Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering
Abstract
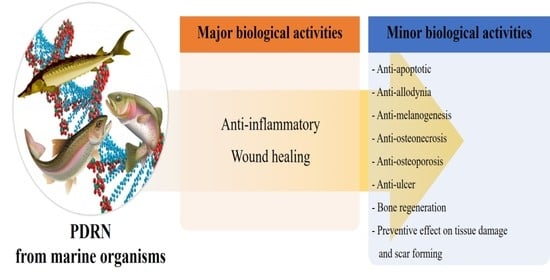
:1. Introduction
2. Results
2.1. PDRN-Related Publication Outputs by Year
2.2. Source of PDRN Extraction
2.3. Therapeutic Effects of PDRN
2.3.1. Characterization of PDRN Bioactivities Based on In Vitro Studies
2.3.2. Characterization of PDRN Based on In Vivo Studies
2.3.3. Characterization of PDRN Based on Clinical Studies
3. Methods
3.1. Search Strategy
3.2. Exclusion Criteria
3.3. Inclusion Criteria
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pallio, G.; Bitto, A.; Pizzino, G.; Galfo, F.; Irrera, N.; Squadrito, F.; Squadrito, G.; Pallio, S.; Anastasi, G.P.; Cutroneo, G.; et al. Adenosine receptor stimulation by polydeoxyribonucleotide improves tissue repair and symptomology in experimental colitis. Front. Pharmacol. 2016, 7, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallio, G.; Bitto, A.; Ieni, A.; Irrera, N.; Mannino, F.; Pallio, S.; Altavilla, D.; Squadrito, F.; Scarpignato, C.; Minutoli, L. Combined treatment with polynucleotides and hyaluronic acid improves tissue repair in experimental colitis. Biomedicines 2020, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological activity and clinical use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Kim, C.-J.; Han, J.H.; Lee, S.; Kim, H.I.; Shin, H.P.; Jeon, J.W. Polydeoxyribonucleotide exerts protective effect against CCl4-Induced acute liver injury through inactivation of NF-κB/MAPK signaling pathway in mice. Int. J. Mol. Sci. 2020, 21, 7894. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.R.; Park, G.-Y.; Lee, S.C. Treatment of full-thickness rotator cuff tendon tear using umbilical cord blood-derived mesenchymal stem cells and polydeoxyribonucleotides in a rabbit model. Stem Cells Int. 2018, 2018, 7146384. [Google Scholar] [CrossRef]
- An, J.; Park, S.H.; Ko, I.-G.; Jin, J.-J.; Hwang, L.; Ji, E.-S.; Kim, S.-H.; Kim, C.-J.; Park, S.Y.; Hwang, J.-J.; et al. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced lung injury by inhibiting apoptotic cell death in rats. Int. J. Mol. Sci. 2017, 18, 1847. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-W.; Hyun, H.; Lee, S.; Kim, S.Y.; Kim, G.-T.; Um, S.; Hong, S.O.; Chun, H.J.; Yang, D.H. The effect of polydeoxyribonucleotide extracted from salmon sperm on the restoration of bisphosphonate-related osteonecrosis of the jaw. Mar. Drugs 2019, 17, 51. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.Y.; Park, J.-U.; Choi, M.-H.; Kim, S.; Kim, H.-E.; Jeong, S.-H. Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci. Rep. 2020, 10, 16811. [Google Scholar] [CrossRef]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar prevention and enhanced wound healing induced by polydeoxyribonucleotide in a rat incisional wound-healing model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef]
- Atef, M.; Ojagh, S.M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Ketnawa, S.; Suwal, S.; Huang, J.-Y.; Liceaga, A.M. Selective separation and characterization of dual ACE and DPP-IV inhibitory peptides from rainbow trout (Oncorhynchus mykiss) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 1062–1073. [Google Scholar] [CrossRef]
- Noman, A.; Xu, Y.; AL-Bukhaiti, W.Q.; Abed, S.M.; Ali, A.H.; Ramadhan, A.H.; Xia, W. Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochem. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- Ko, I.-G.; Kim, S.-E.; Jin, J.-J.; Hwang, L.; Ji, E.-S.; Kim, C.-J.; Han, J.-H.; Hong, I.T.; Kwak, M.S.; Yoon, J.Y.; et al. Combination therapy with polydeoxyribonucleotide and proton pump inhibitor enhances therapeutic effectiveness for gastric ulcer in rats. Life Sci. 2018, 203, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Huh, C.-K.; Lee, J.-H.; Kim, K.-W.; Kim, M.-Y. Histologic study of bone-forming capacity on polydeoxyribonucleotide combined with demineralized dentin matrix. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mun, J.-U.; Cho, H.R.; Bae, S.M.; Park, S.K.; Choi, S.I.; Seo, M.S.; Lim, Y.S.; Rn, S.H.W.; Kim, Y.U. Effect of polydeoxyribonucleotide injection on pes anserine bursitis. Medicine 2017, 96, e8330. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.; Ko, I.-G.; Jin, J.-J.; Kim, S.-H.; Kim, C.-J.; Hwang, J.-J.; Choi, C.W.; Chang, B.S. Attenuation effect of polydeoxyribonucleotide on inflammatory cytokines and apoptotic factors induced by particulate matter (PM10) damage in human bronchial cells. J. Biochem. Mol. Toxicol. 2020, 35, e22635. [Google Scholar] [CrossRef]
- Baek, A.; Kim, M.G.; Kim, S.H.; Cho, S.-R.; Kim, H.J. Anti-inflammatory effect of DNA polymeric molecules in a cell model of osteoarthritis. Inflammation 2018, 41, 677–688. [Google Scholar] [CrossRef]
