Extraction, Enrichment, and LC-MSn-Based Characterization of Phlorotannins and Related Phenolics from the Brown Seaweed, Ascophyllum nodosum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fractionation of Phenolic Material Using Solid Phase Extraction (SPE) and Sephadex LH-20
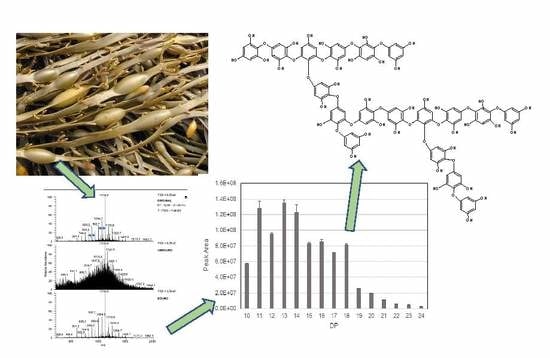
2.2. Liquid Chromatography Mass Spectrometric (LC-MSn) Analysis
2.3. Differences between Positve and Negative Mode MS Data
2.4. Re-Examination of Data Using the LTQ-Orbitrap XL FT-MS System
2.5. Structural Information from MS2 Fragmentation Data
2.6. Structural Information from MS3 Fragmentation Data
3. Conclusions
4. Materials and Methods
4.1. Materials and General Methods
4.2. Solid Phase Extraction
4.3. Fractionation on Sephadex LH-20
4.4. Liquid Chromatography-Mass Spectrometry (LC-MSn) Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragan, M.A.; Glombitza, K.W. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 129–241. [Google Scholar]
- Martínez, J.H.; Castaneda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromat. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Jormalainen, V.; Honkanen, T.; Koivikko, R.; Eränen, J. Induction of phlorotannin production in a brown alga: Defense or resource dynamics? Oikos 2003, 103, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res. 2006, 43, 1–91. [Google Scholar]
- Svensson, C.J.; Pavia, H.; Toth, G.B. Do plant density, nutrient availability, and herbivore grazing interact to affect phlorotannin plasticity in the brown seaweed Ascophyllum nodosum. Marine Biol. 2007, 151, 2177–2181. [Google Scholar] [CrossRef]
- Creis, E.; Delage, L.; Charton, S.; Goulitquer, S.; Leblanc, C.; Potin, P.; Ar Gall, E. Constitutive or inducible protective mechanisms against UV-B radiation in the brown alga Fucus vesiculosus? A study of gene expression and phlorotannin content responses. PLoS ONE 2015, 10, e0128003. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, P.D. Seasonal variation in the relationship between growth rate and phlorotannin. production in the kelp Ecklonia radiata. Oecologia 1995, 102, 169–173. [Google Scholar] [CrossRef]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.B. Influence of light and nitrogen on the phlorotannin content of the brown seaweeds Ascophyllum nodosum and Fucus vesiculosus. Hydrobiology 2000, 440, 299–307. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Pete, R.; Küpper, F.C.; Glombitza, K.-W.; König, G.M. Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur. J. Phycol. 2009, 44, 331–338. [Google Scholar] [CrossRef]
- Arnold, T.M.; Targett, N.M. To grow and defend: Lack of tradeoffs for brown algal phlorotannins. Oikos 2003, 100, 406–408. [Google Scholar] [CrossRef]
- Nakamura, T.; Nagayama, K.; Uchida, K.; Tanaka, R. Antioxidant activity of phlorotannins isolated from the brown alga Eisenia bicyclis. Fisheries Sci. 1996, 62, 923–926. [Google Scholar] [CrossRef] [Green Version]
- Farvin, S.K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef]
