Antitumor Profile of Carbon-Bridged Steroids (CBS) and Triterpenoids
Abstract
:1. Introduction
2. Cyclopropane Containing Steroids and Triterpenoids
3. Sterols and Triterpenoids with Cyclopropane Ring in the Side Chain
4. Cyclobutane Containing Steroids and Triterpenoids
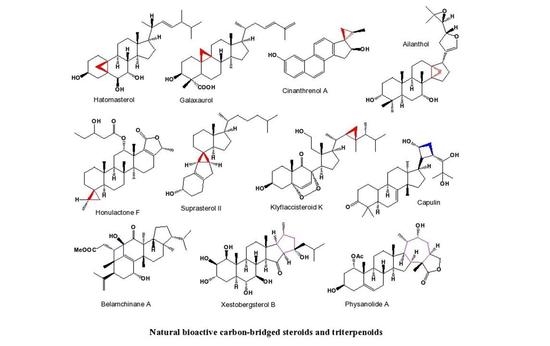
5. Miscellaneous Cyclosteroids and Triterpenoids Derived from Marine and Terrestrial Sources
6. Comparison of Biological Activities of Natural and Synthetic CBS and Triterpenoids
6.1. Antitumor Activity of Cyclopropane-Containing CBS and Triterpenoids
6.2. Antitumor Activity of Cyclobutane-Containing CBS and Triterpenoids
6.3. Miscellaneous Cyclosteroids and Triterpenoids
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moss, G.P. The nomenclature of steroids. Eur. J. Biochem. 1989, 186, 429–458. [Google Scholar]
- Burger, A. Cyclopropane compounds of biological interest. Prog. Drug Res. 1971, 15, 227–270. [Google Scholar]
- Schoenheimer, R.; Evans, E.A., Jr. The chemistry of the steroids. Ann. Rev. Biochem. 1937, 6, 139–162. [Google Scholar] [CrossRef]
- Ruigh, W.L. The chemistry of the steroids. Ann. Rev. Biochem. 1945, 14, 225–262. [Google Scholar] [CrossRef]
- Bergmann, W.; McLean, M.J.; Lester, D. Contributions to the study of marine products. XIII. Sterols from various marine invertebrates. J. Org. Chem. 1943, 8, 271–282. [Google Scholar] [CrossRef]
- Koch, F.C. The steroids. Ann. Rev. Biochem. 1944, 13, 263–294. [Google Scholar] [CrossRef]
- Kokke, W.C.M.C.; Epsteing, S.; Lookll, S.A.; Raull, G.H.; Fenicall, W.; Djerassi, C. On the origin of terpenes in symbiotic associations between marine invertebrates and algae (Zooxanthellae). J. Biol. Chem. 1984, 259, 8168–8173. [Google Scholar] [CrossRef]
- Ermolenko, E.V.; Imbs, A.B.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Chemical diversity of soft coral steroids and their pharmacological activities. Mar. Drugs 2020, 18, 613. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, L.S. Sterol and diterpenoid production by zooxanthellae in coral reefs: A review. Biol. Oceanograph. 1989, 6, 363–374. [Google Scholar]
- Kanazawa, A. Sterols in marine invertebrates. Fisheries Sci. 2001, 67, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Ikekawa, N.; Kanazawa, A.; Ando, T. Identification of 23-demethylacanthasterol in an asteroid, Acanthaster planci and its synthesis. Steroids 1980, 36, 65–71. [Google Scholar]
- Lopanik, N.B. Chemical defensive symbioses in the marine environment. Funct. Ecol. 2014, 28, 328–340. [Google Scholar] [CrossRef]
- Gascoigne, R.M.; Simes, J.J.H. The tetracyclic triterpenes. Quarterly Rev. Chem. Soc. 1955, 9, 328–361. [Google Scholar] [CrossRef]
- Henry, J.A. Chemistry of Cycloartenol and Cyclolaudenol. Ph.D. Theses, Glasgow University, Glasgow, UK, June 1954. [Google Scholar]
- Djerassi, C.; McCrindle, R. Terpenoids. Part LI. The isolation of some new cyclopropane-containing triterpenes from Spanish moss (Tillandsia usneoides, L.). J. Chem. Soc. 1962, 4034–4039. [Google Scholar] [CrossRef]
- De Meijere, A. Introduction: Cyclopropanes and related rings. Chem. Rev. 2003, 103, 931–932. [Google Scholar] [CrossRef] [Green Version]
- Wessjohann, L.A.; Brandt, W.; Thiemann, T. Biosynthesis and metabolism of cyclopropane rings in natural compounds. Chem. Rev. 2003, 103, 1625–1648. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A. Astonishing diversity of carbon-bridged steroids and their biological activities: A brief review. Eur. J. Biotechnol. Biosci. 2018, 6, 6–23. [Google Scholar]
- Fan, Y.Y.; Gao, X.H.; Yue, J.M. Attractive natural products with strained cyclopropane and/or cyclobutane ring systems. Sci. China Chem. 2016, 59, 1126–1141. [Google Scholar] [CrossRef]
- Wang, M.; Lu, P. Catalytic approaches to assemble cyclobutane motifs in natural product synthesis. Org. Chem. Front. 2018, 5, 254–259. [Google Scholar] [CrossRef]
- Namyslo, J.C.; Dieter, E. Kaufmann. The application of cyclobutane derivatives in organic synthesis. Chem. Rev. 2003, 103, 1485–1537. [Google Scholar] [CrossRef] [PubMed]
- Kilimnik, A.; Dembitsky, V.M. Anti-melanoma agents derived from fungal species. Mathews J. Pharm. Sci. 2016, 1, 002. [Google Scholar]
- Levitsky, D.O.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Naturally occurring isocyano/isothiocyanato compounds: Their pharmacological and SAR activities. Mathews J. Pharm. Sci. 2016, 1, 003. [Google Scholar]
- Kuklev, D.V.; Dembitsky, V.M. Chemistry, origin, antitumor and other activities of fungal homo-dimeric alkaloids. Mathews J. Pharm. Sci. 2016, 1, 004. [Google Scholar]
- Kilimnik, A.; Kuklev, D.V.; Dembitsky, V.M. Antitumor acetylenic lipids. Mathews J. Pharm. Sci. 2016, 1, 005. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological and predicted activities of natural azo compounds. Nat. Prod. Bioprospect. 2017, 6, 1–19. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Biological activities of nitro steroids. J. Pharm. Res. Intern. 2017, 18, 1–19. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological and predicted activities of natural azo compounds. Nat. Prod. Bioprospect. 2017, 7, 151–169. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological activities of epithio steroids. J. Pharm. Res. Intern. 2017, 18, 1–19. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Biological activities of organometalloid (As, At, B, Ge, Si, Se, Te) steroids. J. Appl. Pharm. Sci. 2017, 7, 184–202. [Google Scholar]
- Dembitsky, V.M.; Savidov, N.; Poroikov, V.V.; Gloriozova, T.A.; Imbs, A.B. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotech. 2018, 102, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Savidov, N. Steroid phosphate esters and phosphonosteroids and their biological activities. Appl. Microbiol. Biotech. 2018, 102, 7679–7692. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Gloriozova, T.A.; Poroikov, V.V.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Peroxy steroids derived from plant and fungi and their biological activities. Appl. Microbiol. Biotech. 2018, 102, 7657–7667. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring marine α,β-epoxy steroids: Origin and biological activities. Vietnam. J. Chem. 2018, 56, 409–433. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Savidov, N.; Gloriozova, T.A. Sulphur containing steroids: Structures and biological activities. Vietnam. J. Chem. 2018, 56, 540–582. [Google Scholar] [CrossRef]
- Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124. [Google Scholar] [CrossRef]
- Vil, V.; Terent’ev, A.O.; Al Quntar, A.A.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Oxetane-containing metabolites: Origin, structures and biological activities. Appl. Microbiol. Biotech. 2019, 103, 2449–2467. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Gloriozova, T.A.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Hydroperoxides derived from marine sources: Origin and biological activities. Appl. Microbiol. Biotech. 2019, 103, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Gloriozova, T.A.; Poroikov, V.V.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Naturally occurring of α, β-diepoxy-containing compounds: Origin, structures, and biological activities. Appl. Microbiol. Biotech. 2019, 103, 3249–3264. [Google Scholar] [CrossRef]
- Vil, V.A.; Terent’ev, A.O.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Pounina, T.A.; Dembitsky, V.M. Hydroperoxy steroids and triterpenoids derived from plant and fungi: Origin, structures and biological activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87. [Google Scholar] [CrossRef]
- Vil, V.A.; Gloriozova, T.A.; Terent’ev, A.O.; Zhukova, N.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from terrestrial and aquatic sources: Origin, structures and biological activities. Vietnam. J. Chem. 2019, 57, 1–15. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Antitumor and hepatoprotective activity of natural and synthetic neo steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’yakonov, D. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 524, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Sikorsky, T.V.; Ermolenko, E.V.; Gloriozova, T.A.; Dembitsky, V.M. Mini Review: Anticancer activity of diterpenoid peroxides. Vietnam. J. Chem. 2020, 58, 273–280. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological profile of natural and synthetic compounds with rigid adamantane-based scaffolds as potential agents for the treatment of neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2020, 529, 1225–1241. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Honecker, H. Marine compounds and cancer: Updates 2020. Mar. Drugs 2020, 18, 643. [Google Scholar] [CrossRef]
- Mitome, H.; Shirato, N.; Hoshino, A.; Miyaoka, H.; Yamada, Y.; Yamada, Y.; Van Soest, R.W.M. New polyhydroxylated sterols stylisterols A–C and a novel 5, 19-cyclosterol hatomasterol from the Okinawan marine sponge Stylissa sp. Steroids 2005, 70, 63–70. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Zhong, H.M.; Che, C.T. Cycloartanes from the red alga Galaxaura sp. J. Asian Nat. Prod. Res. 2005, 7, 59–65. [Google Scholar] [CrossRef]
- Goad, L.J.; Goodwin, T.W. Studies in phytosterol biosynthesis: Observations on the biosynthesis of fucosterol in the marine brown alga Fucus spiralis. Eur. J. Biochem. 1969, 7, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, S.; Johnson, A.J. Antidiabetes constituents, cycloartenol and 24-methyl-enecycloartanol, from Ficus krishnae. PLoS ONE 2020, 15, e0235221. [Google Scholar]
- Gibbons, G.F.; Goad, L.J.; Goodwin, T.W. The identification of 28-isofucosterol in the marine green algae Enteromorpha intestinalis and Ulva lactuca. Phytochemistry 1968, 7, 983–988. [Google Scholar] [CrossRef]
- Andinq, C.; Brandt, R.D.; Ourisson, G. Sterol biosynthesis in Euglena gracilis Z. Sterol precursors in light-grown and dark-grown Euglena gtacilis Z. Eur. J. Biochem. 1971, 24, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Mercer, E.I.; Harries, W.B. The mechanism of alkylation at C-24 during clionasterol biosynthesis in Monodus subterraneus. Phytochemistry 1975, 14, 439–443. [Google Scholar] [CrossRef]