- Han, J.-H.; Jung, J.; Hwang, L.; Ko, I.-G.; Nam, O.H.; Kim, M.S.; Lee, J.W.; Choi, B.-J.; Lee, D.-W. Anti-inflammatory effect of polydeoxyribonucleotide on zoledronic acid-pretreated and lipopolysaccharide-stimulated RAW 264.7 cells. Exp. Ther. Med. 2018, 16, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Castellini, C.; Belletti, S.; Govoni, P.; Guizzardi, S. Anti inflammatory property of PDRN-an in vitro study on cultured macrophages. Adv. Biosci. Biotechnol. 2017, 8, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Noh, T.K.; Chung, B.Y.; Kim, S.Y.; Lee, M.H.; Kim, M.J.; Youn, C.S.; Lee, M.W.; Chang, S.E. Novel anti-melanogenesis properties of polydeoxyribonucleotide, a popular wound healing booster. Int. J. Mol. Sci. 2016, 17, 1448. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Shrestha, S.M.; Pradhan, A.; Aryal, S. Platelet rich fibrin and bone graft in the treatment of intrabony defect in periodontitis patients. J. Nepal. Soc. Periodontol. Oral Implantol. 2018, 2, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Baek, A.; Kim, Y.; Lee, J.W.; Lee, S.C.; Cho, S.-R. Effect of polydeoxyribonucleotide on angiogenesis and wound healing in an in vitro model of osteoarthritis. Cell Transplant. 2018, 27, 1623–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Kim, M.-J.; Kweon, D.-K.; Lim, S.-T.; Lee, S.-J. Polydeoxyribonucleotide activates mitochondrial biogenesis but reduces MMP-1 activity and melanin biosynthesis in cultured skin cells. Appl. Biochem. Biotechnol. 2019, 191, 540–554. [Google Scholar] [CrossRef]
- Hwang, J.-J.; Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Jeon, J.W.; Paik, S.S.; Chang, B.S.; Choi, C.W. Combination therapy with polydeoxyribonucleotide and pirfenidone alleviates symptoms of acute respiratory distress syndrome in human lung epithelial A549 cells. Int. Neurourol. J. 2020, 24 (Suppl. 1), S56–S64. [Google Scholar] [CrossRef]
- Jeong, H.; Chung, J.-Y.; Ko, I.-G.; Kim, S.-H.; Jin, J.-J.; Hwang, L.; Moon, E.J.; Lee, B.J.; Yi, J.W. Effect of polydeoxyribonucleotide on lipopolysaccharide and sevoflurane-induced postoperative cognitive dysfunction in human neuronal SH-SY5Y cells. Int. Neurourol. J. 2019, 23 (Suppl. 2), S93–S101. [Google Scholar] [CrossRef]
- Koo, Y.; Yun, Y. Effects of polydeoxyribonucleotides (PDRN) on wound healing: Electric cell-substrate impedance sensing (ECIS). Mat. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 554–560. [Google Scholar] [CrossRef]
- Hwang, K.-H.; Kim, J.-H.; Park, E.Y.; Cha, S.-K. An effective range of polydeoxyribonucleotides is critical for wound healing quality. Mol. Med. Rep. 2018, 18, 5166–5172. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.-R.; Han, S.W.; Kim, J.H.; Lee, B.C.; Kim, J.M.; Hong, J.Y.; Kim, B.J. Polydeoxyribonucleotides improve diabetic wound healing in mouse animal model for experimental validation. Ann. Dermatol. 2019, 31, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, C.-J.; Kim, S.-H.; Han, J.-H.; Jeon, J.W. Polydeoxyribonucleotide exerts therapeutic effect by increasing VEGF and inhibiting inflammatory cytokines in ischemic colitis rats. BioMed Res. Int. 2020, 2020, 2169083. [Google Scholar] [CrossRef] [PubMed]
- Lolignier, S.; Eijkelkamp, N.; Wood, J.N. Mechanical allodynia. Pflüg. Arch. 2015, 467, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Huo, B.-B.; Zheng, M.-X.; Hua, X.-Y.; Shen, H.; Lu, Y.-C.; Jiang, D.-L.; Shan, C.-L.; Xu, J.-G. Evaluation of neuropathic pain in a rat model of total brachial plexus avulsion from behavior to brain metabolism. Pain Physician 2019, 22, E215–E224. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, R.; Tilley, D.M.; Vogel, L.; Benyamin, R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010, 10, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yoo, S.H.; Lee, H.-J.; Han, D.; Lee, J.; Jeon, S.H.; Cho, E.-A.; Park, H.J. Anti-allodynic effects of polydeoxyribonucleotide in an animal model of neuropathic pain and complex regional pain syndrome. J. Korean Med. Sci. 2020, 35, e225. [Google Scholar] [CrossRef] [PubMed]
- Karahan, N.; Arslan, I.; Oztermeli, A.; Orak, M.M.; Midi, A.; Alp Yücel, I.I. Comparison of intra-articular application of polydeoxyribonucleic acid and hyaluronic acid in experimentally induced osteoarthritis in rats. J. Musculoskelet. Res. 2020, 23, 2050009. [Google Scholar] [CrossRef]
- Buffoli, B.; Favero, G.; Borsani, E.; Boninsegna, R.; Sancassani, G.; Labanca, M.; Rezzani, R.; Nocini, P.F.; Albaness, M.; Rodella, L.F. Sodium-DNA for bone tissue regeneration: An experimental study in rat calvaria. BioMed Res. Int. 2017, 2017, 7320953. [Google Scholar] [CrossRef] [Green Version]
- Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Kim, C.-J.; Han, J.H.; Kwak, M.S.; Yoon, J.Y.; Jeon, J.W. Evaluating the mucoprotective effect of polydeoxyribonucleotide against indomethacin-induced gastropathy via the MAPK/NF-κB signaling pathway in rats. European. J. Pharmacol. 2020, 874, 172952. [Google Scholar] [CrossRef]
