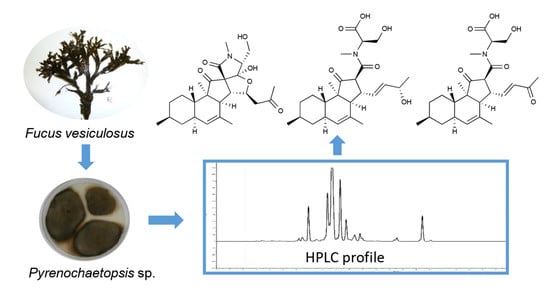
Pyrenosetin D, a New Pentacyclic Decalinoyltetramic Acid Derivative from the Algicolous Fungus Pyrenochaetopsis sp. FVE-087
Abstract
:1. Introduction
2. Results
2.1. Strain Identification and Cultivation
2.2. Extraction, Bioactivity Testing, and Isolation
2.3. Structure Elucidation
2.4. Bioactivity Tests
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Strain Identification and Cultivation
4.3. Extraction and Isolation
4.4. Computational Details
4.5. Biological Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flewelling, A.J.; Johnson, J.A.; Gray, C.A. Isolation and bioassay screening of fungal endophytes from North Atlantic marine macroalgae. Bot. Mar. 2013, 56, 287–297. [Google Scholar] [CrossRef]
- Zuccaro, A.; Schoch, C.L.; Spatafora, J.W.; Kohlmeyer, J.; Draeger, S.; Mitchell, J.I. Detection and identification of fungi intimately associated with the brown seaweed Fucus serratus. Appl. Environ. Microbiol. 2008, 74, 931–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding seaweed–bacteria interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Li, X.; Wang, B.-G. Secondary metabolites from the marine algal-derived endophytic fungi: Chemical diversity and biological activity. Planta Med. 2016, 82, 832–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, B.W.; Choi, J.S.; Kim, J.C.; Nam, K.W.; Kim, D.-S.; Chung, H.Y.; Kang, J.S.; Choi, H.D. Parasitenone, a new epoxycyclohexenone related to gabosine from the marine-derived fungus Aspergillus parasiticus. J. Nat. Prod. 2002, 65, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Elsaedi, S.; Kehraus, S.; König, G.M. Novel bisabolane sesquiterpenes from the marine-derived fungus Verticillium tenerum. Nat. Prod. Commun. 2010, 5, 507–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.-M.; Li, X.-M.; Meng, L.; Li, C.-S.; Huang, C.-G.; Wang, B.-G. 7-Nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspergillus ochraceus EN-31. J. Nat. Prod. 2010, 73, 1780–1784. [Google Scholar] [CrossRef]
- Komatsu, K.; Shigemori, H.; Kobayashi, J. Dictyonamides A and B, new peptides from marine-derived fungus. J. Org. Chem. 2001, 66, 6189–6192. [Google Scholar] [CrossRef]
- Du, F.-Y.; Li, X.-M.; Li, C.-S.; Shang, Z.; Wang, B.-G. Cristatumins A–D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Klemke, C.; König, G.M.; Wright, A.D. Two new xanthone derivatives from the algicolous marine fungus Wardomyces anomalus. J. Nat. Prod. 2003, 66, 706–708. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Spiteller, M. Natural products containing ‘decalin’ motif in microorganisms. Nat. Prod. Rep. 2014, 31, 1175–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolevskaya, M.P.; Leshchenko, E.V.; Hoai, T.P.T.; Denisenko, V.A.; Dyshlovoy, S.A.; Kirichuk, N.N.; Khudyakova, Y.V.; Kim, N.Y.; Berdyshev, D.V.; Pislyagin, E.A.; et al. Pallidopenillines: Polyketides from the alga-derived fungus Penicillium thomii Maire KMM 4675. J. Nat. Prod. 2016, 79, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Tanaka, A.; Nehira, T.; Nishii, T.; Kikuchi, T. Altercrasins A–E, decalin derivatives, from a sea-urchin-derived Alternaria sp.: Isolation and structural analysis including stereochemistry. Mar. Drugs 2019, 17, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, K.M.; Toske, S.G.; Jensen, P.R.; Fenical, W. Solanapyrones E-G, antialgal metabolites produced by a marine fungus. Phytochemistry 1998, 49, 2299–2304. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Zhang, D.; Lee, U.; Kang, J.S.; Choi, H.D.; Son, B.W. Dehydroxychlorofusarielin B, an antibacterial polyoxygenated decalin derivative from the marine-derived fungus Aspergillus sp. J. Nat. Prod. 2007, 70, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, T.; Kawatani, M.; Uramoto, M.; Okano, A.; Aono, H.; Futamura, Y.; Koshino, H.; Takahashi, S.; Osada, H. Pyrrolizilactone, a new pyrrolizidinone metabolite produced by a fungus. J. Antibiot. 2013, 66, 621–623. [Google Scholar] [CrossRef]
- Osterhage, C.; Kaminsky, R.; König, G.M.; Wright, A.D. Ascosalipyrrolidinone A, an antimicrobial alkaloid, from the obligate marine fungus Ascochyta salicorniae. J. Org. Chem. 2000, 65, 6412–6417. [Google Scholar] [CrossRef]
- Yamada, T.; Mizutani, Y.; Umebayashi, Y.; Inno, N.; Kawashima, M.; Kikuchi, T.; Tanaka, R. Tandyukisin, a novel ketoaldehyde decalin derivative, produced by a marine sponge-derived Trichoderma harzianum. Tetrahedron Lett. 2014, 55, 662–664. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Goetz, M.A.; Dombrowski, A.W.; Polishook, J.D.; Hazuda, D.J. Equisetin and a novel opposite stereochemical homolog phomasetin, two fungal metabolites as inhibitors of HIV-1 integrase. Tetrahedron Lett. 1998, 39, 2243–2246. [Google Scholar] [CrossRef]
- Alfatafta, A.A.; Gloer, J.B.; Scott, J.A.; Malloch, D. Apiosporamide, a new antifungal agent from the coprophilous fungus Apiospora montagnei. J. Nat. Prod. 1994, 57, 1696–1702. [Google Scholar] [CrossRef]
- Duong, T.-H.; Nguyen, H.-H.; Le, T.-T.; Tran, T.-N.; Sichaem, J.; Nguyen, T.-T.; Nguyen, T.-P.; Mai, D.-T.; Nguyen, H.-H.; Le, H.-D. Subnudatones A and B, new trans-decalin polyketides from the cultured lichen mycobionts of Pseudopyrenula subnudata. Fitoterapia 2020, 142, 104512–104526. [Google Scholar] [CrossRef]
- Fan, B.; Parrot, D.; Blümel, M.; Labes, A.; Tasdemir, D. Influence of OSMAC-based cultivation in metabolome and anticancer activity of fungi associated with the brown alga Fucus vesiculosus. Mar. Drugs 2019, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Dewapriya, P.; Li, F.; Blümel, M.; Tasdemir, D. Pyrenosetins A–C, new decalinoylspirotetramic acid derivatives isolated by bioactivity-based molecular networking from the seaweed-derived fungus Pyrenochaetopsis sp. FVE-001. Mar. Drugs 2020, 18, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, 3rd ed.; Plenum Press: New York, NY, USA, 1990; p. 158. [Google Scholar]
- Moosmann, P.; Ueoka, R.; Grauso, L.; Mangoni, A.; Morinaka, B.I.; Gugger, M.; Piel, J. Cyanobacterial ent-sterol-like natural products from a deviated ubiquinone pathway. Angew. Chem. Int. Ed. Engl. 2017, 56, 4987–4990. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell′Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L. Stereochemical studies on ovatoxin-a. Chem. Eur. J. 2012, 18, 16836–16843. [Google Scholar] [CrossRef] [Green Version]