- Karunen, P.; Mikola, H.; Ekman, R. Separation and analysis of steryl and wax esters from Dicranum elongatum. Physiol. Plantarum 1980, 49, 351–353. [Google Scholar] [CrossRef]
- Miller, M.B.; Haubrich, B.A.; Wang, Q.; Snell, W.J.; Nes, W.D. Evolutionarily conserved 25(27) -olefin ergosterol biosynthesis pathway in the alga Chlamydomonas reinhardtii. J. Lipid Res. 2012, 53, 1636–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, L.B.; Patterson, G.W. The metabolism of cycloartenol, lanosterol, 24-methylene-cholesterol and fucosterol in Chlorella ellipsoidea. Phytochemistry 1976, 15, 1131–1133. [Google Scholar] [CrossRef]
- Nes, W.D.; Norton, R.A.; Crumley, F.G.; Madigan, S.J.; Katz, E.R. Sterol phylogenesis and algal evolution. Proc. Natl. Acad. Sci. USA 1990, 87, 7565–7569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Ioki, M.; Matsuura, H.; Hashimoto, A.; Hashimoto, A.; Kaya, K.; Nobuyoshi, N. Diverse steroidogenic pathways in the marine alga Aurantiochytrium. J. Appl. Phycol. 2020, 32, 1631–1642. [Google Scholar] [CrossRef]
- Calegario, G.; Pollier, J.; Arendt, P.; de Oliveira, L.S.; Thompson, C.; Soares, A.R. Cloning and functional characterization of cycloartenol synthase from the red seaweed Laurencia dendroidea. PLoS ONE 2016, 11, e0165954. [Google Scholar] [CrossRef] [Green Version]
- Raederstorff, D.; Rohmer, M. Sterols of the unicellular algae Nematochrysopsis roscoffensis and Chrysotila lamellosa: Isolation of (24E)-24-n-propylidenecholesterol and 24-n-propylcholesterol. Phytochemistry 1984, 298, 631–634. [Google Scholar] [CrossRef]
- Raederstorff, D.; Rohmer, M. Sterol biosynthesis via cycloartenol and other biochemical features related to photosynthetic phyla in the amoebae Naegleria lovaniensis and Naegleria gruberi. Eur. J. Biochem. 1987, 164, 427–434. [Google Scholar] [CrossRef]
- Milankovic, M. Probing Sterol Biosynthesis Chokepoint Enzymes in Naegleria gruberi for Treatment of Amoeba Diseases. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, May 2017. [Google Scholar]
- Raederstorff, D.; Rohmer, M. Sterol biosynthesis de novo via cycloartenol by the soil amoeba Acanthamoeba polyphaga. Biochem. J. 1985, 231, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, M.P.; Tan, L.T.; Jensen, P.R.; Fenical, W. Capisterones A and B from the tropical green alga Penicillus capitatus: Unexpected anti-fungal defences targeting the marine pathogen Lindra thallasiae. Tetrahedron 2004, 60, 7035–7039. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Killmer, L.; Breen, A.; Johnson, R.K. A new cycloartanol sulfate from the green alga Tuemoya sp.: An inhibitor of VZV protease. Nat. Prod. Lett. 1997, 9, 209–215. [Google Scholar] [CrossRef]
- Govindan, M.; Abbas, S.A.; Schmitz, F.J.; Lee, R.H.; Papkoff, J.S.; Slate, D.L. New cycloartanol sulfates from the alga Tydemania expeditionis: Inhibitors of the protein tyrosine kinase pp60v-src. J. Nat. Prod. 1994, 57, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.A.; Nguyen, V.M.; Nguyen, T.A.N.; Nguyen, D.H.T.; Tran, D.H.; Bui, T.P.T.; Pham, V.T.; Nguyen, T.N. New triterpene sulfates from Vietnamese red alga Tricleocarpa fragilis and their α-glucosidase inhibitory activity. J. Asian Nat. Prod. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Stonik, V.A.; Kapustina, I.I.; Boguslavsky, V.M.; Dmitrenoik, A.S.; Kalinin, V.I.; Cordeiro, M.L.; Djerassi, C. Biosynthetic studies of marine lipids. 42. Biosynthesis of steroid and triterpenoid metabolites in the sea cucumber Eupentacta fraudatrix. Steroids 1993, 58, 508–517. [Google Scholar] [CrossRef]
- Wu, Z.H.; Liu, T.; Gu, C.X. Steroids and triterpenoids from the brown alga Kjellmaniella crassifolia. Chem. Nat. Compd. 2012, 48, 158–160. [Google Scholar] [CrossRef]
- Kikuchi, T.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Watanabe, K.; Nishino, H. Cancer chemopreventive effects of cycloartane-type and related triterpenoids in in vitro and in vivo models. J. Nat. Prod. 2007, 70, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Xinping, H.; Xiaobin, Z.; Liping, D.; Zhiwei, D.; Wenhan, L. Cycloartane triterpenes from marine green alga Cladophora fascicularis. Chin. J. Ocean. Limnol. 2006, 24, 443–448. [Google Scholar] [CrossRef]
- Wang, N.; Xu, G.; Fang, Y.; Yang, T.; Zhao, H.; Li, G. New flavanol and cycloartane glucosides from Landoltia punctata. Molecules 2014, 19, 6623–6634. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, F.; Wu, X.; Cao, S. A new 24-homo-30-nor-cycloartane triterpenoid from a Hawaiian endophytic fungal strain. Tetrahedron Lett. 2020, 61, 151508. [Google Scholar] [CrossRef]
- Han, M.J.; Qin, D.; Ye, T.T.; Yan, X.; Wang, J.Q.; Duan, X.X. An endophytic fungus from Trichoderma harzianum SWUKD3.1610 that produces nigranoic acid and its analogues. Nat. Prod. Res. 2019, 33, 2079–2087. [Google Scholar] [CrossRef]
- Wang, L.; Qin, D.; Zhang, K.; Huang, Q.; Liu, S.; Han, M.J.; Dong, J.Y. Metabolites from the co-culture of nigranoic acid and Umbelopsis dimorpha SWUKD3.1410, an endophytic fungus from Kadsura angustifolia. Nat. Prod. Res. 2017, 31, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Ondeyka, J.G.; Jayasuriya, H.; Herath, K.B.; Guan, Z.; Schulman, M. Steroidal and triterpenoidal fungal metabolites as ligands of liver X receptors. J. Antibiot. 2005, 58, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akihisa, T.; Watanabe, K.; Yoneima, K.; Suzuki, T.; Kimura, Y. Biotransformation of cycloartane-type triterpenes by the fungus Glomerella fusarioides. J. Nat. Prod. 2006, 69, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Berti, G.; Bottari, F.; Marsili, A.; Morelli, I.; Polvani, M.; Mandelbaum, A. 31-Norcycloartanol and cycloartanol from Polypodium vulgare. Tetrahedron Lett. 1967, 8, 125–130. [Google Scholar] [CrossRef]
- Aljubiri, S.M.; Mahgou, S.A.; Almansour, A.I.; Shaaban, M.; Shaker, K.H. Isolation of diverse bioactive compounds from Euphorbia balsamifera: Cytotoxicity and antibacterial activity studies. Saudi J. Biol. Sci. 2021, 28, 417–426. [Google Scholar] [CrossRef]
- Tavarez-Santamaría, Z.T.; Jacobo-Herrera, N.J.; Rocha-Zavaleta, L.; Zentella-Dehesa, A.; del Carmen Couder-García, B.; Martínez-Vázquez, M. A higher frequency administration of the nontoxic cycloartane-type triterpene argentatin A improved its anti-tumor activity. Molecules 2020, 25, 1780. [Google Scholar] [CrossRef] [Green Version]
- Shehla, N.; Li, B.; Cao, L.; Zhao, J.; Jian, Y.; Daniya, M. Xuetonglactones A–F: Highly oxidized lanostane and cycloartane triterpenoids from Kadsura heteroclita Roxb. Craib. Front. Chem. 2020, 7, 935. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.J.; Djerassi, C. Isolation, stereochemistry, and biosynthesis of Šormosterol, a novel cyclopropane-containing sponge sterol. Coll. Czech. Chem. Comm. 1991, 56, 1093–1105. [Google Scholar] [CrossRef]
- Sun, H.; Liu, B.; Hu, J. Novel cycloartane triterpenoid from Cimicifuga foetida (Sheng ma) induces mitochondrial apoptosis via inhibiting Raf/MEK/ERK pathway and Akt phosphorylation in human breast carcinoma MCF-7 cells. Chin. Med. 2016, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, F.; Liu, H.; Duan, H.; Chen, P.; Lu, S.J.; Yang, G.Z.; Lei, X.X. Isolation, structural elucidation of three new triterpenoids from the stems and leaves of Schisandra chinensis (Turcz) Baill. Molecules 2018, 23, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nian, Y.; Yang, J.; Liu, T.T.; Luo, Y.; Zhang, J.H.; Qiu, M.H. New anti-angiogenic leading structure discovered in the fruit of Cimicifuga yunnanensis. Scient. Rep. 2015, 5, 9026. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Pu, J.X.; Wen, J.; Li, X.N.; He, F.; Su, J.; Li, Y.; Sun, H.D. Unusual cycloartane triterpenoids from Kadsura ananosma. Phytochemistry 2015, 109, 36–42. [Google Scholar] [CrossRef]
- Kuang, H.; Su, Y.; Yang, B.; Xia, Y.; Wang, Q.; Wang, Z.; Yu, Z. Three new cycloartenol triterpenoid saponins from the roots of Cimicifuga simplex Wormsk. Molecules 2011, 16, 4348–4357. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Kant, R.; Maulik, P.R.; Sarkar, J.; Kanojiya, S.; Kumar, K.R. Cytotoxic cycloartane triterpene and rare isomeric bisclerodane diterpenes from the leaves of Polyalthia longifolia var. pendula. Bioorg. Med. Chem. Lett. 2020, 20, 5767–5771. [Google Scholar] [CrossRef]
- Wang, W.-H.; Nian, Y.; He, Y.-J.; Wan, L.-S.; Bao, N.-M.; Zhu, G.-L.; Wang, F.; Qiu, M.H. New cycloartane triterpenes from the aerial parts of Cimicifuga heracleifolia. Tetrahedron 2015, 71, 8018–8025. [Google Scholar] [CrossRef]
- Zheng, D.-J.; Zhou, J.; Liu, Q.; Yao, W.; Zhang, M.-Z.; Shao, B.-H.; Mo, J.-X.; Zhou, C.-X.; Gan, L.-S. Five new cycloartane triterpenoids from Beesia calthifolia. Fitoterapia 2015, 103, 283–288. [Google Scholar] [CrossRef]
- Wang, G.-W.; Lv, C.; Fang, X.; Tian, X.-H.; Ye, J.; Li, H.-L.; Shan, L.; Shen, Y.-H.; Zhang, W.-D. Eight pairs of epimeric triterpenoids involving a characteristic spiro-E/F ring from Abies faxoniana. J. Nat. Prod. 2015, 78, 50–60. [Google Scholar] [CrossRef]
- Gilardoni, G.; Chiriboga, X.; Vita Finzi, P.; Vidari, G. New 3,4-secocycloartane and 3,4-secodammarane triterpenes from the Ecuadorian plant Coussarea macrophylla. Chem. Biodiver. 2015, 12, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Hitotsuyanagi, Y.; Ozeki, A.; Choo, C.Y.; Chan, K.L.; Itokawa, H.; Takeya, K. Malabanones A and B, novel nortriterpenoids from Ailanthus malabarica DC. Tetrahedron 2001, 57, 7477–7480. [Google Scholar] [CrossRef]
- Thongnest, S.; Boonsombat, J.; Prawat, H.; Mahidol, C.; Ruchirawat, S. Ailanthusins A-G and nor-lupane triterpenoids from Ailanthus triphysa. Phytochemistry 2017, 134, 98–105. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Torres, O.B.; Bernardo, L.O.; Mandia, E.H.; Don, M.J.; Shen, C.C. Glabretal-type triterpenoids from Dysoxylum mollissimum. Phytochem. Lett. 2013, 6, 514–518. [Google Scholar] [CrossRef]