- Kirke, D.A.; Smyth, T.J.; Rai, D.K.; Kenny, O.; Stengel, D.B. The chemical and antioxidant stability of isolated low molecular weight phlorotannins. Food Chem. 2017, 221, 1104–1112. [Google Scholar] [CrossRef]
- Yang, Y.I.; Woo, J.H.; Seo, Y.J.; Lee, K.T.; Lim, Y.; Choi, J.H. Protective effect of brown alga phlorotannins against hyper-inflammatory responses in lipopolysaccharide-induced sepsis models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef]
- Lopes, G.; Andrade, P.B.; Valentão, P. Phlorotannins: Towards new pharmacological interventions for Diabetes Mellitus Type 2. Molecules 2016, 22, 56. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A.M. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [Green Version]
- Eom, S.-H.; Kim, Y.-M.; Kim, S.-K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.K. Potential anti-HIV agents from marine resources: An overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [Green Version]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Pantidos, N.; Boath, A.; Lund, V.; Conner, S.; McDougall, G.J. Phenolic-rich extracts from the edible seaweed, Ascophyllum nodosum, inhibit α-amylase and α-glucosidase: Potential anti-hyperglycemic effects. J. Funct. Foods 2014, 10, 201–209. [Google Scholar] [CrossRef]
- Austin, C.; Stewart, D.; Allwood, J.W.; McDougall, G.J. Extracts from the edible seaweed, Ascophyllum nodosum, inhibit lipase activity in vitro: Contributions of phenolic and polysaccharide components. Food Funct. 2018, 24, 502–510. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of polyphenols and vitamin c in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drugs 2019, 17, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Tierney, M.S.; Soler-Vila, A.; Rai, D.K.; Croft, A.K.; Brunton, N.P.; Smyth, T.J. UPLC-MS profiling of low molecular weight phlorotannin polymers in Ascophyllum nodosum, Pelvetia canaliculata and Fucus spiralis. Metabolomics 2014, 10, 524–535. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurized liquid extraction and solid–liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Han, J.-S.; Kim, H.-J.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef]
- Steevensz, A.J.; Mackinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry. Phytochem. Anal. 2012, 23, 547–553. [Google Scholar] [CrossRef]
- Vissers, A.M.; Caligiani, A.; Sforza, S.; Vincken, J.-P.; Gruppen, H. Phlorotannin composition of Laminaria digitata. Phytochem. Anal. 2017, 28, 487–495. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, A.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling phlorotannins from Fucus spp. of the Northern Portuguese coastline: Chemical approach by HPLC-DAD-ESI/MS and UPLC-ESI-QTOF/MSn. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [Green Version]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Meslet-Cladière, L.; Delage, L.; Leroux, C.J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell 2013, 25, 3089–3103. [Google Scholar] [CrossRef] [Green Version]
