; Clark, L.G.; Sasse, E.C.; Clark, O.A. Amifostine reduces side effects and improves complete response rate during radiotherapy: Results of a meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 784–791. [Google Scholar] [CrossRef]
- Brizel, D.M.; Wasserman, T.H.; Henke, M.; Strnad, V.; Rudat, V.; Monnier, A.; Eschwege, F.; Zhang, J.; Russell, L.; Oster, W. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J. Clin. Oncol. 2000, 18, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Trifan, A.; Gille, E.; Petreus, T.; Bordeianu, G.; Miron, A. Can phytochemicals be a bridge to develop new radioprotective agents? Phytochem. Rev. 2015, 14, 555–566. [Google Scholar] [CrossRef]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Gómez-García, F.; Carrillo, N.G.; Valle-Rodríguez, E.; Xerafin, A.; Vicente-Ortega, V.; Surgery, M. Radioprotective effects of lycopene and curcumin during local irradiation of parotid glands in Sprague Dawley rats. Br. J. Oral Maxillofac Surg. 2016, 54, 275–279. [Google Scholar] [CrossRef]
- Patil, S.L.; Mallaiah, S.H.; Patil, R.K. Antioxidative and radioprotective potential of rutin and quercetin in Swiss albino mice exposed to gamma radiation. J. Med. Phys. 2013, 38, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Saha, C.; Dey, S.K. Studies on black tea (Camellia sinensis) extract as a potential antioxidant and a probable radioprotector. Radiat. Environ. Biophys. 2013, 52, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Dhaker, A.; Adhikari, J.; Ivanov, V.; Singh, V.; Chawla, R.; Kumar, R.; Sharma, R.; Karamalakova, Y.; Gadjeva, V.; et al. In vitro studies on radioprotective efficacy of silymarin against γ-irradiation. Int. J. Radiat. Biol. 2013, 89, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.A.; Li, F.; Chung, E.J.; Hudak, K.; White, A.; Krausz, K.; Gonzalez, F.; Citrin, D. Quercetin inhibits radiation-induced skin fibrosis. Radiat. Res. 2013, 180, 205–215. [Google Scholar] [CrossRef]
- Ryu, S.H.; Park, E.Y.; Kwak, S.; Heo, S.H.; Ryu, J.W.; Park, J.H.; Choi, K.C.; Lee, S.W. Protective effect of α-lipoic acid against radiation-induced fibrosis in mice. Oncotarget 2016, 7, 15554–15565. [Google Scholar] [CrossRef] [Green Version]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, J.; Moon, C.; Kim, S.H.; Hyun, J.W.; Park, J.W.; Shin, T. Radioprotective effects of fucoidan in mice treated with total body irradiation. Phytother. Res. 2008, 22, 1677–1681. [Google Scholar] [CrossRef]
- Lee, K.H.; Bae, S.; Cho, C.H.; Rhee, K.H. Fucoidan Protects human skin fibroblast cell line HS68 against-radiation-induced damage. Open Nat. Prod. J. 2009, 2, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Byon, Y.Y.; Kim, M.H.; Yoo, E.S.; Hwang, K.K.; Jee, Y.; Shin, T.; Joo, H.G. Radioprotective effects of fucoidan on bone marrow cells: Improvement of the cell survival and immunoreactivity. J. Vet. Sci. 2008, 9, 359–365. [Google Scholar] [CrossRef]
- Rhee, K.H.; Lee, K.H. Protective effects of fucoidan against γ-radiation-induced damage of blood cells. Arch. Pharm. Res. 2011, 34, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, W.; Zhang, Q.; Jia, Y.; Sun, Y.; Weng, L.; Luo, D.; Zhou, H.; Yang, B. Low molecular weight fucoidan ameliorates diabetic nephropathy via inhibiting epithelial-mesenchymal transition and fibrotic processes. Am. J. Transl. Res. 2015, 7, 1553–1563. [Google Scholar] [PubMed]
- Li, J.; Chen, K.; Li, S.; Feng, J.; Liu, T.; Wang, F.; Zhang, R.; Xu, S.; Zhou, Y.; Zhou, S.; et al. Protective effect of fucoidan from Fucus vesiculosus on liver fibrosis via the TGF-beta1/Smad pathway-mediated inhibition of extracellular matrix and autophagy. Drug Des. Devel. Ther. 2016, 10, 619–630. [Google Scholar] [PubMed] [Green Version]