- Marini, H.R.; Puzzolo, D.; Micali, A.; Adamo, E.B.; Irrera, N.; Pisani, A.; Pallio, G.; Trichilo, V.; Malta, C.; Bitto, A.; et al. Neuroprotective effects of polydeoxyribonucleotide in a murine model of cadmium toxicity. Oxid. Med. Cell Longev. 2018, 2018, 4285694. [Google Scholar] [CrossRef]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laurà, R.; et al. Polydeoxyribonucleotide, an adenosine-A2A receptor agonist, preserves blood testis barrier from cadmium-induced injury. Front. Pharmacol. 2017, 7, 537. [Google Scholar] [CrossRef] [Green Version]
- Ko, I.-G.; Hwang, J.J.; Chang, B.S.; Kim, S.-H.; Jin, J.-J.; Hwang, L.; Kim, C.-J.; Choi, C.W. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced acute lung injury via modulation of the MAPK/NF-κB signaling pathway in rats. Int. Immunopharmacol. 2020, 83, 106444. [Google Scholar] [CrossRef]
- Lee, S.; Won, K.Y.; Joo, S. Protective effect of polydeoxyribonucleotide against CCl4-induced acute liver injury in mice. Int. Neurourol. J. 2020, 24 (Suppl. 2), 88–95. [Google Scholar] [CrossRef]
- Kwon, D.R.; Park, G.-Y.; Moon, Y.S.; Lee, S.C. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cells combined with polydeoxyribonucleotides on full-thickness rotator cuff tendon tear in a rabbit model. Cell Transplant. 2018, 27, 1613–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.R.; Moon, Y.S. Synergic regenerative effects of polydeoxyribonucleotide and microcurrent on full-thickness rotator cuff healing in a rabbit model. Ann. Phys. Rehabili. Med. 2020, 63, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Choi, M.S.; Kim, H.K.; Kim, W.S.; Bae, T.H.; Kim, M.K.; Chang, S.H. Polydeoxyribonucleotide improves tendon healing following achilles tendon injury in rats. J. Orthop. Res. 2018, 36, 1767–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rho, J.H.; Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Chung, J.-Y.; Hwang, T.-J.; Han, J.-H. Polydeoxyribonucleotide ameliorates inflammation and apoptosis in Achilles tendon-injury rats. Int. Neurourol. J. 2020, 24 (Suppl. 2), 79–87. [Google Scholar] [CrossRef]
- Heo, J.W.; Kim, Y.H.; Kim, E.S.; Kim, S.W.; Kim, J. Effect of polydeoxyribonucleotide on chondrocutaneous composite grafts survival. Aesth. Plast. Surg. 2019, 43, 1071–1077. [Google Scholar] [CrossRef]
- Yu, M.; Lee, J.Y. Polydeoxyribonucleotide improves wound healing of fractional laser resurfacing in rat model. J. Cosmet. Laser Ther. 2017, 19, 43–48. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, J.W.; Byun, J.H.; Lee, W.M.; Kim, M.H.; Wu, W.H. Comparison of wound healing effects between Oncorhynchus keta-derived polydeoxyribonucleotide (PDRN) and Oncorhynchus mykiss-derived PDRN. Arch. Craniofac. Surg. 2018, 19, 20–34. [Google Scholar] [CrossRef] [Green Version]
- Zucchi, A.; Cai, T.; Cavallini, G.; D’Achille, G.; Pastore, A.L.; Franco, G.; Lepri, L.; Costantini, E. Genital lichen sclerosus in male patients: A new treatment with polydeoxyribonucleotide. Urol. Int. 2016, 97, 98–103. [Google Scholar] [CrossRef]
- Yoon, Y.C.; Lee, D.-H.; Lee, M.Y.; Yoon, S.-H. Polydeoxyribonucleotide injection in the treatment of chronic supraspinatus tendinopathy: A case-controlled, retrospective, comparative study with 6-month follow-up. Arch. Phys. Med. Rehabil. 2017, 98, 874–880. [Google Scholar] [CrossRef]
- Ryu, K.; Ko, D.; Lim, G.; Kim, E.; Lee, S.H. Ultrasound-guided prolotherapy with polydeoxyribonucleotide for painful rotator cuff tendinopathy. Pain Res. Manag. 2018, 2018, 82861980. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.J.; Park, D. Usefulness of polydeoxyribonucleotide as an alternative to corticosteroids in patients with lateral epicondyitis: A case series. Medicine 2018, 97, e10809. [Google Scholar] [CrossRef]
- Lee, D.-O.; Yoo, J.-H.; Cho, H.-I.; Cho, S.; Cho, H.R. Comparing effectiveness of polydeoxyribonucleotide injection and corticosteroid injection in plantar fasciitis treatment: A prospective randomized clinical study. Foot Ankle Surg. 2020, 26, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Podlesko, A.M.; Ramacciati, N.; Panzolini, S.; Saldi, S.; Fiorucci, S.; Pierini, D.; Mancini, M.; Merolla, M.S.; Lancellotta, V.; Aristei, C. Effects of topical polydeoxyribonucleotide on radiation-induced oral mucositis. Tech. Innov. Patient Support Radiat. Oncol. 2018, 7, 17–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.; Lim, H.S.; Lee, D.-W. Polydeoxyribonucleotide, as a novel approach for the management of medication-related osteonecrosis of the jaw: A preliminary observational study. J. Korean Dent. Sci. 2018, 11, 57–61. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Choi, J.; Jeong, W.; Kwon, S. Polydeoxyribonucleotide improves peripheral tissue oxygenation and accelerates angiogenesis in diabetic foot ulcers. Arch. Plast. Surg. 2017, 44, 482–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, T.H.; Cho, S.B. Adjuvant therapy for revision rhinoplasty of contracted nose using polydeoxyribonucleotide and invasive bipolar radiofrequency. Plast. Reconstr. Surg. Glob. Open. 2018, 6, e1645. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, J.J.; Lee, Y.I.; Lee, W.J.; Lee, C.; Chung, W.Y.; Nam, K.-H.; Lee, J.H. Preventive effect of polynucleotide on post-thyroidectomy scars: A randomized, double-blinded, controlled trial. Lasers Surg. Med. 2018, 50, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kang, J.J.; Kim, J.; Park, S.; Kim, J.M. Efficacy and safety of intra-articular injections of hyaluronic acid combined with polydeoxyribonucleotide in the treatment of knee osteoarthritis. Ann. Rehabil. Med. 2019, 43, 204–214. [Google Scholar] [CrossRef]