- Kasper, P.T.; Rojas-Chertó, M.; Mistrik, R.; Reijmers, T.; Hankemeier, T.; Vreeken, R.J. Fragmentation trees for the structural characterization of metabolites. Rapid Comms. Mass Spectr. 2012, 26, 2275–2286. [Google Scholar] [CrossRef] [Green Version]








| RT | m/z [M − H]− | MS2 | Exact Mass Formulae as [M − H]− | Putative Identity |
|---|---|---|---|---|
| 1.78 | 191.0195 | 173, 111 * | C6H7O7 | Citric acid |
| 3.37 | 276.0184 | 259, 231, 215, 196, 179, 150, 135 | C9H10O7NS | Dihydroxyphenylalanine (DOPA)-sulphate (PC 178810) |
| 5.73 | 246.9914 | 203, 121 | C12H7O6 | Dibenzodioxin-1,3,6,8-tetraol (PC 14309078) |
| 7.45 | 277.0924 | 185, 167, 141, 97 | C11H17O8 | Unknown |
| 9.08 | 318.0284 | 300, 276, 238, 192 | C11H12NSO8 | Unknown |
| 8.93 | 216.9809 | 173, 137, 97 | C7H5O6S | Hydroxybenzoic acid sulphate |
| 9.89 | 230.9967 | 187, 151 | C8H7O6S | Phenolic acid sulphate |
| 12.96 | 591.0075 | 511, 385 | C24H15O16S | Diphlorethohydroxycarmalol sulphate |
| 14.48 | 511.0506 | 385 | C24H15O13 | Diphlorethohydroxycarmalol (PC 16075395) |
| Phlorotannin | MS Properties | Phlorotannin | MS Properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m/z [M − 2H]2− | Calc m/z | DP | MS2 | NL | ΔM − 2H2− | NeutralLoss | m/z [M − 2H]2− | Calc. m/z | DP | MS2 | NL | ΔM − 2H2− | Neutral Loss |
| 621 | 1241 | 10 | 1097 | 144 | PG+H2O | 683 | 1365 | 11 | 1117 | 248 | 2PG * | ||
| 993 | 248 | 2PG* | 975 | 390 | 2PG-THB | ||||||||
| 831 | 410 | UK | 851 | 514 | 3PG-THB | ||||||||
| 603 | 638 | 18 | 4PG-THB | 664 | 701 | 18 | ? | ||||||
| 495 | 746 | 125 | 6PG | 610 | 755 | 72 | ? | ||||||
| 477 | 764 | 144 | 6PG+H2O | 477 | 888 | 205 | 7PG+H2O | ||||||
| 247 | 994 | 373 | 8PG | 371 | 994 | 311 | 8PG | ||||||
| 229 | 1012 | 391 | 8PG+H2O | 355 | 1010 | 327 | 7PG-THB | ||||||
| 229 | 1136 | 453 | 9PG+H2O | ||||||||||
| 745 | 1489 | 12 | 1345 | 144 | PG+H2O | ||||||||
| 1223 | 266 | PG-THB | 807 | 1613 | 13 | 1469 | 144 | PG+H2O | |||||
| 975 | 514 | 3PG-THB | 1223 | 390 | 2PG-THB | ||||||||
| 727 | 762 | 18 | 5PG-THB | 955 | 658 | ? | |||||||
| 672 | 817 | 72 | ? | 788 | 825 | 18 | ? | ||||||
| 477 | 1012 | 267 | 8PG+H2O | 725 | 888 | 81 | 7PG+H2O | ||||||
| 229 | 1260 | 515 | 10PG+H2O | 477 | 1136 | 329 | 9PG+H2O | ||||||
| 353 | 1260 | 453 | 10PG+H2O | ||||||||||
| 869 | 1737 | 14 | 1471 | 266 | PG-THB | ||||||||
| 1223 | 514 | 3PG-THB | 931 | 1861 | 15 | 1700 | 161 | ? | |||||
| 975 | 762 | 5PG-THB | 1595 | 266 | PG-THB | ||||||||
| 851 | 887 | 18 | 7PG+H2O * | 1347 | 514 | 3PG-THB | |||||||
| 787 | 950 | 81 | ? | 1099 | 762 | 5PG-THB | |||||||
| 477 | 1260 | 391 | 10PG+H2O | 912 | 949 | 18 | ? | ||||||
| 353 | 1384 | 515 | 11PG+H2O | 849 | 1012 | 81 | 8PG+H2O | ||||||
| 477 | 1384 | 453 | 11PG+H2O | ||||||||||
| 993 | 1985 | 16 | 1595 | 390 | 2PG-THB | 355 | 1506 | 575 | 11PG-THB | ||||
| 1223 | 762 | 5PG-THB | |||||||||||
| 974 | 1011 | 18 | 8PG+H2O * | 1055 | 2109 | 17 | 1929 | 180 | ? | ||||
| 911 | 1074 | 81 | ? | 1719 | 390 | 2PG-THB | |||||||
