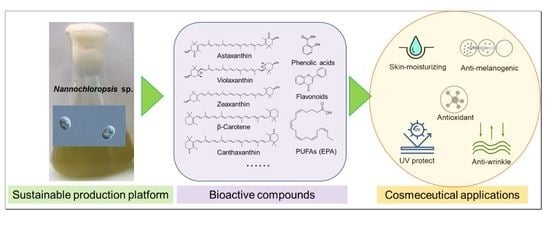
Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications
Abstract
:1. Introduction
2. Results
2.1. Isolation of Nannochloropsis sp. G1-5 and Analysis of Biochemical Composition
2.2. In Vitro Cytotoxicity of NG15 Extract
2.3. Anti-Melanogenic Activity of NG15 Extract
2.4. Antioxidant, Anti-Inflammatory, and UV-Protection Activities of NG15 Extract
2.5. Skin Moisturizing and Anti-Wrinkle Activities of NG15 Extract
3. Discussion
4. Materials and Methods
4.1. Isolation, Identification, and Cultivation of Microalgae
4.2. Preparation of the NG15 Extract
4.3. Analysis of Carotenoids and Fatty Acid Methyl Esters
4.4. Determination of Total Phenolic and Total Flavonoid Content
4.5. Cell Culture
4.6. Cell Viability Assay
4.7. Determination of Antioxidant Activity
4.8. Determination of Tyrosinase and Elastase Inhibition Activity
4.9. Determination of Melanin Content
4.10. Determination of Anti-Inflammatory Activity
4.11. Determination of Cell Viability after UV Radiation
4.12. Quantitative Real-Time PCR
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dabrowska, M.; Mielcarek, A.; Nowak, I. Evaluation of sex-related changes in skin topography and structure using innovative skin testing equipment. Skin Res. Technol. 2018, 24, 614–620. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and function of the skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 1, pp. 1–14. [Google Scholar]
- Lorz, L.R.; Yoo, B.C.; Kim, M.Y.; Cho, J.Y. Anti-Wrinkling and anti-melanogenic effect of Pradosia mutisii methanol extract. Int. J. Mol. Sci. 2019, 20, 1043. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an attractive source for cosmetics to counter environmental stress. Sci. Total. Environ. 2021, 772, 144905. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta 2013, 1832, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora Huertas, A.C.; Schmelzer, C.E.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016, 128-129, 163–173. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Schikowski, T.; Huls, A. Air pollution and skin aging. Curr. Environ. Health Rep. 2020, 7, 58–64. [Google Scholar] [CrossRef]
- Chung, J.H.; Hanft, V.N.; Kang, S. Aging and photoaging. J. Am. Acad. Dermatol. 2003, 49, 690–697. [Google Scholar] [CrossRef]
- Uitto, J. Understanding premature skin aging. N. Engl. J. Med. 1997, 337, 1463–1465. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Chung, J.H.; Kang, S.; Varani, J.; Lin, J.; Fisher, G.J.; Voorhees, J.J. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J. Investig. Dermatol. 2000, 115, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Longas, M.O.; Russell, C.S.; He, X.Y. Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Carbohydr. Res. 1987, 159, 127–136. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef] [Green Version]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003, 16, 629–638. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. New Insights on Unique features and role of nanostructured materials in cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G. Enigmatic microalgae from aeroterrestrial and extreme habitats in cosmetics: The potential of the untapped natural sources. Cosmetics 2020, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.A.; Dario, M.F. Bioactive Cosmetics. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar]