- Dallari, D.; Sabbioni, G.; Del Piccolo, N.; Carubbi, C.; Veronesi, F.; Torricelli, P.; Milena, F. Efficacy of intra-articular polynucleotides associated with hyaluronic acid versus hyaluronic acid alone in the treatment of knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Clin. J. Sport Med. 2020, 30, 1–7. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, J.-M.; Park, J.-S.; Park, S.; Lee, B.J.; Park, D. Ultrasound-guided peri-brachial plexus polydeoxyribonucleotide injection for a patient with postherpetic brachial plexopathy: A case report. Medicine 2019, 98, e16694. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, D. Effect of polydeoxyribonucleotide injection in a patient with carpal tunnel syndrome. Am. J. Phys. Med. Rehabil. 2018, 97, e93–e95. [Google Scholar] [CrossRef]
- Huh, J.; Shim, K.S.; Cho, H.-J.; Lee, B.J.; Park, D. Polydeoxyribonucleotide injection in the treatment of patients with carpal tunnel syndrome: Retrospective preliminary study. Medicine 2019, 98, e17522. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Plaza-Manzano, G. Carpal tunnel syndrome: Just a peripheral neuropathy? Pain Manag. 2018, 8, 209–216. [Google Scholar] [CrossRef]
- Park, D.; Yu, K.J.; Cho, J.Y.; Woo, S.B.; Park, J.; Lee, Z.; Kim, J.M. The effectiveness of 2 consecutive intra-articular polydeoxyribonucleotide injections compared with intra-articular triamcinolone for hemiplegic shoulder pain: A STROBE-complaint retrospective study. Medicine 2017, 96, e8741. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Park, K.D.; Park, Y. The effect of polydeoxyribonucleotide on the treatment of radiating leg pain due to cystic mass lesion in inner aspect of right sciatic foramen: A CARE compliant case report. Medicine 2018, 97, e12794. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.S.; Kim, H.S. Treatment for acute stage complex regional pain syndrome type II with polydeoxyribonucleotide injection. J. Korean Neurosurg. Soc. 2016, 59, 529–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Mysore, V. Classifications of patterned hair loss: A review. J. Cutan. Aesthet. Surg. 2016, 9, 3–12. [Google Scholar] [CrossRef]
- Choi, Y.J.; Cho, S.; Kim, Y.K.; Kim, D.S. Improvement of hair graying during a treatment of male pattern hair loss using 1927-nm fractionated thulium laser energy and polydeoxyribonucleotide injections. Med. Laser 2017, 6, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.B.; Zheng, Z.; Kang, J.-S.; Kim, H. Therapeutic efficacy of 1927-nm fractionated thulium laser energy and polydeoxyribonucleotide on pattern hair loss. Med. Laser 2016, 5, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.-H.; Cho, H.R.; Kang, K.N.; Rhyu, C.J.; Chon, S.W.; Lim, Y.S.; Yoo, J.I.; Kim, J.-W.; Kim, Y.U. The effect of polydeoxyribonucleotide prolotherapy on posterior tibial tendon dysfunction after ankle syndesmotic surgery: A case report. Medicine 2016, 95, e5346. [Google Scholar] [CrossRef]
- Do, H.-K.; Lee, J.-H.; Lim, J.-Y. Polydeoxyribonucleotide injection in the patients with partial-thickness tear of supraspinatus tendon: A prospective and pilot study using ultrasound. Physician Sportsmed. 2018, 46, 213–220. [Google Scholar] [CrossRef]
- Park, D. Application of ultrasound-guided C5 nerve root block using polydeoxyribonucleotide in traumatic C5 nerve root injury caused by fracture of the articular process of the cervical spine: A case report. Medicine 2017, 96, e8728. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Park, G.; Lee, J.; Bae, H. The effect of polydeoxyribonucleotide on chronic non-healing wound of an amputee: A case report. Ann. Rehabil. Med. 2018, 42, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Belmontesi, M. Polydeoxyribonucleotide for the improvement of a hypertrophic retracting scar-An interesting case report. J. Cosmet. Dermatol. 2020, 19, 2982–2986. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lim, Y.M.; Lew, D.H.; Song, S.Y. Salvage of unilateral complete ear amputation with continuous local hyperbaric oxygen, platelet-rich plasma and polydeoxyribonucleotide without micro-revascularization. Arch. Plast. Surg. 2017, 44, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, A.; Jan, A.; Wajid, M.; Tariq, S. Management of chronic non-healing wounds by hirudotherapy. World J. Plast. Surg. 2017, 6, 9–17. [Google Scholar]