| 848 | 1137 | 144 | 9PG+H2O | 1469 | 640 | 5PG+H2O | |||||||
| 477 | 1508 | 515 | 12PG+H2O | 1223 | 886 | 6PG-THB | |||||||
| 353 | 1632 | 639 | 13PG+H2O | 1036 | 1073 | 18 | ? | ||||||
| 973 | 1136 | 81 | 9PG+H2O | ||||||||||
| 1117 | 2233 | 18 | 1985 | 248 | 2PG * | 831 | 1278 | 223 | 10PG+ 2H2O | ||||
| 1719 | 514 | 3PG-THB | 477 | 1632 | 577 | 13PG+H2O | |||||||
| 1451 | 782 | ? | 353 | 1756 | 701 | 14PG+H2O | |||||||
| 1223 | 1010 | 7PG-THB | |||||||||||
| 1098 | 1135 | 18 | 9PG+H2O* | 1179 | 2357 | 19 | 1825 | 532 | ? | ||||
| 1089 | 1144 | 28 | ? | 1595 | 762 | 6PG+H2O | |||||||
| 1035 | 1198 | 81 | ? | 1223 | 1134 | 8PG-THB | |||||||
| 709 | 1524 | 407 | ? | 1160 | 1197 | ? | |||||||
| 477 | 1756 | 639 | 14PG+H2O | 1151 | 1206 | 27 | ? | ||||||
| 337 | 1896 | 779 | ? | 973 | 1384 | 205 | 11PG+H2O | ||||||
| 601 | 1756 | 577 | 14PG+H2O | ||||||||||
| 1241 | 2481 | 20 | 1967 | 514 | 3PG-THB | 479 | 1878 | 699 | 14PG-THB | ||||
| 1719 | 762 | 5PG-THB | 355 | 2002 | 823 | 15PG-THB | |||||||
| 1489 | 992 | 8PG | |||||||||||
| 1222 | 1259 | 18 | 10PG+H2O* | 1303 | 2605 | 21 | 1805 | 800 | ? | ||||
| 1159 | 1322 | 81 | ? | 1595 | 1010 | 7PG-THB | |||||||
| 955 | 1526 | 285 | ? | 1284 | 1321 | 18 | ? | ||||||
| 727 | 1754 | 513 | 13PG-THB | 1275 | 1330 | 27 | ? | ||||||
| 477 | 2004 | 763 | 16PG+H2O | 1221 | 1384 | 81 | 11PG+H2O | ||||||
| 355 | 2126 | 885 | 16PG-THB | 955 | 1650 | 347 | ? | ||||||
| 831 | 1774 | 471 | 14PG+2 H2O | ||||||||||
| 1365 | 2729 | 22 | 1967 | 762 | 5PG-THB | 479 | 2126 | 823 | 16PG-THB | ||||
| 1719 | 1010 | 7PG-THB | 365 | 2240 | 937 | ? | |||||||
| 1451 | 1278 | ? | |||||||||||
| 1337 | 1392 | 27 | ? | ||||||||||
| 1283 | 1446 | 81 | ? | 1427 | 2853 | 23 | None | ||||||
| 955 | 1774 | 409 | ? | ||||||||||
| 727 | 2002 | 637 | 15PG-THB | ||||||||||
| 477 | 2252 | 887 | 18PG+H2O | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allwood, J.W.; Evans, H.; Austin, C.; McDougall, G.J. Extraction, Enrichment, and LC-MSn-Based Characterization of Phlorotannins and Related Phenolics from the Brown Seaweed, Ascophyllum nodosum. Mar. Drugs 2020, 18, 448. https://doi.org/10.3390/md18090448
Allwood JW, Evans H, Austin C, McDougall GJ. Extraction, Enrichment, and LC-MSn-Based Characterization of Phlorotannins and Related Phenolics from the Brown Seaweed, Ascophyllum nodosum. Marine Drugs. 2020; 18(9):448. https://doi.org/10.3390/md18090448
Chicago/Turabian StyleAllwood, J. William, Huw Evans, Ceri Austin, and Gordon J. McDougall. 2020. "Extraction, Enrichment, and LC-MSn-Based Characterization of Phlorotannins and Related Phenolics from the Brown Seaweed, Ascophyllum nodosum" Marine Drugs 18, no. 9: 448. https://doi.org/10.3390/md18090448
APA StyleAllwood, J. W., Evans, H., Austin, C., & McDougall, G. J. (2020). Extraction, Enrichment, and LC-MSn-Based Characterization of Phlorotannins and Related Phenolics from the Brown Seaweed, Ascophyllum nodosum. Marine Drugs, 18(9), 448. https://doi.org/10.3390/md18090448