- Grauso, L.; Teta, R.; Esposito, G.; Menna, M.; Mangoni, A. Computational prediction of chiroptical properties in structure elucidation of natural products. Nat. Prod. Rep. 2019, 36, 1005–1030. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, T.; Kato, N.; Shimizu, T.; Okano, A.; Futamura, Y.; Takahashi, S.; Osada, H. Wakodecalines A and B, new decaline metabolites isolated from a fungus Pyrenochaetopsis sp. RK10-F058. J. Antibiot. 2017, 71, 123–128. [Google Scholar] [CrossRef] [PubMed]
- De Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Redisposition of Phoma-like anamorphs in Pleosporales. Stud. Mycol. 2013, 75, 1–36. [Google Scholar] [CrossRef] [Green Version]
- De Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 2010, 102, 1066–1081. [Google Scholar] [CrossRef]
- Klemke, C.; Kehraus, S.; Wright, A.D.; König, G.M. New secondary metabolites from the marine endophytic fungus Apiospora montagnei. J. Nat. Prod. 2004, 67, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wiese, J.; Labes, A.; Kramer, A.; Schmaljohann, R.; Imhoff, J.F. Lindgomycin, an unusual antibiotic polyketide from a marine fungus of the Lindgomycetaceae. Mar. Drugs 2015, 13, 4617–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Han, X.; Wang, D.; Liu, M.; Gou, J.; Peng, Y.; Liu, J.; Li, Y.; Cao, F.; Zhang, C. Bioactive 3-decalinoyltetramic acids derivatives from a marine-derived strain of the fungus Fusarium equiseti D39. Front. Microbiol. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Leshchenko, E.V.; Berdyshev, D.V.; Sobolevskaya, M.P.; Antonov, A.S.; Denisenko, V.A.; Popov, R.S.; Pivkin, M.V.; Udovenko, A.A.; Pislyagin, E.A.; et al. Zosteropenillines: Polyketides from the marine-derived fungus Penicillium thomii. Mar. Drugs 2017, 15, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Uehara, H.; Matsunami, K.; Aoki, S.; Kitagawa, I. Trichoharzin, a new polyketide produced by the imperfect fungus Trichoderma harzianum separated from the marine sponge Micale cecilia. Tetrahedron Lett. 1993, 34, 7925–7928. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Huang, M.; Liu, L.; Wang, J.; Lin, Y. Six new polyketide decalin compounds from mangrove endophytic fungus Penicillium aurantiogriseum 328#. Mar. Drugs 2015, 13, 6306–6318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; Kikuchi, T.; Tanaka, R. Altercrasin A, a novel decalin derivative with spirotetramic acid, produced by a sea urchin-derived Alternaria sp. Tetrahedron Lett. 2015, 56, 1229–1232. [Google Scholar] [CrossRef]
- Jang, J.-H.; Asami, Y.; Jang, J.-P.; Kim, S.-O.; Moon, D.O.; Shin, K.-S.; Hashizume, D.; Muroi, M.; Saito, T.; Oh, H. Fusarisetin A, an acinar morphogenesis inhibitor from a soil fungus, Fusarium sp. FN080326. J. Am. Chem. Soc. 2011, 133, 6865–6867. [Google Scholar] [CrossRef]
- Pornpakakul, S.; Roengsumran, S.; Deechangvipart, S.; Petsom, A.; Muangsin, N.; Ngamrojnavanich, N.; Sriubolmas, N.; Chaichit, N.; Ohta, T. Diaporthichalasin, a novel CYP3A4 inhibitor from an endophytic Diaporthe sp. Tetrahedron Lett. 2007, 48, 651–655. [Google Scholar] [CrossRef]