- Sato, T.; Ichioka, S. Microsurgical replantation of an amputated ear. Acute Med. Surg. 2017, 4, 373–374. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Y. Biomedical engineering for health research and development. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 220–224. [Google Scholar]
- Zhong, Q.; Ding, H.; Gao, B.; He, Z.; Gu, Z. Advances of microfluidics in biomedical engineering. Adv. Mater. Technol. 2019, 4, 1800663. [Google Scholar] [CrossRef]
- Hashimoto, S. Multidisciplinary learning extends communication skill, and helps cross cultural understandings: Biomedical engineering. J. Syst. Cybern. Inf. 2017, 15, 106–112. [Google Scholar]
- Repanas, A.; Andriopoulou, S.; Glasmacher, B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J. Drug Deliv. Sci. Technol. 2016, 31, 137–146. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jung, W.-K. R&D Trends of brown algae as potential candidates in biomedical application. J. Mar. Biosci. Biotechnol. 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Ko, S.C.; Nam, S.Y.; Oh, J.; Kim, Y.-M.; Kim, J.-I.; Kim, N.; Yi, M.; Jung, W.-K. Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, H.S.; Oh, S.-J.; Hwang, C.-W.; Jung, W.-K. Phlorotannins ameliorate extracellular matrix production in human vocal fold fibroblasts and prevent vocal fold fibrosis via aerosol inhalation in a laser-induced fibrosis model. J. Tissue Eng. Regen. Med. 2020, 14, 1918–1928. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, M.-S.; Ko, S.-C.; Heo, S.-Y.; Kang, H.W.; Kim, S.W.; Hwang, C.W.; Lee, K.D.; Oak, C.; Jung, M.J.; et al. Fabrication and biological activity of polycaprolactone/phlorotannin endotracheal tube to prevent tracheal stenosis: An in vitro and in vivo study. J. Biomed. Mater. Res. Part B 2020, 108, 1046–1056. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, W.; Zha, K.; Liu, G.; Yang, S.; Ye, S.; Liu, Y.; Xiong, Y.; Wu, Y.; Cao, F. Treatment of chronic ulcer in diabetic rats with self assembling nanofiber gel encapsulated-polydeoxyribonucleotide. Am. J. Transl. Res. 2016, 8, 3067–3076. [Google Scholar] [PubMed]
- Han, K.; Ha, J.; Hur, W. Preparation Process for Polynucleotide Fragments from Plant and Non-Aqueous Composition Containing the Same. KR Patent 10-2016-0012504, 1 February 2016. [Google Scholar]
- Kim, J.S.; Shin, D.K. Cosmetic Composition for Anti-Aging Activity Comprising DNA Originated Strawberry. KR Patent 10-2015882, 8 April 2019. [Google Scholar]
- Ki, K.H.; Lee, J.E.; Kim, Y.E.; Joo, I.W. Method of Producing DNA Derived from Aloe Genus Plant, and Composition Containing DNA Derived from Aloe Genus Plant for Anti-Aging and Anti-Inflammation. KR Patent 10-2019-0021328, 22 February 2019. [Google Scholar]



| Kind of Study | Source | Article Number | Therapeutic Effect |
|---|---|---|---|
| In vitro | O. keta | 1 | Alleviative effect on disease symptoms |
| 1 | Biological effect (Anti-MMP, anti-melanogenesis, activating mitochondrial biogenesis) | ||
| 1 | Dysfunctional tissue treatment | ||
| 1 | OA treatment | ||
| O. mykiss | 1 | Anti-melanogenesis | |
| 1 | OA treatment | ||
| In vivo | O. keta | 1 | Anti-osteonecrosis |
| 1 | Anti-ulcer | ||
| 1 | OA treatment | ||
| 3 | Preventive effect on tissue damage | ||
| 1 | RCTT treatment | ||
| 1 | Tendon tear treatment | ||
| O. mykiss | 1 | Anti-Allodynic | |
| 1 | Bone regeneration | ||
| 1 | OA treatment | ||
| 2 | RCTT treatment | ||
| A. sinensis | 1 | Bone regeneration | |
| 1 | Preventive effect on tissue damage | ||
| Clinical | O. keta | 1 | Adjuvant therapy |
| 1 | Alleviative effect on limb pain | ||
| 1 | Alleviative effect on leg pain | ||
| 1 | Alleviative effect on shoulder pain | ||
| 1 | Anti-osteonecrosis | ||
| 1 | Anti-ulcer | ||
| 2 | CRPS treatment | ||
| 1 | Preventive effect on PHL | ||
| 1 | Preventive effect on scar forming | ||
| O. mykiss | 1 | CRPS treatment | |
| 1 | Dysfunctional tissue treatment | ||
| 1 | RCTT treatment | ||
| 2 | OA treatment | ||
| 1 | Preventive effect on PHL |
| PublicationYear | Source | Stimulator | Cell | Outcomes | Target | Ref |
|---|---|---|---|---|---|---|
| 2016 | O. keta & O. mykiss | - | Mel-Ab & Co-culture of human melanocyte- keratinocyte model | ↓ Synthesis of melanin ↓ Intracellular activity of tyrosinase ↓ Protein expression of MITF, tyrosinase, and TRP-1 ↑ Phosphorylation of ERK and AKT | Etc | [20] |
| 2016 | O. keta | - | U2OS &HDF | ↑ Cell motilities | Wound healing | [8] |
| 2017 | O. mykiss | E. coli LPS (1& 10 ng/mL) | RAW 264.7 | ↓ Production of NO ↓ Secretion of IL-12 and TNF-α ↑ Secretion of IL-10 and VEGF-A | Inflammatory disease | [19] |
| 2018 | O. keta | IL-1β (10 ng/mL) | SW1353 | ↓ mRNA expression of CCL3, CCL4, CCL8, CXCL10, CXCL11, IL-1β, IL-6, IL-8, and TLR3 ↓ Protein level of CCL3, IL-1β, IL-6, and IL-8 | Inflammatory disease | [17] |
| 2018 | O. keta | H2O2 (2 mM) | CHON-001 | ↓ Cell damage induced by H2O2 ↓ Expression of COX-2, PGE2 and TNF-α | Degenerative joint disease | [21] |
| 2018 | O. keta | ZA (10 μM) & E. coli LPS (0.1 μg/mL) | RAW 264.7 | ↑ Cell viability ↓ Production of NO ↓ Expression of IL-1β, IL-6, iNOS, and TNF-α ↑ Expression of VEGF and A2AR | Inflammatory disease | [18] |