- Choi, A.R.; Lee, I.K.; Woo, E.E.; Kwon, J.W.; Yun, B.S.; Park, H.R. New glabretal triterpenes from the immature fruits of Poncirus trifoliata and their selective cytotoxicity. Chem. Pharm. Bull. 2015, 63, 1065–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.; Cho, K.W.; Hong, S.S.; Hwang, B.Y.; Chun, T.; Lee, D. Antiproliferative glabretal-type triterpenoids from the root bark of Dictamnus dasycarpus. Bioorg. Med. Chem. Lett. 2015, 25, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Su, B.N.; Chai, H.; Mi, Q.; Riswan, S.; Kardono, L.B.S.; Afriastini, J.J.; Santarsiero, B.D.; Mesecar, A.D.; Farnsworth, N.R.; Cordell, G.A.; et al. Activity-guided isolation of cytotoxic constituents from the bark of Aglaia crassinervia collected in Indonesia. Bioorg. Med. Chem. 2006, 14, 960–972. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, A.C.; da Paz Lima, M.; Ferreira, A.G.; Tadei, W.P.; da Silva Pinto, A.C. Constituintes quimicos do caule de Spathelia excelsa (Rutaceae) e atividade frente a Aedes aegypti. Quim. Nova 2009, 32, 2068–2072. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Fujioka, T.; Chang, J.J.; Chen, I.S.; Mihashi, K.; Lee, K.H. Anti-tumor agents. 136. Cumingianosides A-F, potent antileukemic new triterpene glucosides, and cumindysosides A and B, trisnor- and tetranortriterpene glucosides with a 14,18-cycloapoeuphane-type skeleton from Dysoxylum cumingianum. J. Org. Chem. 1992, 57, 6946–6953. [Google Scholar] [CrossRef]
- Fujioka, T.; Sakurai, A.; Mihashi, K.; Kashiwada, Y.; Chen, I.S.; Lee, K.H. Antitumor agents. 168. Dysoxylum cumingianum. IV. The structures of cumingianosides G-O, new triterpene glucosides with a 14,18-cycloapotirucallane-type skeleton from Dysoxylum cumingianum, and their cytotoxicity against human cancer cell lines. Chem. Pharm. Bull. 1997, 45, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulholl, D.A.; Nair, J.J. Glabretal triterpenoids from Dysoxylum muelleri. Phytochemistry 1996, 42, 1667–1671. [Google Scholar] [CrossRef]
- Mulholl, D.A.; Nair, J.J. Triterpenoids from Dysoxylum pettigrewianum. Phytochemistry 1994, 37, 1409–1411. [Google Scholar] [CrossRef]
- Addae-Mensah, I.; Waibel, R.; Asunka, S.A.; Oppong, I.V.; Achenbach, H. The dichapetalins—A new class of triterpenoids. Phytochemistry 1996, 43, 649–656. [Google Scholar] [CrossRef]
- Osei-Safo, D.; Chama, M.A.; Addae-Mensah, I.; Waibel, R.; Asomaning, W.A.; Oppong, I.V. Dichapetalin M from Dichapetalum madagascariensis. Phytochem. Lett. 2008, 1, 147–150. [Google Scholar] [CrossRef]
- Tuchinda, P.; Kornsakulkarn, J.; Pohmakotr, M.; Kongsaeree, P.; Prabpai, S.; Yoosook, C.; Kasisit, J.; Napaswad, C.; Sophasan, S.; Reutrakul, V. Dichapetalin-Type Triterpenoids and lignans from the aerial parts of Phyllanthus acutissima. J. Nat. Prod. 2008, 71, 655–663. [Google Scholar] [CrossRef]
- Kurimoto, S.I. Chemical Studies on Meliaceous Plants (Dysoxylum cumingianum, Azadirachta indica) and a Lamiaceous Plant (Scutellaria coleifolia). Ph.D. Thesis, University of Tokushima, Tokushima, Japan, 2014. [Google Scholar]
- Lafont, R.; Dauphin-Villemant, C.; Warren, J.T.; Rees, H.H. Ecdysteroid Chemistry and Biochemistry. In Reference Module in Life Sciences; Roitberg, B.D., Ed.; Elsevier: New York, NY, USA, 2017; pp. 125–195. [Google Scholar]
- Harmatha, J.; Budesınsky, M.; Vokac, K. Photochemical transformation of 20-hydroxyecdysone: Production of monomeric and dimeric ecdysteroid analogues. Steroids 2002, 67, 127–135. [Google Scholar] [CrossRef]
- Machida, K.; Abe, T.; Arai, D.; Okamoto, M.; Shimizu, I.; de Voogd, N.J.; Fusetani, N.; Nakao, Y. Cinanthrenol A, an estrogenic steroid containing phenanthrene nucleus, from a marine sponge Cinachyrella sp. Org. Lett. 2014, 16, 1539–1541. [Google Scholar] [CrossRef]
- Huang, S.X.; Li, R.T.; Liu, J.P.; Lu, Y.; Chang, Y. Isolation and characterization of biogenetically related highly oxygenated nortriterpenoids from Schisandra chinensis. Org. Lett. 2007, 9, 2079–2082. [Google Scholar] [CrossRef]
- Hu, K.; Li, X.R.; Tang, J.W.; Li, X.N.; Puno, P.T. Structural determination of eleven new preschisanartane-type schinortriterpenoids from two Schisandra species and structural revision of preschisanartanin J using NMR computation method. Chin. J. Nat. Med. 2019, 17, 970–981. [Google Scholar] [CrossRef]
- Toda, F.; Garratt, P. Four-membered ring compounds containing bis(methylene)-cyclobutene or tetrakis(methylene)cyclobutane moieties. Benzocyclobutadiene, benzo-dicyclobutadiene, biphenylene, and related compounds. Chem. Rev. 1992, 92, 1685–1707. [Google Scholar] [CrossRef]
- Shi, Y.M.; Wang, X.B.; Li, X.N.; Luo, X.; Shen, Z.Y. Lancolides, antiplatelet aggregation nortriterpenoids with tricyclo[6.3.0.02,11]undecane-bridged system from Schisandra lancifolia. Org. Lett. 2013, 15, 5068–5071. [Google Scholar] [CrossRef]
- Chen, J.J.; Li, Z.M.; Gao, K.; Chang, J.; Yao, X.J. Vladimuliecins A and B: Cytotoxic pentacyclic pregnanols from Vladimiria muliensis. J. Nat. Prod. 2009, 72, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bazzill, J.; Gallagher, R.J.; Subramanian, C.; Grogan, P.T.; Day, V.W.; Kindscher, K.; Cohen, M.S.; Timmermann, B.N. Antiproliferative withanolides from Datura wrightii. J. Nat. Prod. 2013, 76, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Cao, C.M.; Gallagher, R.J.; Day, V.W.; Kindscher, K.; Timmermann, B.N. Withanolides from Physalis coztomatl. Phytochemistry 2015, 109, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Ando, J.; Takagi, M.; Ikeda, T.; Yoshimitsu, A.; Nohara, T. Four novel withanolide-type steroids from the leaves of Solanum cilistum. Chem. Pharm. Bull. 2001, 49, 1440–1443. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.K.; Petrow, V.; Stuart-Webb, I.A. 133. 6β-Hydroxy-3,5-cyclopregnan-20-one and some related compounds. J. Chem. Soc. 1957, 8, 665–668. [Google Scholar] [CrossRef]
- Misico, R.I.; Nicotra, V.E.; Oberti, J.C.; Barboza, G.; Gil, R.R.; Burton, G. Withanolides and related steroids. Prog. Chem. Org. Nat. Prod. 2011, 94. [Google Scholar] [CrossRef]
- Makino, B.; Kawai, M.; Kito, K.; Yamamura, H.; Butsugan, Y. New Physalins possessing an additional carbon-carbon bond from Physalis alkekengi var. francheti. Tetrahedron 1995, 51, 12529. [Google Scholar] [CrossRef]
- Yokosuka, A.; Mimaki, Y.; Sashida, Y. Four new 3,5-cyclosteroidal saponins from Dracaena surculosa. Chem. Pharm. Bull. 2002, 50, 992–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.-R.; Huang, Y.-J.; Lu, S.-Y.; Yang, J.; Qiu, M.-H. Ganolearic acid A, a hexanorlanostane triterpenoid with a 3/5/6/5-fused tetracyclic skeleton from Ganoderma cochlear. J. Org. Chem. 2018, 83, 13178. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Fujita, M.; Nagaoka, H.; Mitome, H.; Yamada, Y. Aragusterol A: A potent antitumor marine steroid from the okinawan sponge of the genus, Xestospongia. Tetrahedron Lett. 1993, 34, 6277–6280. [Google Scholar] [CrossRef]
- Iguchi, K.; Shimura, H.; Taira, S.; Yokoo, C.; Matsumoto, K.; Yamada, Y. Aragusterol B and D, new 26,27-cyclosterols from the Okinawan marine sponge of the genus Xestospongia. J. Org. Chem. 1994, 59, 7499–7502. [Google Scholar] [CrossRef]
- Giner, J.-L.; Gunasekera, S.P.; Pomponi, S.A. Sterols of the marine sponge Petrosia weinbergi: Implications for the absolute configurations of the antiviral orthoesterols and weinbersterols. Steroids 1999, 64, 820–824. [Google Scholar] [CrossRef]
- Kokke, W.C.M.C.; Shoolery, J.N.; Fenical, W.; Djerassi, C. Biosynthetic studies of marine lipids. 4. Mechanism of side chain alkylation in E-24-propylidenecholesterol by a Chrysophyte alga. J. Org. Chem. 1984, 49, 3742–3752. [Google Scholar] [CrossRef]
- Giner, J.L.; Zimmerman, M.P.; Djerassi, C. Synthesis of (24R,28R)-and (24S, 28S)-24,28-methylene-5-stigmasten-3.beta.-ol and biosynthetic implications of cyclopropyl cleavage to 24-substituted cholesterols. J. Org. Chem. 1988, 53, 5895–5902. [Google Scholar] [CrossRef]
- Pailee, P.; Mahidol, C.; Ruchirawat, S.; Prachyawarakorn, V. Sterols from Thai marine sponge Petrosia (Strongylophora) sp. and their cytotoxicity. Mar. Drugs 2017, 15, 54. [Google Scholar] [CrossRef] [Green Version]
- Fukuoka, K.; Yamagishi, T.; Ichihara, T.; Nakaike, S.; Iguchi, K.; Yamada, Y. Mechanism of action of aragusterol a (YTA0040), a potent anti-tumor marine steroid targeting the G1 phase of the cell cycle. Int. J. Cancer 2000, 88, 810–819. [Google Scholar] [CrossRef]
- Levina, E.V.; Kalinovsky, A.I.; Andriyashchenko, P.V.; Dmitrenok, P.S.; Aminin, D.L.; Stonik, V.A. Phrygiasterol, a cytotoxic cyclopropane-containing polyhydroxysteroid, and related compounds from the Pacific starfish Hippasteria phrygiana. J. Nat. Prod. 2005, 68, 1541–1544. [Google Scholar] [CrossRef]
- Sheikh, Y.M.; Djerassi, C.; Tursch, B.M. Acansterol: A cyclopropane-containing marine sterol from Acanthaster planci. J. Chem. Soc. 1971, 2, 217–218. [Google Scholar] [CrossRef]
- Alam, M.; Martin, G.E.; Ray, S.M. Dinoflagellate sterols. 2. Isolation and structure of 4-methylgorgostanol from the dinoflagellate Glenodinium foliaceum. J. Org. Chem. 1979, 44, 4466–4467. [Google Scholar] [CrossRef]
- Withers, N.W.; Kokke, W.C.M.C.; Rohmer, M.; Fenical, W.H.; Djerassi, C. Isolation of sterols with cyclopropyl-containing side chains from the cultured marine alga Peridinium foliaceum. Tetrahedron Lett. 1979, 18, 3605–3609. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishizaka, T.; Mitsuhashi, H. Marine sterols X. Minor constituents of the sterols of the soft coral Sarcophyton glaucum. Steroids 1982, 40, 209–221. [Google Scholar] [CrossRef]
- Calabro, K.; Kalahroodi, E.L.; Rodrigues, D.; Díaz, C.; de la Cruz, M.; Cautain, B.; Laville, R.; Reyes, F.; Pérez, T.; Soussi, B.; et al. Poecillastrosides, steroidal saponins from the Mediterranean deep-sea sponge Poecillastra compressa (Bowerbank, 1866). Mar. Drugs 2017, 15, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.; Rho, J.; Cho, K.; Sim, C.J.; Shin, J. Isolation of epidioxysteroids from a sponge of the genus Tethya. Bull. Korean Chem. Soc. 1997, 18, 631–635. [Google Scholar]
- Gunasekera, S.P.; Cranick, S.; Pomponi, S.A. New sterol ester from a deep-water marine sponge, Xestospongia sp. J. Nat. Prod. 1991, 54, 1119–1122. [Google Scholar] [CrossRef]
- Ha, T.B.; Djerassi, C. Minor and trace sterols in marine invertebrates 52. isolation, structure elucidation and partial synthesis of 24-propyl-24, 28-methylenecholest-5-en-3β-ol. Tetrahedron Lett. 1985, 26, 4031–4034. [Google Scholar]
- Giner, J.-L.; Djerassi, C. Biosynthetic studies of marine lipids. 40. Generation of the cyclopropane ring of sormosterol. Acta Chem. Scand. 1992, 46, 678–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwashima, M.; Nara, K.; Iguchi, K. New marine steroids, yonarasterols, isolated from the Okinawan soft coral, Clavularia viridis. Steroids 2000, 65, 130–137. [Google Scholar] [CrossRef]