- Hwang, P.A.; Yan, M.D.; Kuo, K.L.; Phan, N.N.; Lin, Y.C. A mechanism of low molecular weight fucoidans degraded by enzymatic and acidic hydrolysis for the prevention of UVB damage. J. Appl. Phycol. 2017, 29, 521–529. [Google Scholar] [CrossRef]
- Hwang, P.A.; Yan, M.D.; Lin, H.T.V.; Li, K.L.; Lin, Y.C. Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Sue, Y.M.; Cheng, C.Y.; Chen, Y.C.; Liu, C.T.; Hsu, Y.H.; Hwang, P.A.; Huang, N.J.; Chen, T.H. Oligo-fucoidan prevents renal tubulointerstitial fibrosis by inhibiting the CD44 signal pathway. Sci. Rep. 2017, 7, 40183. [Google Scholar] [CrossRef] [Green Version]
- Prise, K.M.; Schettino, G.; Folkard, M.; Held, K.D. New insights on cell death from radiation exposure. Lancet Oncol. 2005, 6, 520–528. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Jonas, U.; Jhurry, D.; Schönherr, H. Enhanced differentiation of human preosteoblasts on electrospun blend fiber mats of polydioxanone and anionic sulfated polysaccharides. ACS Biomater. Sci. Eng. 2017, 3, 3447–3458. [Google Scholar] [CrossRef]
- Song, Y.S.; Li, H.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Choi, H.R.; Park, K.C.; Kim, D.S. Fucoidan promotes the reconstruction of skin equivalents. Korean J. Physiol. Pharmacol. 2014, 18, 327–331. [Google Scholar] [CrossRef]
- Kobashigawa, S.; Kashino, G.; Mori, H.; Watanabe, M. Relief of delayed oxidative stress by ascorbic acid can suppress radiation-induced cellular senescence in mammalian fibroblast cells. Mech. Ageing Dev. 2015, 146, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Marampon, F.; Gravina, G.L.; Festuccia, C.; Popov, V.M.; Colapietro, E.A.; Sanita, P.; Musio, D.; De Felice, F.; Lenzi, A.; Jannini, E.A.; et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Invest. 2016, 39, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Pohlers, D.; Brenmoehl, J.; Loffler, I.; Muller, C.K.; Leipner, C.; Schultze-Mosgau, S.; Stallmach, A.; Kinne, R.W.; Wolf, G. TGF-beta and fibrosis in different organs-molecular pathway imprints. BBA Mol. Basis Dis. 2009, 1792, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchida, K.; Zhu, Y.Q.; Siva, S.; Dunn, S.R.; Sharma, K. Role of Smad4 on TGF-beta-induced extracellular matrix stimulation in mesangial cells. Kidney Int. 2003, 63, 2000–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Xiao, P.; Lei, S.; Deng, F.; Xiao, G.G.; Liu, Y.; Chen, X.; Li, L.; Wu, S.; Chen, Y.; et al. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim. Biophys. Sin. 2008, 40, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Lee, E.K.; Lee, M.J.; Kim, J.H.; Yang, W.S. Fucoidan inhibits activation and receptor binding of transforming growth factor-beta 1. Biochem. Biophys. Res. Commun. 2013, 432, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic-Bussod, M.; Balzaretti-Maggi, V.S.; Gadbois, D.M. Extracellular matrix and radiation G1 cell cycle arrest in human fibroblasts. Cancer Res. 1999, 59, 4843–4847. [Google Scholar]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Bio. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar]
- Vaughan, M.B.; Howard, E.W.; Tomasek, J.J. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp. Cell Res. 2000, 257, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Judge, J.L.; Owens, K.M.; Pollock, S.J.; Woeller, C.F.; Thatcher, T.H.; Williams, J.P.; Phipps, R.P.; Sime, P.J.; Kottmann, R.M. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L879–L887. [Google Scholar] [CrossRef] [Green Version]