| 2018 | IL-1β (10 ng/mL) | SW1353 | ↑ Protein expression of aggrecan, ANG-2, PDGF, and VEGF ↓ Protein expression of endostatin, angiostatin, and MMP13 ↑ Cell migration | Degenerative joint disease | [22] | |
| 2018 | O. keta | - | CCD-986SK | ↑ Phosphorylation of ERK, FAK, and JNK ↑ Cell migration | Wound healing | [27] |
| 2019 | O. keta | - | HGF (neonatal) | ↑ Cell proliferation ↑ Expression of VEGF and CD31 | Wound healing | [28] |
| 2019 | O. keta | E. coli LPS (1 μg/mL) | SH-SY5Y | ↑ Cell proliferation ↓ Production and expression of IL-1β, IL-6, and TNF-α ↑ Phosphorylation of CREB and cAMP ↑ Expression of BDNF, cAMP and VEGF | Inflammatory disease | [25] |
| 2020 | O. keta | E. coli LPS (1 μg/mL) & TGF-β (5 ng/mL) | A549 | ↑ Cell viability reduced by LPS and TGF-β ↓ Expression of CTGF and hydroxyproline ↓ Expression of COL I, FGF, IL-6, and TNF-α | Etc | [24] |
| 2020 | O. keta | - | HDF & Diabetic HDF & HUVECs | ↑ Cell proliferation and migration ↑ Expression of FGF and VEGF ↑ Vessel formation and density ↑ Total vessel network length | Wound healing | [8] |
| 2020 | O. keta | - | B16-F10 & CCD-986SK | ↓ Activity of mushroom tyrosinase and the cellular tyrosinase ↓ Intracellular content and cellular level of melanin ↓ mRNA and protein expression of MITF, tyrosinase, TRP1, and TRP2 ↑ Mitochondrial density and the mtDNA contents ↓ Activity of in vitro collagenase and elastase ↓ Level of cellular MMP1 ↑ mRNA expression for mtDNA Antioxidant activity | Etc | [23] |
| 2020 | O. keta | PM10 (100 μg/mL) | NCI-H358 | ↓ Cytotoxicity induced by PM10 ↑ Concentration of cAMP ↓ Level of Caspase-3 and -9, IL-1β, IL-6, and TNF-α ↑ Phosphorylation of CREB and PKA ↓ Expression ration of Bax/Bcl-2 ↓ Expression of Cyt c and Apaf-1 | Etc | [16] |
| PublicationYear | Source | Treatment | Model | Outcomes | Target | Ref |
|---|---|---|---|---|---|---|
| 2016 | O. mykiss | Treatment of fractional ablative CO2 laser | Skin wounded SD rats | ↑ Wound healing ↑ Epithelial confluence ↑ Score on granulation tissue thickness ↑ Number of VEGF-positive cells ↓ Erythema and crusting ↑ Number of PECAM-1/CD31-positive microvessels ↑ Production of VEGF | Wound healing | [46] |
| 2016 | O. mykiss | - | Subcutaneous implanted nude mice | ↑ Number of osteoblast and fibroblast attached to the surface of each particle ↑ Area ratio of NB ↑ Number of bone-forming cells ↑ Deposition and calcification of NB matrix ↑ Development of the blood vessels Newly formed COL matrix Observed a fibrous capsule in vicinity of dentin particles | Etc | [36] |
| 2016 | O. mykiss | Intra-colonic injection of DNBS (25 mg) or Oral administration of DSS (8%) | Colitis-induced SD rats | ↓ Colitis-induced damage ↓ Lipid peroxidation and neutrophil infiltration ↓ Serum level of IL-1β and TNF-α ↓ Bcl-2 expression ↑ Activation of A2AR | Inflammatory disease | [1] |
| 2017 | O. mykiss | CdCl2 challenge (2 mg/kg) | BTB integrity-induced C57 BL/6J mice | ↓ Phosphorylation of ERK ↓ Level of FSH and LH ↑ Serum concentration of TE and inhibin B ↑ Size of seminiferous tubules ↑ Johnsen’s score ↑ Number of spermatozoa ↓ Isolation of peripheral positive germ cell and macrophages ↓Immunoreactivity on Claudin-11, N-Cadherin, occludin, and TGF-β3 ↓ Fragmented junctions between adjacent sertoli cells | Etc | [38] |
| 2017 | O. mykiss | Intra-tracheal instillation of E. coli LPS (5 mg/kg) | Lung injured SD rats | ↓ Lung injury score and DNA fragmentation ↓ Expression of caspase-3, -8, and -9 ↑ Bax/Bcl-2 expression ratio ↑ Expression of A2AR, IL-6, and TNF-α Observed patch intra-alveolar macrophages Observed normal-looking alveolar structures except hyperplasia of type II pneumocytes | Inflammatory disease | [6] |
| 2017 | O. mykiss | - | Skin wounded SD rats | ↓ Infiltration and number of inflammatory cells ↓ Scar size ↓ Expression of HMGB-1 ↑ Expression of COL I and III | Wound healing | [9] |
| 2017 | A. sinensis | - | Calvarial defected Wistar rats | ↑ Percentage of NB ↑ Production of OCN and OPN | Etc | [13] |
| 2017 | O. mykiss | - | Tendon injured SD rats | ↑ CSA of the laceration sites ↑ Resistant to mechanical stress ↑ Stored energy | Wound healing | [43] |
| 2018 | O. keta | Injection of MIA (60 mg/mL) | OA induced SD rats | ↓ Expression of IL-1β, MMP-3, MMP-7 ↓ Phosphorylation of ERK ↓ Production of COX-2, PGE2, and TNF-α ↑ Regularity in chondrocyte distribution | Etc | [21] |
| 2018 | O. mykiss | - | Skin wounded ICR mice | ↑ Wound healing↓ Infiltration of inflammatory cells ↑ Expression of p63, TGF-β, and VEGF ↑ Neovascularization Observed clear re-epithelialization and granulation tissue proliferation | Wound healing | [47] |
| 2018 | O. keta | - | Tendon injured New Zealand white rabbits | ↑ Regeneration of COL fibers ↑ Number of VEGF-positive cells and density of PECAM-1-positive microvessel ↓ Tendon tear size | Etc | [5] |