- He, H.; Kulanthaivel, P.; Baker, B.J.; Kalter, K.; Darges, J.; Cofield, D.; Wolff, L.; Adams, L. New antiproliferative and antiinflammatory 9,11-secosterols from the gorgonian Pseudopterogorgia sp. Tetrahedron 1995, 51, 51–58. [Google Scholar] [CrossRef]
- Jagodzinska, B.M.; Trimmer, J.D.; Fenical, W.; Djerassi, C. Sterols in marine invertebrates. 51. Isolation and structure elucidation of C-18 functionalized sterols from the soft coral Sinularia dissecta. J. Org. Chem. 1985, 50, 2988–2992. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Huang, C.Y.; Tseng, W.R.; Chiang, P.L.; Hwang, T.L.; Sue, J.H.; Sunge, P.J.; Dai, C.F.; Sheu, J.H. Klyflaccisteroids K–M, bioactive steroidal derivatives from a soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2017, 27, 1220–1224. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; Scalona, O.; Sodano, G.; Fattorusso, E.; Magno, S.; Mayol, L.; Santacroce, C. Metabolism in Porifera. VII. Conversion of [7,7-3H2]-fucosterol into calysterol by the sponge Calyx niceaensis. Experientia 1977, 33, 1550–1552. [Google Scholar] [CrossRef]
- Fatturosso, E.; Magno, S.; Mayol, L.; Santacrove, C.; Sioa, D. Calysterol: A C-29 cyclopropene-containing marine sterol from the sponge Calyx nicaensis. Tetrahedron 1975, 31, 1715–1716. [Google Scholar] [CrossRef]
- O’Connor, J.M.; Pu, L.; Chadha, R.K. Metallacycle annelation: Reaction of a metallacycle alpha-substituent and a vinylidene ligand to give a bicyclic metallalactone complex. J. Am. Chem. Soc. 1990, 112, 9627–9628. [Google Scholar] [CrossRef]
- Li, L.N.; Li, H.T.; Lang, R.W.; Itoh, T.; Sica, D.; Djerassi, C. Minor and trace sterols in marine invertebrates. 31. Isolation and structure elucidation of 23H-isocalysterol, a naturally occurring cyclopropene. Some comparative observations on the course of hydrogenolytic ring opening of steroidal cyclopropenes and cyclopropanes. J. Am. Chem. Soc. 1982, 104, 6726–6732. [Google Scholar]
- Jiménez, J.I.; Yoshida, W.; Scheuer, P.J.; Lobkovsky, E.; Clardy, J.; Kelly, M. Honulactones: New bishomoscalarane sesterterpenes from the Indonesian sponge Strepsichordaia aliena. J. Org. Chem. 2000, 65, 6837–6840. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.I.; Yoshida, W.Y.; Scheuer, P.J.; Kelly, M. Scalarane-based sesterterpenes from an Indonesian sponge Strepsichordaia aliena. J. Nat. Prod. 2000, 63, 1388–1392. [Google Scholar] [CrossRef]
- Holick, M.F.; Smith, E.; Pincus, S. Skin as the site of vitamin D synthesis and target tissue for 1,25-dihydroxyvitamin D3 use of calcitriol (1,25-dihydroxyvitamin D3) for treatment of psoriasis. Arch. Dermatol. 1987, 123, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Kalaras, M.D. Production of Ergocalciferol (vitamin D2) and Related Sterols in Mushrooms with Exposure to Pulsed Ultraviolet Light. Ph.D. Thesis, Pennsylvania State University, State College, PE, USA, January 2012. [Google Scholar]
- Tapavicza, E.; Meyera, A.M.; Furche, F. Unravelling the details of vitamin D photosynthesis by non-adiabatic molecular dynamics simulations. Phys. Chem. Chem. Phys. 2011, 13, 20986–20998. [Google Scholar] [CrossRef] [PubMed]
- Severino, V.G.P.; de Freitas, S.D.L.; Braga, P.A.C.; Forim, M.R.; da Silva, M.F.G.F.; Fernandes, J.B.; Vieira, P.C.; Venâncio, T. New limonoids from Hortia oreadica and unexpected coumarin from H. superba using chromatography over cleaning sephadex with sodium hypochlorite. Molecules 2014, 19, 12031–12047. [Google Scholar] [CrossRef] [Green Version]
- Mulholland, D.A.; McFarland, K.; Randrianarivelojosia, M.; Rabarison, H. Cedkathryns A and B, pentanortriterpenoids from Cedrelopsis gracilis (Ptaeroxylaceae). Phytochemistry 2004, 65, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, J.S.; Luo, J.G.; Wang, X.B.; Kong, L.Y. Velutabularins A–J, phragmalin-type limonoids with novel cyclic moiety from Chukrasia tabularis var. velutina. Tetrahedron 2011, 67, 2942–2948. [Google Scholar] [CrossRef]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxic constituents of propolis from Myanmar and their structure–activity relationship. Biol. Pharm. Bull. 2009, 32, 2075–2078. [Google Scholar] [CrossRef] [Green Version]
- Djoumessi, A.V.B.; Sandjo, L.P.; Liermann, J.C.; Schollmeyer, D.; Vincent, V.K. Donellanic acids A–C: New cyclopropanic oleanane derivatives from Donella ubanguiensis (Sapotaceae). Tetrahedron 2012, 68, 4621–4627. [Google Scholar] [CrossRef]
- Xu, W.H.; Jacob, M.R.; Agarwal, A.K.; Clark, A.M.; Liang, Z.S.; Li, X.C. Verbesinosides A–F, 15,27-cyclooleanane saponins from the American native plant Verbesina virginica. J. Nat. Prod. 2009, 72, 1022–1027. [Google Scholar] [CrossRef] [Green Version]
- Huffman, M.N. 3,5-cyclo Steroids and the Production Thereof. U.S. Patent 2860147A, 11 November 1958. [Google Scholar]
- Gibb, B.C. The Synthesis and Structural Examination of 3a,5-cyclo-5a-Androstane Steroids. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, October 1992. [Google Scholar]
- Hutfman, M.N. 3, 5-Cyclo Steroids and the Production Thereof. U.S. Patent 2860147, 31 May 1951. [Google Scholar]
- Jeger, O. Cyclosteroid Compounds and Process for Their Manufacture. U.S. Patent 3014050, 19 December 1961. [Google Scholar]
- Yates, P.; Winnik, F.M. The synthesis of bridged steroids with a bicycle [2,2,1] heptane ring A system. Can. J. Chem. 1981, 59, 1641–1650. [Google Scholar] [CrossRef]
- Templeton, J.F.; Ling, Y.; Lin, W.; Majgier-Baranowska, H.; Marat, K. 19-Hydroxy-5β,19-cyclosteroids: Synthesis, isomerization and ring opening. J. Chem. Soc. Perkin Trans. 1 1997, 21, 1895–1904. [Google Scholar] [CrossRef]
- Bartlett, P.T.; Wingrove, A.S.; Owyang, R. Cycloaddition. VII. Competitive 1, 2-and 1, 4-addition to cis-fixed cyclic dienes. J. Am. Chem. Soc. 1968, 90, 6067–6070. [Google Scholar] [CrossRef]
- Ottow, E.; Schwede, W.; Halfbrodt, W.; Fritzemeier, K.-H.; Krattenmacher, R. Progestationally Active 19,11-Bridged 4-Estrenes. U.S. Patent 5703066A, 4 June 1997. [Google Scholar]
- Akhrem, A.A.; Titov, Y.A. Chemistry of 19-norsteroids. Russ. Chem. Rev. 1964, 33, 77–91. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive cyclobutane-containing alkaloids. J. Nat. Med. 2008, 62, 1–33. [Google Scholar] [CrossRef]
- Sergeiko, A.; Poroikov, V.V.; Hanuš, L.O.; Dembitsky, V.M. Cyclobutane-containing alkaloids: Origin, synthesis, and biological activities. Open Med. Chem. J. 2008, 2, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembitsky, V.M. Chemistry and biodiversity of the biologically active natural glycosides. Chem. Biodiver. 2004, 1, 673–781. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Vil, V.A. Medicinal chemistry of stable and unsTable 1,2-dioxetanes: Origin, formation, and biological activities. Sci. Synthesis Knowl. Updates 2019, 3, 333–381. [Google Scholar]
- Dembitsky, V.M.; Dor, I.; Shkrob, I.; Aki, M. Branched alkanes and other apolar compounds produced by the cyanobacterium Microcoleus vaginatus from the Negev desert. Russ. J. Bioorg. Chem. 2001, 27, 110–119. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Naturally occurring bioactive cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomedicine 2014, 21, 1559–1581. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, N.B.; Vitousek, P.M. Fungal endophyte communities reflect environmental structuring across a Hawaiian landscape. Proc. Natl. Acad. Sci. USA 2012, 109, 13022–13027. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gao, K.; Bian, M.; Ding, H. Recent advances in the total synthesis of cyclobutane-containing natural products. Org. Chem. Front. 2020, 7, 136–154. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Řezanka, T. Metabolites produced by nitrogen fixing Nostoc species. Folia Microbiol. 2005, 50, 363–391. [Google Scholar] [CrossRef]
- Řezanka, T.; Dembitsky, V.M. Metabolites produced by cyanobacteria belonging to several species of the family Nostocaceae. Folia Microbiol. 2006, 51, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Miao, J.H.; Qin, F.Y.; Yan, Y.M.; Yang, J.; Cheng, Y.X. Belamchinanes A–D from Belamcanda chinensis: Triterpenoids with an unprecedented carbon skeleton and their activity against age-related renal fibrosis. Org. Lett. 2018, 20, 5506–5509. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Liu, J.Q.; Yan, Y.X.; Chen, J.C.; Lu, Y.; Guo, Y.H.; Qiu, M.H. Three new triterpenoids containing four-membered ring from the fruiting body of Ganoderma sinense. Org. Lett. 2010, 12, 1656–1659. [Google Scholar] [CrossRef]
- Fossen, T.; Rasoanaivo, P.; Manjovelo, C.S.; Raharinjato, H.F.; Sviatlana Yahorava, S.; Yahorau, A.; Wikberg, J.E.S. A new protolimonoid from Capuronianthus mahafalensis. Fitoterapia 2012, 83, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.; Driessen, R.A.J.; de Gelder, R.; Goubitz, K. Elucidation of the structure of solanoeclepin A, a natural hatching factor of potato and tomato cyst nematodes, by single-crystal x-ray diffraction. Croat. Chem. Acta 1999, 72, 593–606. [Google Scholar]
- Luo, J.; Tian, X.; Zhang, H.; Zhou, M.; Li, J.; Kong, L. Two rare limonoids from the stem barks of Entandrophragma utile. Tetrahedron Lett. 2016, 57, 5334–5337. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Schwikkard, S.L.; Sandor, P.; Nuzillard, J.M. Delevoyin C, a tetranortriterpenoid from Entandrophragma delevoyi. Phytochemistry 2000, 53, 465–468. [Google Scholar] [CrossRef]
- Achanta, P.S.; Gattu, R.K.; Belvotagi, A.R.V.; Akkinepally, R.R.; Rao, A.; Achanta, V.N. New malabaricane triterpenes from the oleoresin of Ailanthus malabarica. Fitoterapia 2015, 100, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.H.; Junior, J.N.S.; Guedes, M.L.S.; Braz-Filho, R.; Costa-Lotufo, L.V.; Araujo, A.J.; Silveira, E.R.; Lima, M.A.S. New terpenoids from Croton limae (Euphorbiaceae). J. Braz. Chem. Soc. 2015, 26, 1565–1572. [Google Scholar]
- Li, H.-J.; Amagata, T.; Tenny, K.; Crews, P. Additional scalarane sesterterpenes from the sponge Phyllospongia papyracea. J. Nat. Prod. 2007, 70, 802. [Google Scholar] [CrossRef]