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; de Carvalho, J.C.M.; Santos de Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio)Technological aspects of microalgae pigments for cosmetics. Appl. Microbiol. Biotechnol. 2020, 104, 9513–9522. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Jahan, A.; Ahmad, I.Z.; Fatima, N.; Ansari, V.A.; Akhtar, J. Algal bioactive compounds in the cosmeceutical industry: A review. Phycologia 2017, 56, 410–422. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Whitelam, G.C.; Codd, G.A. Damaging effects of light on microorganisms. In Microbes in Extreme Environments; Herbert, R.A., Codd, G.A., Eds.; Academic Press: London, UK, 1986; pp. 129–169. [Google Scholar]
- Spijkerman, E.; Wacker, A.; Weithoff, G.; Leya, T. Elemental and fatty acid composition of snow algae in arctic habitats. Front. Microbiol. 2012, 3, 380. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and therapeutic applications of fish oil’s fatty acids on the skin. Mar. Drugs. 2018, 16, 256. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Antiinflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat. Commun. 2012, 3, 686. [Google Scholar] [CrossRef] [Green Version]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; González-del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Zanella, L.; Vianello, F. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Letsiou, S.; Kalliampakou, K.; Gardikis, K.; Mantecon, L.; Infante, C.; Chatzikonstantinou, M.; Labrou, N.E.; Flemetakis, E. Skin protective effects of Nannochloropsis gaditana extract on H2O2-stressed human dermal fibroblasts. Front. Mar. Sci. 2017, 4, 221. [Google Scholar] [CrossRef] [Green Version]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; Oulad El Majdoub, Y.; Kounnoun, A.; Miceli, N.; Fernanda Taviano, M.; Mondello, L.; et al. The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical applications of twenty-five species of rapid-growing green-microalgae as indicated by their antibacterial, antioxidant and mineral content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Abinandan, S.; Perera, I.A.; Subashchandrabose, S.R.; Venkateswarlu, K.; Cole, N.; Megharaj, M. Acid-adapted microalgae exhibit phenotypic changes for their survival in acid mine drainage samples. FEMS Microbiol. Ecol. 2020, 96, fiaa113. [Google Scholar] [CrossRef]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Kanto, H.; Kavanagh, R.; Abdel-Malek, Z. Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival. Ann. N. Y. Acad. Sci. 2003, 994, 359–365. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef]
- Ismail, N.S.; Pravda, E.A.; Li, D.; Shih, S.C.; Dallabrida, S.M. Angiopoietin-1 reduces H2O2-induced increases in reactive oxygen species and oxidative damage to skin cells. J. Investig. Dermatol. 2010, 130, 1307–1317. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.; Visscher, M.; Laruffa, A.; Wickett, R. Natural moisturizing factors (NMF) in the stratum corneum (SC). II. Regeneration of NMF over time after soaking. J. Cosmet. Sci. 2010, 61, 23–29. [Google Scholar]
- Jokela, T.A.; Karna, R.; Makkonen, K.M.; Laitinen, J.T.; Tammi, R.H.; Tammi, M.I. Extracellular UDP-glucose activates P2Y14 receptor and induces signal transducer and activator of transcription 3 (STAT3) TYP705 phosphorylation and binding to hyaluronan synthase 2 (HAS2) promoter, stimulating hyaluronan synthesis of keratinocytes. J. Biol. Chem. 2014, 289, 18569–18581. [Google Scholar] [CrossRef] [Green Version]