| 2018 | O. keta | Oral administration of IND (20 mg/kg) | GU induced SD rats | ↓ mRNA and protein expression of A2AR, IL-1β, IL-6, TNF-α, and VEGF ↑ cAMP concentration ↑ Phosphorylation of CREB and PKA Regenerated GU-induced tissue | Etc | [35] |
| 2018 | A. sinensis | CdCl2 challenge (2 mg/kg) | Brain damaged C57 BL/6J mice | ↓ Level of MDA ↑ Level of GSH and BDNF ↓ Expression of mTOR ↓ Brain edema and ↓ Neuronal morphological changes | Etc | [14] |
| 2018 | O. mykiss | - | Tendon injured New Zealand white rabbits | ↓ Mean tendon tear size ↑ Number of VEGF-positive cells and density of PECAM-1 positive microvessel ↑ Walking distance and fast walking time | Etc | [41] |
| 2018 | O. keta | - | Skin wounded hairless mice | ↑ COL production ↓ Lipid accumulation Observed a normal wound healing process | Wound healing | [27] |
| 2018 | O. keta | Removal of ovary and uterus & Administration of ZA (0.6 mg/mL) | Osteoporosis inducedSD rats | ↓ Severity of the osteonecrosis ↓ Necrotic bone formation at defect sites ↑ Number of blood vessels ↑ Number of attached osteoclasts ↓ Number of detached osteoclasts ↑ Recovery bone remodeling | Etc | [7] |
| 2019 | O. keta | Chondrocutaneous section of composite tissue | New Zealand White rabbits | ↑ Average viable area ↑ Number of capillaries Observed the blood flow signal at the margin of the composite grafts | Wound healing | [45] |
| 2019 | O. keta | Skin wounded Cg-+Leprdb/+Leprdb & m+/+Leprdb mice | ↑ Granulation tissue and capillary blood vessels ↓ Diabetic wound depth ↑ Proliferation of fibroblasts and ↑ Thickness of the epidermis ↑ Closure of diabetic wound ↑ Epithelialization of the epidermis | Wound healing | [28] | |
| 2019 | O. keta | Devascularization of the descending colon | Ischemic colitisinduced SD rats | ↓ Mucosal damage ↓ COL deposition in the colonic tissue ↓ Expression of A2AR, caspase-3, COX-2, IL-1β, IL-6, TNF- α and VEGF ↑ Expression ratio of Bcl-2/Bax ↑ Phosphorylation of ERK | Inflammatory disease | [29] |
| 2020 | O. keta | Oral administration of IND (20 mg/kg) | GU induced SD rats | ↑ Mucosal tissue regeneration ↓ Histological score and ulcer index ↓ Expression and level of IL-1β, IL-6, and TNF-α ↑ Expression and level of cAMP expression ↓ Activation of NF-κB and MAPK pathway ↓ Expression ratio of Bax/Bcl-2 ↑ Activation of A2AR | Etc | [37] |
| 2020 | O. keta | Intratracheal instillation of E. coli LPS (5 mg/kg) | Lung injured SD rats | ↓ Lung injury score ↓ Number of WBC and BALF ↓ Expression of, cleaved caspase-3 and -9, IL-1β, IL-6, TNF-α ↑ Expression of cAMP ↓ Expression ratio of Bax/Bcl-2 ↑ Phosphorylation of CREB and PKA ↓ Activation of NF-κB and MAPK pathways ↓ DNA fragmentation | Etc | [39] |
| 2020 | O. mykiss | SNL or CPIP | Neuropathic pain induced C57/Bl6 mice | ↓ Mechanical allodynia ↓ Expression of GFAP | Etc | [33] |
| 2020 | O. mykiss | Injury on ACL | OA inducedWistar albino rats | ↓ Total Mankin scores and structural integrity ↓ Cellular changes and tidemark continuity | Etc | [34] |
| 2020 | O. mykiss | Intra-colonic instillation of DNBS (25 mg/0.8 mL) | Colitis induced SD rats | ↑ Colon length ↓ Abnormal condition of colon ↓ Extent and severity of colon injury and epithelial and mucosal alterations ↓ Ulceration, size of colitis, and hyperaemia ↑ Bcl-2 positivity ↓ Activity of MPO and lipid peroxidation | Inflammatory disease | [2] |
| 2020 | O. keta | I.p. injection of CCl4 (10 mL/kg) | Liver injured C57BL/6 mice | ↓ Expression of CYP2E1 and UCP2 ↓ Gross morphology of the liver ↓ Liver weight, index, and histopathological score ↓ Concentration of TNF-α, IL-1 and IL-6 ↓ Activation of NF-κB and MAPK pathway ↓ Expression of cleaved caspase-3 and -9 ↓ Expression ratio of Bax/Bcl-2 | Etc | [4] |
| 2020 | O. keta | - | Skin wounded C57BLKS/J-db/db mice | ↑ Wound closure rate ↑ Thickness of the granulation tissue ↓ Number of inflammatory cells ↑ COL density of the regenerated tissue ↓ Expression of MPO and TGF-β ↑ Expression of VEGF and α-SMA | Wound healing | [8] |
| 2020 | O. mykiss | - | Tendon injured New Zealand white rabbits | ↓ Mean STTS ↑ COL synthesis ↑ Density of PECAM-1-positive microvessel ↑ Fast walking time and walking distance and speed | Etc | [42] |
| 2020 | O. mykiss | I.p. injection of CCl4 (10 mL/kg) | Lung injured ICR mice | ↓ AST and ALT concentrations ↑ Expression of A2AR ↓ Expression of IL-1β, IL-6, and TNF-α ↓ Expression ratio of Bax/Bcl-2 ↓ Percentage of TUNEL-positive cells | Wound healing | [40] |
| 2020 | O. keta | - | Tendon injured SD rats | ↑ Tactile threshold for the von Frey filament test ↑ Paw withdrawal latency ↓ Concentration of IL-6 and TNF-α ↓ Number of cleaved caspase-3- and -9-positive cells ↑ Expression of cAMP ↑ Phosphorylation of CREB and PKA ↓ Expression ratio of Bax/Bcl-2 | Etc | [44] |
| PublicationYear | Source | Patient | Outcomes | Target | Ref |
|---|---|---|---|---|---|
| 2016 | O. mykiss | Patients with PHL (n = 8, Both) | ↑ Number and thickness of hair ↓ Possibility of side effects | Etc | [69] |