- Jahn, T.; König, G.M.; Wright, A.D. Three new scalaranebased sesterterpenes from the tropical marine sponge Strepsichordaia lendenfeldi. J. Nat. Prod. 1999, 62, 375–377. [Google Scholar] [CrossRef]
- Hassan, M.H.A.; Rateb, M.E.; Hetta, M.; Abdelaziz, T.A.; Sleim, M.A.; Jaspars, M.; Mohammed, R. Scalarane sesterterpenes from the Egyptian Red Sea sponge Phyllospongia lamellosa. Tetrahedron 2015, 71, 577–583. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Kaisin, M.; Moussiaux, B. Ichthyotoxic sesterterpenoids from the neo guinean sponge Carteriospongia foliascens. Tetrahedron 1985, 41, 4603–4614. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Kaisin, M.; Moussiaux, B. Erratum: Ichthyotoxic sesterterpenoids from the neo guinean sponge Carteriospongia foliascens. Tetrahedron 1986, 42, 445–448. [Google Scholar]
- Braekman, J.C.; Daloze, D. Chemical defence in sponges. Pure Appl. Chem. 1986, 58, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Cao, F.; Wu, Z.H.; Shao, C.L.; Pang, S.; Liang, X.Y.; de Voogd, N.J.; Wang, C.Y. Cytotoxic scalarane sesterterpenoids from the South China Sea sponge Carteriospongia foliascens. Org. Biomol. Chem. 2015, 13, 4016–4024. [Google Scholar] [CrossRef]
- Hoffmann, P.; Kathriarachchi, H.S.; Wurdack, K.J. A phylogenetic classification of phyllanthaceae. Kew Bulletin. 2006, 61, 37–53. [Google Scholar]
- Xia, Y.; Luo, H.; Liu, J.P.; Gluud, C. Phyllanthus species versus antiviral drugs for chronic hepatitis B virus infection. Cochrane Database Syst. Rev. 2013, 4, CD009004. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.Y.; Zhang, H.; Zhou, Y.; Liu, H.B.; Tang, W.; Zhou, B.; Zuo, J.P.; Yue, J.M. Phainanoids A–F, a new class of potent immunosuppressive triterpenoids with an unprecedented carbon skeleton from Phyllanthus hainanensis. J. Am. Chem. Soc. 2015, 137, 138–141. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Gan, L.S.; Liu, H.C.; Li, H.; Xu, C.H.; Zuo, J.P.; Ding, J.; Yue, J.M. Phainanolide A, highly modified and oxygenated triterpenoid from Phyllanthus hainanensis. Org. Lett. 2017, 19, 4580–4583. [Google Scholar] [CrossRef]
- Maher, S.; Rasool, S.; Mehmood, R.; Perveen, S.; Tareen, R.B. Trichosides A and B, new withanolide glucosides from Tricholepis eburnean. Nat. Prod. Res. 2018, 32, 1–6. [Google Scholar] [CrossRef]
- Ganesh, M.; Mohankumar, M. Extraction and identification of bioactive components in Sida cordata (Burm.f.) using gas chromatography–mass spectrometry. J. Food Sci. Technol. 2017, 54, 3082–3091. [Google Scholar] [CrossRef]
- Jacobs, H.J.C. Photochemistry of conjugated trienes: Vitamin D revisited. Pure Appl. Chem. 1995, 67, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Khripacha, V.A.; Zhabinskii, V.N.; Fando, G.P.; Kuchto, A.I.; Khripacha, N.B.; Groen, M.B.; van der Louw, J.; de Groot, A. A new type of steroids with a cyclobutane fragment in the AB-ring moiety. Steroids 2006, 71, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Wammer, K.H.; Anderson, K.C.; Erickson, P.R.; Kliegman, S.; Moffatt, M.E.; Berg, S.M.; Heitzman, J.A. Environmental photochemistry of altrenogest: Photoisomerization to a bioactive product with increased environmental persistence via reversible photohydration. Environ. Sci. Technol. 2016, 50, 7480–7488. [Google Scholar] [CrossRef]
- Yan, P.; Zhou, Q.; Chen, J.; Lu, P. Controllable skeleton rearrangement of 3-substituted cyclobutanones under basic conditions. Chin. J. Chem. 2020, 38, 1103–1106. [Google Scholar] [CrossRef]
- Kamernitskii, A.V.; Ignatov, V.N.; Levina, I.S. Photochemical methods for the construction of an additional four-membered carbocycle in steroids. Russ. Chem. Rev. 1988, 57, 270–282. [Google Scholar] [CrossRef]
- Muller, E. Methoden der Organischen Chemie (Houben-Wcyl); G. Thieme Verlag: Stuttgart, Germany, 1971. [Google Scholar]
- Peet, N.P.; Johnston, J.O.; Burkhart, J.P.; Wright, C.L. A-ring bridged steroids as potent inhibitors of aromatase. J. Steroid Biochem. Mol. Biol. 1993, 44, 409–420. [Google Scholar] [CrossRef]
- Cross, A.D. Process for Conversion of 2,19-cyclo Steroids into 10 alpha-Steroids. U.S. Patent 3,139,426, 30 June 1964. [Google Scholar]
- Nagata, W.; Narisada, M.; Wakabashi, T.; Hayase, Y.; Murakami, M. Synthesis of bridged steroids. VI. B-norsteroids having a gibbane B-C-D ring system. Synthesis of 5-cyano-B-norsteroids via hydrocyanation. Chem. Pharm. Bull. 1971, 19, 1567–1581. [Google Scholar] [CrossRef] [Green Version]
- Di Chenna, P.H.; Veleiro, A.S.; Sonego, J.M.; Ceballos, N.R.; Garland, M.T.; Baggiod, R.F.; Burton, B. Synthesis of 6,19-cyclopregnanes. Constrained analogues of steroid hormones. Org. Biomol. Chem. 2007, 5, 2453–2457. [Google Scholar] [CrossRef]
- Johnston, J.O.; Wright, C.L.; Burkhart, J.P.; Peet, N.P. Biological characterization of A-ring steroids. J. Steroid Biochem. Mol. Biol. 1993, 44, 623–631. [Google Scholar] [CrossRef]
- Shoji, N.; Umeyama, A.; Shin, K.; Takeda, K.; Arihara, S.; Kobayashi, J.; Takei, M. Two unique pentacyclic steroids with cis C/D ring junction from Xestospongia bergquistia Fromont, powerful inhibitors of histamine release. J. Org. Chem. 1992, 57, 2996–2997. [Google Scholar] [CrossRef]
- Kobayashi, J.; Shinonaga, H.; Shigemori, H.; Umeyama, A.; Shoji, N.; Arihara, S. Xestobergsterol C, a new pentacyclic steroid from the Okinawan marine sponge Ircinia sp. and absolute stereochemistry of xestobergsterol A. J. Nat. Prod. 1995, 58, 312–318. [Google Scholar] [CrossRef]
- Nicotra, V.E.; Gil, R.R.; Vaccarini, C.; Oberti, J.C.; Burton, G. 15,21-Cyclowithanolides from Jaborosa bergii. J. Nat. Prod. 2003, 66, 1471–1479. [Google Scholar] [CrossRef]
- Amagata, T.; Minoura, K.; Numata, A. Gymnasterones, novel cytotoxic metabolites produced by a fungal strain from a sponge. Tetrahedron Lett. 1998, 39, 3773. [Google Scholar] [CrossRef]
- Chakravarty, A.K.; Pakrashi, S.C. Solanocastrine, a unique 16,23-cyclo-22,26-epiminocholestane from Solanum capsicastrum. Tetrahedron Lett. 1987, 28, 4753–4756. [Google Scholar] [CrossRef]
- Monteagudo, E.S.; Oberti, J.C.; Gros, E.G.; Burton, G. A spiranic withanolide from Jaborosa odonelliana. Phytochemistry 1990, 29, 933–939. [Google Scholar] [CrossRef]
- Cirigliano, A.M.; Veleiro, A.S.; Bonetto, G.M.; Oberti, J.C.; Burton, G. Spiranoid withanolides from Jaborosa runcinata and Jaborosa araucana. J. Nat. Prod. 1996, 59, 717–724. [Google Scholar] [CrossRef]
- Cirigliano, A.M.; Veleiro, A.S.; Oberti, J.C.; Burton, G. Spiranoid withanolides from Jaborosa odonelliana. J. Nat. Prod. 2002, 65, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.M.; Misico, R.I. Spiranoid withanolides from Jaborosa odonelliana and Jaborosa runcinata. Z. Nat. B Chem. Sci. 2005, 60, 867–871. [Google Scholar] [CrossRef] [Green Version]
- Guella, G.; Dini, F.; Pietra, F. Metabolites with a novel C30 backbone from marine ciliates. Angew. Chem. Int. Ed. 1999, 38, 1134–1136. [Google Scholar] [CrossRef]
- Nicolau, K.C.; Zhang, H.; Ortiz, A.; Dagneau, P. Total synthesis of the originally assigned structure of vannusal B. Angew. Chem. Int. Ed. 2008, 47, 8605–8610. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, K.C.; Zhang, H.; Ortiz, A. The true structure of the vannusals, part 1: Initial forays into suspected and intelligence gathering. Angew. Chem. Int. Ed. 2009, 48, 5642–5647. [Google Scholar] [CrossRef]
- Nicolau, K.C.; Ortiz, A.; Zhang, H. The true structures of the vannusals, part 2: Total synthesis and revised structure of vannusal B. Angew. Chem. Int. Ed. 2009, 48, 5648–5652. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, K.C.; Ortiz, A.; Zhang, H.; Dagneau, P.; Lanver, A.; Jennings, M.P.; Arseniyadis, S.; Faraoni, R.; Lizos, D.E. Total synthesis and structural revision of vannusals A and B: Synthesis of the originally assigned structure of vannusal B. J. Am. Chem. Soc. 2010, 132, 7138–7152. [Google Scholar] [CrossRef] [Green Version]
- Nicolau, K.C.; Ortiz, A.; Zhang, H.; Guella, G. Total synthesis and structural revision of vannusals A and B: Synthesis of the true structures of vannusals A and B. J. Am. Chem. Soc. 2010, 132, 7153–7176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Ren, F.-C.; Liu, J.-K. Alstonic acids A and B, unusual 2, 3-secofernane triterpenoids from Alstonia scholars. Phytochemistry 2009, 70, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Yates, P.; Douglas, S.P.; Datta, S.K.; Sawyer, J.F. Bridged-ring steroids. V. The total synthesis of 1,4-methano steroids by a modified Torgov sequence. Can. J. Chem. 1988, 66, 2268–2278. [Google Scholar] [CrossRef]
- Yates, P.; Walliser, F.M. The reaction of steroid 2, 4-dienes with acetylenes. J. Chem. 1976, 54, 3508–3516. [Google Scholar] [CrossRef]
- Hanson, J.R. Steroids: Partial synthesis in medicinal chemistry. Nat. Prod. Rep. 2010, 27, 887–899. [Google Scholar] [CrossRef]
- Douglas, S.P.; Sawyer, J.F.; Yates, P. (±)-14[b]-Hydroxy-1[b], 4[b]-methano-5[b], 8[a],9[b]-androstane-7, 17-dione. Acta Crystal. 1987, C43, 1372–1375. [Google Scholar]
- Nagata, W.; Narisada, M.; Sugasawa, T.; Wakabayashi, T. Synthesis of bridged steroids. III. Cholestane derivatives having a bridged bicycle [2.2.2] octane ring system of the atisine type. Chem. Pharm. Bull. 1968, 16, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Hanson, J.R.; Manickavasagar, R.; Thangavelu, V. The stereochemistry of oxidation of some B-norsteroids. J. Chem. Res. 1998, 4, 734–735. [Google Scholar] [CrossRef]
- Yee, S.S.; Du, L.; Risinger, A.L. Taccalonolide microtubule stabilizers. Prog. Chem. Org. Nat. Prod. 2020, 112, 183–206. [Google Scholar]
- Huang, Y.; Liu, J.-K.; Muhuhlbauer, A.; Henkel, T. Three novel taccalonolides from the tropical plant Tacca subflaellata. Helv. Chim. Acta 2002, 85, 2553–2558. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Z.; Gao, Y. Taccalonolides from Tacca plantaginea. Phytochemistry 1996, 42, 891–899. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Shen, J.-H.; Gao, Y.-S.; Wichtl, M. Five taccalonolides from Tacca plantaginea. Planta Med. 1997, 63, 40–46. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Zhao, R.-H.; Chen, C.-X.; Ni, W.; Teng, F.; Hao, X.-J.; Liu, H.-Y. Taccalonolides W-Y, three new pentacyclic steroids from Tacca plantaginea. Helv. Chim. Acta 2008, 91, 1077–1081. [Google Scholar] [CrossRef]