- Lavker, R.; Kaidbey, K. The spectral dependence for UVA-induced cumulative damage in human skin. J. Investig. Dermatol. 1997, 108, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Walter, L.S., Matoira, H.C., Eds.; Springer: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Newman, S.M.; Boynton, J.E.; Gillham, N.W.; Randolph-Anderson, B.L.; Johnson, A.M.; Harris, E.H. Transformation of chloroplast ribosomal RNA genes in Chlamydomonas: Molecular and genetic characterization of integration events. Genetics 1990, 126, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Sanz, N.; García-Blanco, A.; Gavalás-Olea, A.; Loures, P.; Garrido, J.L. Phytoplankton pigment biomarkers: HPLC separation using a pentafluorophenyloctadecyl silica column. Methods Ecol. Evol. 2015, 6, 1199–1209. [Google Scholar] [CrossRef] [Green Version]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids; Birkhäuser: Basel, Switzerland, 2004; pp. 1–647. [Google Scholar]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.S.; Petry, F.C.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. HPLC-PDA-MS/MS as a strategy to characterize and quantify natural pigments from microalgae. Curr. Res. Food Sci. 2020, 8, 100–112. [Google Scholar] [CrossRef]
- Egeland, E.S.; Garrido, J.L.; Clementson, L.; Andersen, K.; Thomas, C.T.; Zapata, M.; Airs, R.; Llewellyn, C.; Newman, G.L.; Rodríguez, F.; et al. Data sheets aiding identification of phytoplankton carotenoids and chlorophylls. In Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Roy, S., Llewellyn, C., Egeland, E.S., Johnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 665–822. [Google Scholar]
- Wang, W.; Li, J.; Zhang, H.; Wang, X.; Fan, J.; Zhang, X. Phenolic compounds and bioactivity evaluation of aqueous and methanol extracts of Allium mongolicum Regel. Food Sci. Nutr. 2019, 7, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Blois, M.L. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]




| FAME Component | Content (mg/g Extract) |
|---|---|
| Myristic acid (C14:0) | 22.93 ± 0.21 |
| Palmitic acid (C16:0) | 215.85 ± 2.80 |
| Palmitoleic acid (C16:1 ω7) | 188.95 ± 2.56 |
| Stearic acid (C18:0) | 7.56 ± 0.02 |
| Oleic acid (C18:1 ω9) | 91.40 ± 1.22 |
| Linoleic acid (C18:2 ω6) | 4.63 ± 0.06 |
| γ-Linolenic acid (C18:3 ω6) | 2.21 ± 0.03 |
| Eicosatrienoic acid (C20:3 ω6) | 1.10 ± 0.02 |
| Arachidonic acid (C20:4 ω6) | 16.02 ± 0.25 |
| Eicosapentaenoic acid (C20:5 ω3) | 31.53 ± 0.58 |
| Sum | 582.19 ± 7.70 |
| Carotenoid Component | Content (mg/g Extract) |
|---|---|
| Vaucheriaxanthin | 0.82 ± 0.02 |
| Violaxanthin | 1.81 ± 0.04 |
| Astaxanthin | 0.78 ± 0.02 |
| Zeaxanthin | 0.13 ± 0.00 |
| Canthaxanthin | 1.93 ± 0.04 |
| Chlorophyll a | 5.39 ± 0.11 |
| β-Carotene | 5.28 ± 0.21 |
| Sum | 16.13 ± 0.44 |
| Total Phenolic Content (mg GAE/g Extract) | Total Flavonoids Content (mg QE/g Extract) |
|---|---|
| 77.29 ± 1.25 | 20.15 ± 0.28 |
| Gene | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| 18S rDNA | CCTGGTTGATCCTGCCAGTA | ACCTTGTTACGACTTCTCCTTC |
| COL1A1 | AGGGCCAAGACGAAGACATC | AGATCACGTCATCGCACAACA |
| HAS-2 | GAAAGGGCCTGTCAGTCTTATTT | TTCGTGAGATGCCTGTCATCACC |
| MMP-1 | TCTGACGTTGATCCCAGAGAGCAG | CAGGGTGACACCAGTGACTGCAC |
| β-actin | GGATTCCTATGTGGGCGACGA | CGCTCGGTGAGGATCTTCATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Kwon, Y.M.; Kim, K.W.; Kim, J.Y.H. Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications. Mar. Drugs 2021, 19, 690. https://doi.org/10.3390/md19120690
Kim SY, Kwon YM, Kim KW, Kim JYH. Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications. Marine Drugs. 2021; 19(12):690. https://doi.org/10.3390/md19120690
Chicago/Turabian StyleKim, Sun Young, Yong Min Kwon, Kyung Woo Kim, and Jaoon Young Hwan Kim. 2021. "Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications" Marine Drugs 19, no. 12: 690. https://doi.org/10.3390/md19120690
APA StyleKim, S. Y., Kwon, Y. M., Kim, K. W., & Kim, J. Y. H. (2021). Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications. Marine Drugs, 19(12), 690. https://doi.org/10.3390/md19120690