| 2016 | O. mykiss | Patients with genital LS (n = 21, Male) | ↑ Quality of life conditions of the patients No adverse reactions to the drug and no pain | Inflammatory disease | [48] |
| 2016 | O. mykiss | Patient with leg numbness (n = 1, Female) | ↓ Allodynia and hyperalgesia ↓ Skin flushing Treatment of the acute inflammatory phase of CRPS type II | Etc | [66] |
| 2016 | O. mykiss | Patient with ankle pain (n = 1, Female) | ↓ NRS score and pain No swelling and tenderness No complications | Etc | [70] |
| 2017 | O. keta | Patient with male PHL (n = 1, Male) | ↑ Satisfaction of patient ↑ Hair counts, thickness, and graying | Etc | [68] |
| 2017 | O. mykiss | Patients with RCD (n = 106, Both) | ↓ SPADI and VAS | Inflammatory disease | [49] |
| 2017 | O. mykiss | Patient with PA bursitis (n = 1, Female) | ↓ NRS and pain No side effects | Inflammatory disease | [51] |
| 2017 | O. keta | Patient with neck and shoulder pain (n = 1, Female) | ↑ Motor power of the left shoulder elevation and elbow flexion ↓ Pain of neck and left shoulder ↑ CMAP of the left musculocutaneous, axillary, and suprascapular nerve ↑ Expression of COL I and III, FGF, and VEGF Showed dense COL fibers with organized patterned arrangement | Wound healing | [72] |
| 2017 | O. keta | Patients with hemiplegic shoulder pain (n = 20, Both) | ↓ Passive ROM and NRS | Etc | [64] |
| 2017 | O. keta | Patients with diabetic foot ulcer (n = 20, Both) | ↓ Transcutaneous oxygen tension ↓ Inflammation and neutrophil pigmentation ↑ Granulation tissue formation | Etc | [55] |
| 2017 | O. mykiss | Patient with partial ear amputation (n = 1, Male) | ↑ Survival of the composite ear graft Almost completely healed after 53 days | Wound healing | [75] |
| 2018 | O. keta | Patients with contracted nose (n = 30, Both) | ↑ Mobility of nasal skin Softened the skin of contracted noses | Etc | [56] |
| 2018 | O. mykiss | Patients with chronic RCD (n = 32, Both) | ↓ VAS score, pain, and SPADI ↑ Function and SANE No complications | Inflammatory disease | [50] |
| 2018 | O. mykiss | Patients with tendon tear (n = 17, Both) | ↓ VAS at active shoulder ↓ DASH subscore and Global DASH ↑ ROM in forward flexion ↓ Tear volume of supraspinatus tendon No adverse events except one patient | Etc | [71] |
| 2018 | O. keta | Patients with lateral epicondylitis (n = 2, Male) | ↓ Pain with decreased NRS ↓ Hypervascularity of common extensor tendon Improvement in the LE symptoms without any complications | Inflammatory disease | [52] |
| 2018 | O. keta | Patient with burn wound (n = 1, Male) | Improved adequately to wear the prosthesis after 4 weeks | Wound healing | [73] |
| 2018 | O. keta | Patients undergoing Thyroidectomy (n = 42, Both) | ↓ VSS, EI, and height and width of scar ↓ Pigmentation and vascularity No adverse events | Etc | [57] |
| 2018 | A. sinensis | Patients with oral mucositis (n = 3, Both) | ↓ Erythema, desquamation, and pain No allergic reactions | Inflammatory disease | [15] |
| 2018 | O. keta | Patient with CTS (n = 1, Female) | ↑ NRS, BCTQ-severity and function scores No side effects | Etc | [61] |
| 2018 | O. keta | Patient with leg pain (n = 1, Male) | Pain relief | Etc | [65] |
| 2018 | O. keta | Patients with MRONJ (n = 5, Both) | Relief of pain and no sign of infection Observed soft tissue coverage of the operation area | Etc | [54] |
| 2019 | O. mykiss | Patients with knee joint pain (n = 29, Both) | ↓ VAS, KSS, and WOMAC except stiffness No TEAEs, ADRs, and any other complications | Etc | [58] |
| 2019 | O. keta | Patient with limb pain (n = 1, Female) | ↓ Left arm pain ↑ Motor strength | Etc | [60] |
| 2019 | O. keta | Patients with CTS (n = 30, Both) | ↓ NRS, the severity score of BCTQ, and CSA | Etc | [62] |
| 2020 | O. mykiss | Patients with Knee OA (n = 98, Both) | ↑ KSS | Etc | [59] |
| 2020 | O. keta | Patients with heel pain (n = 38, Both) | ↓ VAS and MOXFQ scores No complications | Inflammatory disease | [53] |
| 2020 | O. mykiss | Patient with disabling scared (n = 1, Female) | ↓ Pain and prediabetes Aesthetic and functional improvement Restored cutaneous texture Able to walk autonomously No TEAEs | Wound healing | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-H.; Heo, S.-Y.; Oh, G.-W.; Heo, S.-J.; Jung, W.-K. Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering. Mar. Drugs 2021, 19, 296. https://doi.org/10.3390/md19060296
Kim T-H, Heo S-Y, Oh G-W, Heo S-J, Jung W-K. Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering. Marine Drugs. 2021; 19(6):296. https://doi.org/10.3390/md19060296
Chicago/Turabian StyleKim, Tae-Hee, Seong-Yeong Heo, Gun-Woo Oh, Soo-Jin Heo, and Won-Kyo Jung. 2021. "Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering" Marine Drugs 19, no. 6: 296. https://doi.org/10.3390/md19060296
APA StyleKim, T. -H., Heo, S. -Y., Oh, G. -W., Heo, S. -J., & Jung, W. -K. (2021). Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering. Marine Drugs, 19(6), 296. https://doi.org/10.3390/md19060296