- Pitt, J.I.; Miller, J.D. A concise history of mycotoxin research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef]
- Xu, J.; Caro-Diaz, E.J.E.; Lacoske, M.H.; Hung, C.-I.; Jamora, C.; Theodorakis, E.A. Fusarisetin A: Scalable total synthesis and related studies. Chem. Sci. 2012, 3, 3378–3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grauso, L.; Li, Y.; Scarpato, S.; Shulha, O.; Rárová, L.; Strnad, M.; Teta, R.; Mangoni, A.; Zidorn, C. Structure and conformation of zosteraphenols, tetracyclic diarylheptanoids from the seagrass Zostera marina: An NMR and DFT Study. Org. Lett. 2020, 22, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16. Revision C.01.; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Pierens, G.K. 1H and 13C NMR scaling factors for the calculation of chemical shifts in commonly used solvents using density functional theory. J. Comput. Chem. 2014, 35, 1388–1394. [Google Scholar] [CrossRef] [PubMed]


| No. | 1 | ||
|---|---|---|---|
| δH, mult (J in Hz) | δC | ||
| 1 | - | 213.2 | |
| 2 | - | 56.4 | |
| 3 | 2.32, d (10.0) | 54.8 | |
| 4 | - | 133.2 | |
| 5 | 5.25, br s | 128.0 | |
| 6 | 1.84, m | 37.7 | |
| 7 | eq | 1.82, m | 42.9 |
| ax | 0.75, q (11.9) | ||
| 8 | 1.44, m | 33.5 | |
| 9 | eq | 1.71, m | 36.0 |
| ax | 0.83, dq (2.7, 12.6) | ||
| 10 | eq | 1.29, dq (12.9, 3.0) | 25.8 |
| ax | 1.02, m | ||
| 11 | 1.48, ddd (11.8, 10.5, 2.7) | 37.9 | |
| 12 | 0.88, s | 14.2 | |
| 13 | 2.67, dd (10.1, 3.4) | 57.0 | |
| 14 | 4.90, dt (9.3, 3.3) | 83.7 | |
| 15 | a | 2.70, dd (16.2, 3.1) | 50.3 |
| b | 2.52, dd (16.2, 9.4) | ||
| 16 | - | 206.8 | |
| 17 | 2.09, s | 30.8 | |
| 18 | 1.76, br s | 23.2 | |
| 19 | 0.89, d (6.6) | 22.5 | |
| 2′ | - | 170.2 | |
| 3′ | - | 75.3 | |
| 4′ | - | 110.3 | |
| 5′ | 3.44, dd (7.0, 4.1) | 68.3 | |
| 6′ | a | 3.86, dd (11.6, 4.1) | 60.4 |
| b | 3.79, dd (11.6, 6.4) | ||
| 7′ | 2.86, s | 29.5 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.; Dewapriya, P.; Li, F.; Grauso, L.; Blümel, M.; Mangoni, A.; Tasdemir, D. Pyrenosetin D, a New Pentacyclic Decalinoyltetramic Acid Derivative from the Algicolous Fungus Pyrenochaetopsis sp. FVE-087. Mar. Drugs 2020, 18, 281. https://doi.org/10.3390/md18060281
Fan B, Dewapriya P, Li F, Grauso L, Blümel M, Mangoni A, Tasdemir D. Pyrenosetin D, a New Pentacyclic Decalinoyltetramic Acid Derivative from the Algicolous Fungus Pyrenochaetopsis sp. FVE-087. Marine Drugs. 2020; 18(6):281. https://doi.org/10.3390/md18060281
Chicago/Turabian StyleFan, Bicheng, Pradeep Dewapriya, Fengjie Li, Laura Grauso, Martina Blümel, Alfonso Mangoni, and Deniz Tasdemir. 2020. "Pyrenosetin D, a New Pentacyclic Decalinoyltetramic Acid Derivative from the Algicolous Fungus Pyrenochaetopsis sp. FVE-087" Marine Drugs 18, no. 6: 281. https://doi.org/10.3390/md18060281
APA StyleFan, B., Dewapriya, P., Li, F., Grauso, L., Blümel, M., Mangoni, A., & Tasdemir, D. (2020). Pyrenosetin D, a New Pentacyclic Decalinoyltetramic Acid Derivative from the Algicolous Fungus Pyrenochaetopsis sp. FVE-087. Marine Drugs, 18(6), 281. https://doi.org/10.3390/md18060281