- Muhlbauer, A.; Seip, S.; Nowak, A.; Tran, V.S. Five novel taccalonolides from the roots of the Vietnamese plant Tacca paxiana. Helv. Chim. Acta 2003, 86, 2065. [Google Scholar] [CrossRef]
- Li, J.; Risinger, A.L.; Peng, J.; Chen, Z.; Hu, L.; Mooberry, S.L. Potent Taccalonolides, AF and AJ, inform significant structure activity relationships and tubulin as the binding site of these microtubule stabilizers. J. Am. Chem. Soc. 2011, 133, 19064–19067. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.C.; Kuo, T.H.; Damu, A.G.; Su, C.R.; Lee, E.J.; Wu, T.S.; Shu, R.; Chen, C.M.; Bastow, K.F.; Chen, T.H.; et al. Physanolide A, a novel skeleton steroid, and other cytotoxic principles from Physalis angulate. Org. Lett. 2006, 8, 2953–2956. [Google Scholar] [CrossRef]
- Lu, L.; Chen, J.; Nian, Y.; Sun, Y.; Qiu, M. Trinor-cycloartane glycosides from the rhizomes of Cimicifuga foetida. Molecules 2009, 14, 1578–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.C.; Fraser, T.R. The connection of chemical constitution and physiological action. Trans. Roy. Soc. Edinburg 1868, 25, 224–242. [Google Scholar]
- Cros, A.F.A. Action de l’Alcohol Amylique Sur l’Organisme. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 1863. [Google Scholar]
- Richet, M.C. Note sur le rapport entre la toxicité et les propriétes physiques des corps. Compt. Rend. Soc. Biol. 1893, 45, 775–776. [Google Scholar]
- Meyer, H. Zur Theorie der AIkoholnarkose. Arch. Exp. Path. Pharm. 1899, 42, 109–118. [Google Scholar] [CrossRef]
- Overton, C.E. Studien über die Narkose; Fischer: Jena, Germany, 1901. [Google Scholar]
- Hammett, L.P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 1935, 17, 125–136. [Google Scholar] [CrossRef]
- Hammett, L.P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]
- Taft, R.W. Separation of polar, steric and resonance effects in reactivity. In Steric Effects in Organic Chemistry; Newman, M.S., Ed.; Wiley: Hoboken, NJ, USA, 1956; pp. 556–675. [Google Scholar]
- Hansch, C.; Fujita, T. p-σ-π Analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Exploring QSAR; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W., Jr. Computational methods in drug discovery. Pharm. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef] [Green Version]
- Kokh, D.B.; Amaral, M.; Bomke, J.; Grädler, U.; Musil, D. Estimation of drug-target residence times by τ-random acceleration molecular dynamics simulations. J. Chem. Theor. Comput. 2018, 14, 3859–3869. [Google Scholar] [CrossRef]
- Cherkasov, A.M.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I.; Cronin, M.; Dearden, J. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef] [Green Version]
- Burov, Y.V.; Poroikov, V.V.; Korolchenko, L.V. National system for registration and biological testing of chemical compounds: Facilities for new drugs search. Bull. Natl. Center Biol. Act. Comp. 1990, 1, 4–25. [Google Scholar]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.; Filimonov, D.; Poroikov, V.; Oprea, T. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Druzhilovskiy, D.S.; Rudik, A.V. Computer-aided prediction of biological activity spectra for organic compounds: The possibilities and limitations. Russ. Chem. Bull. 2019, 68, 2143–2154. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Druzhilovskiy, D.S.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Dmitriev, A.V.; Pogodin, P.V.; Poroikov, V.V. Computer-aided prediction of biological activity spectra for chemical compounds: Opportunities and limitations. Biom. Chem. Res. Method 2018, 1, e00004. [Google Scholar] [CrossRef] [Green Version]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskiy, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Anusevicius, K.; Mickevicius, V.; Stasevych, M.; Zvarych, V.; Komarovska-Porokhnyavets, O.; Novikov, V.; Tarasova, O.; Gloriozova, T.; Poroikov, V. Design, synthesis, in vitro antimicrobial activity evaluation and computational studies of new N-(4-iodophenyl)—Alanine derivatives. Res. Chem. Intermed. 2015, 41, 7517–7540. [Google Scholar] [CrossRef]
- Druzhilovskiy, D.S.; Rudik, A.V.; Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Poroikov, V.V. Online resources for the prediction of biological activity of organic compounds. Rus. Chem. Bull. 2016, 65, 384–393. [Google Scholar] [CrossRef]
- Murtazalieva, K.A.; Druzhilovskiy, D.S.; Goel, R.K.; Sastry, G.N.; Poroikov, V.V. How good are publicly available web services that predict bioactivity profiles for drug repurposing? SAR QSAR Environ. Res. 2017, 28, 843–862. [Google Scholar] [CrossRef]
- PASS Online URL. Available online: http://www.way2drug.com/passonline/ (accessed on 29 April 2021).
- Lagunin, A.A.; Goel, R.K.; Gawande, D.Y.; Priynka, P.; Gloriozova, T.A.; Dmitriev, A.V.; Ivanov, S.M.; Rudik, A.V.; Konova, V.I.; Pogodin, P.V. Chemo- and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: A critical review. Nat. Prod. Rep. 2014, 31, 1585–1611. [Google Scholar] [CrossRef]
- Goel, R.K.; Poroikov, V.; Gawande, D.; Lagunin, A.; Randhawa, P.; Mishra, A. Revealing medicinal plants useful for comprehensive management of epilepsy and associated co-morbidities through in silico mining of their phytochemical diversity. Planta Med. 2015, 81, 495–506. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring plant isoquinoline N-oxide alkaloids: Their pharmacological and SAR activities. Phytomedicine 2015, 22, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Gawande, D.Y.; Druzhilovsky, D.; Gupta, R.C.; Poroikov, V.; Goel, R.K. Anticonvulsant activity and acute neurotoxic profile of Achyranthes aspera Linn. J. Ethnopharmacol. 2017, 202, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Pounina, T.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Sulfated and sulfur-containing steroids and their pharmacological profile. Mar. Drugs 2021, 19, 240. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Povydysh, M.; Ivkin, D.; Luzhanin, V.; Krasnova, M.; Okovityi, S.; Nosov, A.; Titova, M.; Tomilova, S.; Filimonov, D.; et al. Antihypoxic action of Panax Japonicus, Tribulus Terrestris and Dioscorea Deltoidea cell cultures: In silico and animal studies. Mol. Inform. 2020, 39, 2000093. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Winstead, A.; Yu, H.; Peng, J. Taccalonolides: A novel class of microtubule-stabilizing anticancer agents. Cancers 2021, 13, 920. [Google Scholar] [CrossRef] [PubMed]

























| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 1 | Antineoplastic (0.915) Apoptosis agonist (0.892) Antineoplastic (liver cancer) (0.822) Chemopreventive (0.776) Cytoprotectant (0.611) Prostate cancer treatment (0.557) Antimetastatic (0.528) | Anti-hypercholesterolemic (0.900) Hypolipemic (0.897) Atherosclerosis treatment (0.690) | Anti-osteoporotic (0.861) Anti-eczematic (0.850) Immunosuppressant (0.744) Antiparkinsonian, rigidity relieving (0.720) Anti-inflammatory (0.706) |
| 2 | Chemopreventive (0.968) Apoptosis agonist (0.879) Antineoplastic (0.867) Cytoprotectant (0.645) Antimetastatic (0.578) | Hypolipemic (0.874) Anti-hypercholesterolemic (0.649) Cholesterol synthesis inhibitor (0.614) Lipid metabolism regulator (0.598) Atherosclerosis treatment (0.594) | Anti-eczematic (0.889) Anti-inflammatory (0.860) Antifungal (0.821) Immunosuppressant (0.742) Anti-psoriatic (0.720) |
| 3 | Chemopreventive (0.923) Antineoplastic (0.863) Cytoprotectant (0.704) Antimetastatic (0.655) Antineoplastic (liver cancer) (0.608) Anticarcinogenic (0.553) Proliferative diseases treatment (0.551) Antineoplastic (pancreatic cancer) (0.544) | Hypolipemic (0.879) Anti-hypercholesterolemic (0.847) Cholesterol synthesis inhibitor (0.705) Atherosclerosis treatment (0.674) | Anti-eczematic (0.900) Anti-inflammatory (0.843) Antifungal (0.806) Antipruritic (0.776) Immunosuppressant (0.750) Anti-psoriatic (0.744) Anti-osteoporotic (0.716) |
| 4 | Chemopreventive (0.857) Antineoplastic (0.839) Apoptosis agonist (0.799) Cytoprotectant (0.646) Antimetastatic (0.623) Antineoplastic (pancreatic cancer) (0.514) | Hypolipemic (0.883) Anti-hypercholesterolemic (0.739) Cholesterol synthesis inhibitor (0.731) Atherosclerosis treatment (0.665) | Anti-eczematic (0.871) Anti-fungal (0.823) Anti-inflammatory (0.805) Anti-osteoporotic (0.707) Anti-psoriatic (0.683) |
| 5 | Chemopreventive (0.842) Antineoplastic (0.840) Cytoprotectant (0.680) Antimetastatic (0.647) Proliferative diseases treatment (0.555) Prostatic (benign) hyperplasia treatment (0.540) Antineoplastic (pancreatic cancer) (0.528) | Hypolipemic (0.857) Anti-hypercholesterolemic (0.788) Cholesterol synthesis inhibitor (0.697) Atherosclerosis treatment (0.663) | Anti-eczematic (0.880) Anti-inflammatory (0.808) Anti-fungal (0.781) Anti-psoriatic (0.719) |
| 6 | Chemopreventive (0.866) Antineoplastic (0.715) | Hypolipemic (0.703) Cholesterol synthesis inhibitor (0.521) | Antifungal (0.878) Anti-inflammatory (0.771) |
| 7 | Chemopreventive (0.849) Antineoplastic (0.766) | Hypolipemic (0.676) Cholesterol synthesis inhibitor (0.554) | Antifungal (0.836) Anti-inflammatory (0.737) |
| 8 | Chemopreventive (0.713) Antineoplastic (0.690) Apoptosis agonist (0.584) | Hypolipemic (0.742) Atherosclerosis treatment (0.644) Cholesterol synthesis inhibitor (0.593) | Antifungal (0.850) Anti-inflammatory (0.759) |
| 9 | Chemopreventive (0.949) Apoptosis agonist (0.822) Antineoplastic (0.801) Antimetastatic (0.558) | Hypolipemic (0.788) Cholesterol synthesis inhibitor (0.572) Atherosclerosis treatment (0.508) | Antifungal (0.884) Anti-inflammatory (0.814) |
| 10 | Chemopreventive (0.765) Antineoplastic (0.701) | Hypolipemic (0.711) Cholesterol synthesis inhibitor (0.571) | |
| 11 | Chemopreventive (0.836) Apoptosis agonist (0.763) Antineoplastic (0.755) | Hypolipemic (0.744) Cholesterol synthesis inhibitor (0.546) Atherosclerosis treatment (0.511) | Anti-eczematic (0.701) |
| 12 | Chemopreventive (0.938) Antineoplastic (0.804) Apoptosis agonist (0.623) | Hypolipemic (0.736) Atherosclerosis treatment (0.641) Cholesterol synthesis inhibitor (0.575) | Hepatoprotectant (0.900) |
| 13 | Chemopreventive (0.928) Antineoplastic (0.812) Apoptosis agonist (0.763) | Hypolipemic (0.800) Atherosclerosis treatment (0.609) Cholesterol synthesis inhibitor (0.532) | Hepatoprotectant (0.861) |