- Mutsaers, S.E.; Bishop, J.E.; McGrouther, G.; Laurent, G.J. Mechanisms of tissue repair: From wound healing to fibrosis. Int. J. Biochem. Cell B 1997, 29, 5–17. [Google Scholar] [CrossRef]
- Rhyu, D.Y.; Yang, Y.; Ha, H.; Lee, G.T.; Song, J.S.; Uh, S.; Lee, H.B. Role of reactive oxygen species in TGF-β1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 2005, 16, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dancea, H.C.; Shareef, M.M.; Ahmed, M.M. Role of radiation-induced TGF-beta signaling in cancer therapy. Mol. Cell Pharmacol. 2009, 1, 44. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.M.; Liu, P.Y.; Chen, Y.A.; Tseng, H.Y.; Shen, P.C.; Hwang, P.A.; Hsu, H.L. Oligo-Fucoidan prevents IL-6 and CCL2 production and cooperates with p53 to suppress ATM signaling and tumor progression. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Yoon, Y.M.; Lee, J.H.; Lee, S.H. Protective role of fucoidan on cisplatin-mediated ER stress in renal proximal tubule epithelial cells. Anticancer Res. 2019, 39, 5515–5524. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Yu, H.H.; Chengchuan, K.O.; Chang, C.L.; Yuan, K.S.P.; Wu, A.T.; Shan, Y.S.; Wu, S.Y. Fucoidan inhibits radiation-induced pneumonitis and lung fibrosis by reducing inflammatory cytokine expression in lung tissues. Mar. Drugs 2018, 16, 392. [Google Scholar] [CrossRef] [Green Version]








| Gene | Primer sequence (5′- 3′) | Annealing | ||
|---|---|---|---|---|
| TGF-β1 | Sense Antisense | AGGGGCCTCTAAGAGCAGTC AGGTTGGCATTCCACTTCAC | 94 °C, 30 s 55 °C, 30 s 72 °C, 30 s | 25 cycle |
| Smad3 | Sense Antisense | GGGCCAACAAGTCAACAAGT CTGGCTGGCTAAGGAGTGAC | 94 °C, 30 s 55 °C, 30 s 72 °C, 30 s | 25 cycle |
| Smad4 | Sense Antisense | CGGCCGTGGCAGGGAACA CTGCAGAGCTCGGTGAAGGTGAAT | 94 °C, 30 s 55 °C, 30 s 72 °C, 30 s | 25 cycle |
| Collagen-I | Sense Antisense | CCGTGCTTCTCAGAACATCA CTTGCCCCATTCATTTGTCT | 94 °C, 30 s 55 °C, 30 s 72 °C, 30 s | 25 cycle |
| Fibronectin | Sense Antisense | GTGGCTGCCTTCAACTTCTC TGAATGCCAGTCCTTTAGGG | 94 °C, 30 s 53 °C, 30 s 72 °C, 30 s | 25 cycle |
| α-SMA | Sense Antisense | ACTGGGACGACATGGAAAAG CATCTCCAGAGTCCAGCACA | 94 °C, 30 s 53 °C, 30 s 72 °C, 30 s | 25 cycle |
| GAPDH | Sense Antisense | TGTTCCTACCCCCAATGTGT CCCTGTTGCTGTAGCCGTAT | 94 °C, 30 s 55 °C, 30 s 72 °C, 30 s | 25 cycle |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-Y.; Chen, Y.-T.; Tsai, G.-Y.; Hsu, F.-Y.; Hwang, P.-A. Protective Effect of Low-Molecular-Weight Fucoidan on Radiation-Induced Fibrosis Through TGF-β1/Smad Pathway-Mediated Inhibition of Collagen I Accumulation. Mar. Drugs 2020, 18, 136. https://doi.org/10.3390/md18030136
Wu S-Y, Chen Y-T, Tsai G-Y, Hsu F-Y, Hwang P-A. Protective Effect of Low-Molecular-Weight Fucoidan on Radiation-Induced Fibrosis Through TGF-β1/Smad Pathway-Mediated Inhibition of Collagen I Accumulation. Marine Drugs. 2020; 18(3):136. https://doi.org/10.3390/md18030136
Chicago/Turabian StyleWu, Szu-Yuan, Yu-Ting Chen, Guo-Yu Tsai, Fu-Yin Hsu, and Pai-An Hwang. 2020. "Protective Effect of Low-Molecular-Weight Fucoidan on Radiation-Induced Fibrosis Through TGF-β1/Smad Pathway-Mediated Inhibition of Collagen I Accumulation" Marine Drugs 18, no. 3: 136. https://doi.org/10.3390/md18030136
APA StyleWu, S. -Y., Chen, Y. -T., Tsai, G. -Y., Hsu, F. -Y., & Hwang, P. -A. (2020). Protective Effect of Low-Molecular-Weight Fucoidan on Radiation-Induced Fibrosis Through TGF-β1/Smad Pathway-Mediated Inhibition of Collagen I Accumulation. Marine Drugs, 18(3), 136. https://doi.org/10.3390/md18030136