| 14 | Chemopreventive (0.956) Apoptosis agonist (0.832) Antineoplastic (0.825) | Hypolipemic (0.847) Atherosclerosis treatment (0.657) Cholesterol synthesis inhibitor (0.568) | Hepatic disorders treatment (0.898) |
| 15 | Chemopreventive (0.935) Apoptosis agonist (0.821) Antineoplastic (0.789) | Hypolipemic (0.796) Atherosclerosis treatment (0.623) Cholesterol synthesis inhibitor (0.618) | Hepatoprotectant (0.823) |
| 16 | Chemopreventive (0.944) Apoptosis agonist (0.808) Antineoplastic (0.795) Anticarcinogenic (0.628) | Hypolipemic (0.842) Cholesterol synthesis inhibitor (0.714) Atherosclerosis treatment (0.708) | Hepatoprotectant (0.872) Antifungal (0.831) Anti-inflammatory (0.823) |
| 17 | Apoptosis agonist (0.864) Antineoplastic (0.841) Chemopreventive (0.824) Antimetastatic (0.610) Antineoplastic (melanoma) (0.570) Proliferative diseases treatment (0.537) Bone diseases treatment (0.529) Antineoplastic (pancreatic cancer) (0.516) | Hypolipemic (0.816) Atherosclerosis treatment (0.665) Cholesterol synthesis inhibitor (0.579) | Anti-eczematic (0.865) Antifungal (0.819) |
| 18 | Chemopreventive (0.909) Apoptosis agonist (0.873) Antineoplastic (0.847) Antimetastatic (0.629) | Hypolipemic (0.894) Atherosclerosis treatment (0.670) Cholesterol synthesis inhibitor (0.625) Anti-hypercholesterolemic (0.622) | Hepatic disorders treatment (0.842) Antiinflammatory (0.839) Antieczematic (0.831) Antifungal (0.809) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 19 | Chemopreventive (0.858) Antineoplastic (0.815) Apoptosis agonist (0.811) Antimetastatic (0.620) | Hypolipemic (0.863) Cholesterol synthesis inhibitor (0.536) | Anti-eczematic (0.809) Anti-ulcerative (0.765) |
| 20 | Chemopreventive (0.923) Apoptosis agonist (0.847) Antineoplastic (0.837) Cytoprotectant (0.652) Antimetastatic (0.634) | Hypolipemic (0.861) Atherosclerosis treatment (0.624) Cholesterol synthesis inhibitor (0.613) | Antieczematic (0.837) Antiinflammatory (0.833) Antifungal (0.829) |
| 21 | Antineoplastic (0.894) Chemopreventive (0.851) Apoptosis agonist (0.810) Antimetastatic (0.589) Cytoprotectant (0.576) | Hypolipemic (0.867) Atherosclerosis treatment (0.512) | Anti-eczematic (0.850) Anti-inflammatory (0.755) |
| 22 | Chemopreventive (0.959) Antineoplastic (0.886) Apoptosis agonist (0.858) Cytoprotectant (0.701) Antineoplastic (liver cancer) (0.641) Antimetastatic (0.607) Proliferative diseases treatment (0.554) Prostate cancer treatment (0.510) | Hypolipemic (0.877) Atherosclerosis treatment (0.676) Anti-hypercholesterolemic (0.609) Cholesterol synthesis inhibitor (0.568) Lipid metabolism regulator (0.553) | Hepatic disorders treatment (0.921) Anti-eczematic (0.877) Anti-inflammatory (0.872) Anti-psoriatic (0.808) |
| 23 | Chemopreventive (0.967) Antineoplastic (0.884) Apoptosis agonist (0.881) Cytoprotectant (0.638) Antimetastatic (0.615) | Hypolipemic (0.881) Atherosclerosis treatment (0.654) Cholesterol synthesis inhibitor (0.568) Lipid metabolism regulator (0.544) | Anti-eczematic (0.888) Anti-inflammatory (0.827) Antifungal (0.800) Anti-psoriatic (0.739) |
| 24 | Chemopreventive (0.952) Apoptosis agonist (0.897) Antineoplastic (0.857) Cytoprotectant (0.677) Antimetastatic (0.657) Anticarcinogenic (0.561) Antineoplastic (liver cancer) (0.552) Proliferative diseases treatment (0.538) Antineoplastic (pancreatic cancer) (0.537) | Hypolipemic (0.900) Atherosclerosis treatment (0.689) Cholesterol synthesis inhibitor (0.671) Anti-hypercholesterolemic (0.662) Lipid metabolism regulator (0.529) | Anti-eczematic (0.879) Anti-psoriatic (0.709) |
| 25 | Chemopreventive (0.991) Antineoplastic (0.915) Apoptosis agonist (0.879) Anticarcinogenic (0.787) Proliferative diseases treatment (0.735) Antimetastatic (0.579) Antineoplastic (sarcoma) (0.533) | Hypolipemic (0.825) Anti-hypercholesterolemic (0.816) Atherosclerosis treatment (0.669) | Hepatoprotectant (0.987) Antifungal (0.893) Anti-inflammatory (0.882) |
| 26 | Chemopreventive (0.881) Antineoplastic (0.854) Apoptosis agonist (0.825) Antimetastatic (0.544) | Hypolipemic (0.833) Cholesterol synthesis inhibitor (0.821) Anti-hypercholesterolemic (0.791) Lipoprotein disorders treatment (0.717) | Antifungal (0.867) Anti-eczematic (0.830) Anti-inflammatory (0.804) |
| 27 | Antineoplastic (0.867) Apoptosis agonist (0.742) Chemopreventive (0.707) Cytoprotectant (0.656) Proliferative diseases treatment (0.606) Antimetastatic (0.565) Chemoprotective (0.558) Antineoplastic (pancreatic cancer) (0.544) Anticarcinogenic (0.541) | Hypolipemic (0.698) Atherosclerosis treatment (0.594) Anti-hypercholesterolemic (0.550) Cholesterol synthesis inhibitor (0.521) | Antieczematic (0.886) Hepatoprotectant (0.861) Antipsoriatic (0.714) |
| 28 | Antineoplastic (0.875) Chemopreventive (0.780) Apoptosis agonist (0.768) Proliferative diseases treatment (0.687) Cytoprotectant (0.685) Anticarcinogenic (0.639) Antimetastatic (0.590) Antineoplastic (pancreatic cancer) (0.549) | Anti-hypercholesterolemic (0.714) Hypolipemic (0.698) Antipruritic (0.639) Atherosclerosis treatment (0.582) Cholesterol synthesis inhibitor (0.576) | Hepatoprotectant (0.858) Immunosuppressant (0.751) Hepatic disorders treatment (0.686) |
| 29 | Antineoplastic (0.881) Chemopreventive (0.791) Apoptosis agonist (0.669) Proliferative diseases treatment (0.666) Anticarcinogenic (0.657) Cytoprotectant (0.627) Chemoprotective (0.565) Antimetastatic (0.559) Antineoplastic (pancreatic cancer) (0.547) | Anti-hypercholesterolemic (0.738) Hypolipemic (0.707) Cholesterol synthesis inhibitor (0.559) Atherosclerosis treatment (0.539) | Anti-eczematic (0.898) Hepatoprotectant (0.866) |
| 30 | Antineoplastic (0.814) Apoptosis agonist (0.801) Chemopreventive (0.782) Cytoprotectant (0.604) Antineoplastic (pancreatic cancer) (0.565) Antimetastatic (0.526) | Hypolipemic (0.830) Cholesterol synthesis inhibitor (0.679) Anti-hypercholesterolemic (0.618) Atherosclerosis treatment (0.546) | Anti-eczematic (0.847) Antiinflammatory (0.794) Antifungal (0.789) Immunosuppressant (0.733) Antiosteoporotic (0.727) |
| 31 | Antineoplastic (0.797) Apoptosis agonist (0.766) Chemopreventive (0.762) Cytoprotectant (0.585) Antineoplastic (pancreatic cancer) (0.559) Prostatic (benign) hyperplasia treatment (0.519) Antimetastatic (0.516) | Hypolipemic (0.742) Cholesterol synthesis inhibitor (0.583) | Anti-eczematic (0.831) Antiinflammatory (0.771) Antifungal (0.751) |
| 32 | Antineoplastic (0.803) Apoptosis agonist (0.719) Chemopreventive (0.696) Prostatic (benign) hyperplasia treatment (0.599) Antineoplastic (pancreatic cancer) (0.538) | Erythropoiesis stimulant (0.743) Diuretic (0.629) Anesthetic general (0.611) | |
| 33 | Chemopreventive (0.889) Antineoplastic (0.837) Apoptosis agonist (0.751) Cytoprotectant (0.720) Antineoplastic (pancreatic cancer) (0.563) Antineoplastic enhancer (0.558) Antimetastatic (0.543) | Hypolipemic (0.752) Anti-hypercholesterolemic (0.669) Cholesterol synthesis inhibitor (0.607) Atherosclerosis treatment (0.527) | |
| 34 | Apoptosis agonist (0.854) Antineoplastic (0.846) Chemopreventive (0.831) Cytoprotectant (0.687) Antimetastatic (0.635) Proliferative diseases treatment (0.577) Antineoplastic (pancreatic cancer) (0.559) | Hypolipemic (0.875) Anti-hypercholesterolemic (0.681) Atherosclerosis treatment (0.639) Cholesterol synthesis inhibitor (0.599) | Anti-eczematic (0.900) |
| 35 | Antineoplastic (0.816) Apoptosis agonist (0.799) Chemopreventive (0.738) Cytoprotectant (0.661) Antimetastatic (0.624) Proliferative diseases treatment (0.580) Antineoplastic (pancreatic cancer) (0.547) | Hypolipemic (0.852) Atherosclerosis treatment (0.623) Anti-hypercholesterolemic (0.594) Cholesterol synthesis inhibitor (0.592) | Anti-eczematic (0.880) |
| 36 | Antineoplastic (0.886) Chemopreventive (0.819) Apoptosis agonist (0.769) Antimetastatic (0.630) Antineoplastic (renal cancer) (0.593) Antineoplastic (lymphocytic leukemia) (0.525) Prostate cancer treatment (0.511) | Hypolipemic (0.795) | Diabetic neuropathy treatment (0.884) Antidiabetic symptomatic (0.778) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 37 | Antineoplastic (0.877) Apoptosis agonist (0.771) Antiparasitic (0.631) Chemopreventive (0.629) Antimetastatic (0.577) | Spasmolytic, urinary (0.696) | |
| 38 | Antineoplastic (0.852) Apoptosis agonist (0.785) Chemopreventive (0.665) Antimetastatic (0.578) | Spasmolytic, urinary (0.671) | |
| 39 | Antineoplastic (0.898) Chemopreventive (0.849) Apoptosis agonist (0.823) Antimetastatic (0.554) | Hypolipemic (0.581) | |
| 40 | Antineoplastic (0.785) Chemopreventive (0.715) Apoptosis agonist (0.588) | Hypolipemic (0.556) | |
| 41 | Chemopreventive (0.994) Antineoplastic (0.910) Apoptosis agonist (0.826) | Hypolipemic (0.651) | |
| 42 | Antineoplastic (0.775) Apoptosis agonist (0.716) Chemopreventive (0.626) Antimetastatic (0.583) | Alzheimer’s disease treatment (0.831) Neurodegenerative diseases treatment (0.818) Antiparkinsonian (0.556) | |
| 43 | Antineoplastic (0.842) Apoptosis agonist (0.575) Antimetastatic (0.505) | ||
| 44 | Antineoplastic (0.860) Apoptosis agonist (0.851) Chemopreventive (0.797) Antimetastatic (0.585) Antineoplastic enhancer (0.571) Antineoplastic (sarcoma) (0.548) | Hypolipemic (0.809) | |
| 45 | Antineoplastic (0.857) Chemopreventive (0.731) Apoptosis agonist (0.702) Antimetastatic (0.589) | Hypolipemic (0.787) | |
| 46 | Antineoplastic (0.921) Apoptosis agonist (0.822) Chemopreventive (0.748) Antimetastatic (0.607) Antineoplastic (renal cancer) (0.538) | Hypolipemic (0.590) Cholesterol synthesis inhibitor (0.525) | |
| 47 | Chemopreventive (0.910) Antineoplastic (0.892) Apoptosis agonist (0.887) Anticarcinogenic (0.554) Antineoplastic (sarcoma) (0.554) Antineoplastic (pancreatic cancer) (0.543) | Hypolipemic (0.626) | Antithrombotic (0.689) Alzheimer’s disease treatment (0.540) |
| 48 | Antineoplastic (0.844) Apoptosis agonist (0.814) Chemopreventive (0.790) Antimetastatic (0.602) Antineoplastic (lymphocytic leukemia) (0.524) | Hypolipemic (0.825) Cholesterol synthesis inhibitor (0.622) Atherosclerosis treatment (0.576) | Antiviral (HIV) (0.520) |
| 49 | Chemopreventive (0.967) Antineoplastic (0.906) Apoptosis agonist (0.655) | Hypolipemic (0.646) | |
| 50 | Chemopreventive (0.936) Antineoplastic (0.895) Apoptosis agonist (0.722) Anticarcinogenic (0.604) Antineoplastic (genitourinary cancer) (0.555) Antimetastatic (0.555) | Hypolipemic (0.782) | Diabetic neuropathy treatment (0.696) Antidiabetic (0.610) |
| 51 | Antineoplastic (0.848) Apoptosis agonist (0.767) Chemopreventive (0.607) Antimetastatic (0.587) | Hypolipemic (0.847) | Antiprotozoal (Plasmodium) (0.629) |
| 52 | Antineoplastic (0.820) Chemopreventive (0.735) Cytoprotectant (0.629) Apoptosis agonist (0.598) | Anti-hypercholesterolemic (0.614) Atherosclerosis treatment (0.589) Cholesterol synthesis inhibitor (0.581) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 53 | Apoptosis agonist (0.768) Antineoplastic (0.759) Chemopreventive (0.574) Antimetastatic (0.514) Antineoplastic (pancreatic cancer) (0.509) | Antiprotozoal (Plasmodium) (0.755) | |
| 54 | Apoptosis agonist (0.778) Antineoplastic (0.770) Chemopreventive (0.634) Antineoplastic (pancreatic cancer) (0.562) Antineoplastic (sarcoma) (0.555) Antimetastatic (0.547) | Hypolipemic (0.506) | Antiprotozoal (Plasmodium) (0.724) |
| 55 | Antineoplastic (0.881) Apoptosis agonist (0.692) Antimetastatic (0.602) | Hypolipemic (0.775) | Cardiotonic (0.691) |
| 56 | Antineoplastic (0.752) Apoptosis agonist (0.698) Chemopreventive (0.619) | ||
| 57 | Antineoplastic (0.752) Apoptosis agonist (0.698) | ||
| 58 | Antineoplastic (0.825) Apoptosis agonist (0.690) | ||
| 59 | Antineoplastic (0.881) Apoptosis agonist (0.728) Chemopreventive (0.709) Antineoplastic (genitourinary cancer) (0.594) Antimetastatic (0.546) Antineoplastic (sarcoma) (0.532) Antineoplastic (pancreatic cancer) (0.503) | Hypolipemic (0.805) | |
| 60 | Antineoplastic (0.804) Chemopreventive (0.700) Apoptosis agonist (0.669) Antineoplastic (sarcoma) (0.521) Antineoplastic (renal cancer) (0.512) | Hypolipemic (0.693) Lipid metabolism regulator (0.525) | Alzheimer’s disease treatment (0.571) |
| 61 | Antineoplastic (0.888) Chemopreventive (0.864) Anticarcinogenic (0.569) Antimetastatic (0.559) | Hypolipemic (0.827) | |
| 62 | Antineoplastic (0.869) Chemopreventive (0.862) Antimetastatic (0.560) Antineoplastic (sarcoma) (0.503) | Hypolipemic (0.815) Lipid metabolism regulator (0.520) | Antithrombotic (0.608) |
| 63 | Antineoplastic (0.811) Chemopreventive (0.790) Apoptosis agonist (0.774) Antineoplastic (pancreatic cancer) (0.551) Antimetastatic (0.518) | Hypolipemic (0.503) | Genital warts treatment (0.759) |
| 64 | Antineoplastic (0.837) Apoptosis agonist (0.803) Chemopreventive (0.748) Antineoplastic (myeloid leukemia) (0.704) | Hypolipemic (0.708) Lipid metabolism regulator (0.501) | Immunosuppressant (0.632) |
| 65 | Chemopreventive (0.895) Antineoplastic (0.875) Antineoplastic (myeloid leukemia) (0.677) | Hypolipemic (0.733) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 66 | Chemopreventive (0.950) Antineoplastic (0.846) Proliferative diseases treatment (0.745) Anticarcinogenic (0.743) Apoptosis agonist (0.701) Antimetastatic (0.570) Antineoplastic (myeloid leukemia) (0.557) Antineoplastic (pancreatic cancer) (0.505) | Anti-hypercholesterolemic (0.769) Hypolipemic (0.752) Lipid metabolism regulator (0.730) Atherosclerosis treatment (0.532) | Hepatoprotectant (0.912) |
| 67 | Chemopreventive (0.948) Antineoplastic (0.861) Anticarcinogenic (0.757) Apoptosis agonist (0.740) Proliferative diseases treatment (0.712) Antimetastatic (0.576) Antineoplastic (myeloid leukemia) (0.550) Antineoplastic (lymphocytic leukemia) (0.520) | Hypolipemic (0.744) Anti-hypercholesterolemic (0.650) Lipid metabolism regulator (0.649) | Hepatoprotectant (0.903) |
| 68 | Chemopreventive (0.954) Antineoplastic (0.869) Apoptosis agonist (0.803) Anticarcinogenic (0.706) | Hypolipemic (0.773) Lipid metabolism regulator (0.758) Anti-hypercholesterolemic (0.751) | Hepatoprotectant (0.866) |
| 69 | Chemopreventive (0.943) Antineoplastic (0.835) Proliferative diseases treatment (0.719) Apoptosis agonist (0.690) Anticarcinogenic (0.656) Antineoplastic (pancreatic cancer) (0.549) Antimetastatic (0.544) Antineoplastic (sarcoma) (0.505) | Anti-hypercholesterolemic (0.798) Hypolipemic (0.675) Lipid metabolism regulator (0.513) | Hepatoprotectant (0.834) |
| 70 | Chemopreventive (0.958) Antineoplastic (0.859) Apoptosis agonist (0.713) Anticarcinogenic (0.634) Proliferative diseases treatment (0.597) Antimetastatic (0.562) Antineoplastic (sarcoma) (0.535) Antineoplastic (myeloid leukemia) (0.531) | Hypolipemic (0.754) Anti-hypercholesterolemic (0.606) Lipid metabolism regulator (0.511) | Anti-eczematic (0.955) Anti-psoriatic (0.592) |
| 71 | Chemopreventive (0.974) Antineoplastic (0.844) Anticarcinogenic (0.782) Proliferative diseases treatment (0.718) Antimetastatic (0.567) Antineoplastic (myeloid leukemia) (0.560) Antineoplastic (lymphocytic leukemia) (0.540) | Hypolipemic (0.730) Lipid metabolism regulator (0.599) Anti-hypercholesterolemic (0.519) | Respiratory analeptic (0.894) |
| 72 | Chemopreventive (0.808) Antineoplastic (0.782) Apoptosis agonist (0.683) | Lipid metabolism regulator (0.662) Hypolipemic (0.652) | |
| 73 | Antineoplastic (0.789) Chemopreventive (0.787) Apoptosis agonist (0.761) Antimetastatic (0.576) Proliferative diseases treatment (0.568) Antineoplastic (myeloid leukemia) (0.552) Cytoprotectant (0.509) Anticarcinogenic (0.503) | Lipid metabolism regulator (0.843) Hypolipemic (0.798) Cholesterol synthesis inhibitor (0.635) Anti-hypercholesterolemic (0.628) | Antithrombotic (0.638) |
| 74 | Antineoplastic (0.790) Apoptosis agonist (0.736) Antineoplastic (liver cancer) (0.640) | Hypolipemic (0.597) | Genital warts treatment (0.831) |
| 75 | Antineoplastic (0.764) Chemopreventive (0.677) Antineoplastic (liver cancer) (0.571) Apoptosis agonist (0.531) | Hypolipemic (0.679) | Genital warts treatment (0.630) |
| 76 | Antineoplastic (0.688) | Hypolipemic (0.553) | Genital warts treatment (0.635) |
| 77 | Antineoplastic (0.867) Apoptosis agonist (0.820) Antineoplastic (liver cancer) (0.561) | Hypolipemic (0.590) | Genital warts treatment (0.635) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 78 | Antineoplastic (0.820) | Genital warts treatment (0.780) | |
| 79 | Antineoplastic (0.841) | Antimitotic (0.642) | |
| 80 | Antineoplastic (0.820) | Genital warts treatment (0.780) | |
| 81 | Antineoplastic (0.841) | Prostate disorders treatment (0.650) | |
| 82 | Antineoplastic (0.831) | Genital warts treatment (0.854) | |
| 83 | Antineoplastic (0.866) | Genital warts treatment (0.707) | |
| 84 | Antineoplastic (0.759) Chemopreventive (0.711) Apoptosis agonist (0.644) Cytoprotectant (0.631) Antimetastatic (0.587) | Hypolipemic (0.764) Atherosclerosis treatment (0.600) Cholesterol synthesis inhibitor (0.525) Lipid metabolism regulator (0.521) Anti-hypercholesterolemic (0.519) | |
| 85 | Antineoplastic (0.801) Chemopreventive (0.780) Apoptosis agonist (0.673) Cytoprotectant (0.621) Antimetastatic (0.597) | Hypolipemic (0.765) Cholesterol synthesis inhibitor (0.577) Lipid metabolism regulator (0.500) | Immunosuppressant (0.727) |
| 86 | Antineoplastic (0.773) Apoptosis agonist (0.687) Chemopreventive (0.609) Cytoprotectant (0.583) | Cholesterol synthesis inhibitor (0.556) Hypolipemic (0.511) | Anti-ischemic, cerebral (0.973) |
| 87 | Antineoplastic (0.825) Antineoplastic (myeloid leukemia) (0.645) Apoptosis agonist (0.573) Antineoplastic (carcinoma) (0.504) | Alzheimer’s disease treatment (0.824) Neurodegenerative diseases treatment (0.809) Psychotropic (0.700) | |
| 88 | Antineoplastic (0.889) Apoptosis agonist (0.580) Antimetastatic (0.515) | Hypolipemic (0.508) | Hepatic disorders treatment (0.931) |
| 89 | Antineoplastic (0.870) Apoptosis agonist (0.759) | Hepatic disorders treatment (0.952) Hepatoprotectant (0.514) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 90 | Antineoplastic (0.715) | Immunosuppressant (0.770) Cardiotonic (0.726) | |
| 91 | Antineoplastic (0.744) | Immunosuppressant (0.735) Cardiotonic (0.688) | |
| 92 | Antineoplastic (0.901) Apoptosis agonist (0.818) Chemopreventive (0.732) Cytostatic (0.606) Antimetastatic (0.581) Anticarcinogenic (0.546) Antineoplastic (breast cancer) (0.539) | Anti-hypercholesterolemic (0.625) Hypolipemic (0.617) | Respiratory analeptic (0.902) Antidepressant (0.776) |
| 93 | Antineoplastic (0.839) Proliferative diseases treatment (0.804) Chemopreventive (0.792) Anticarcinogenic (0.722) Apoptosis agonist (0.701) Antineoplastic (sarcoma) (0.567) Antimetastatic (0.503) | Lipoprotein disorders treatment (0.800) Anti-hypercholesterolemic (0.677) | Antidiabetic (0.902) Spasmolytic (0.705) Cardiotonic (0.682) |
| 94 | Antineoplastic (0.877) Chemopreventive (0.709) Apoptosis agonist (0.707) Antineoplastic (sarcoma) (0.673) Proliferative diseases treatment (0.630) Antineoplastic (lymphocytic leukemia) (0.560) Prostate disorders treatment (0.557) Cytostatic (0.557) Anticarcinogenic (0.556) Antineoplastic (pancreatic cancer) (0.538) Antineoplastic (breast cancer) (0.522) Antimetastatic (0.505) | Anti-hypercholesterolemic (0.862) Lipid metabolism regulator (0.549) Hypolipemic (0.532) | Respiratory analeptic (0.950) |
| 95 | Antineoplastic (0.856) Chemopreventive (0.701) Antineoplastic (sarcoma) (0.688) Proliferative diseases treatment (0.640) Apoptosis agonist (0.615) Anticarcinogenic (0.588) Cytostatic (0.584) Antineoplastic (lymphocytic leukemia) (0.569) Antineoplastic (pancreatic cancer) (0.534) Antineoplastic (renal cancer) (0.531) Antimetastatic (0.512) | Anti-hypercholesterolemic (0.806) Lipid metabolism regulator (0.539) | Respiratory analeptic (0.953) Hepatoprotectant (0.901) |
| 96 | Antineoplastic (0.937) Apoptosis agonist (0.827) Chemopreventive (0.757) Anticarcinogenic (0.741) Proliferative diseases treatment (0.718) Antineoplastic (sarcoma) (0.673) Antineoplastic (lymphocytic leukemia) (0.587) Antimetastatic (0.540) Antineoplastic (breast cancer) (0.525) Antineoplastic (pancreatic cancer) (0.524) Antineoplastic (renal cancer) (0.521) | Anti-hypercholesterolemic (0.863) Lipid metabolism regulator (0.555) | Respiratory analeptic (0.952) |
| 97 | Antineoplastic (0.801) Antineoplastic (breast cancer) (0.603) Apoptosis agonist (0.589) | Antidepressant (0.946) Mood disorders treatment (0.944) Psychotropic (0.922) | |
| 98 | Antineoplastic (0.763) Antineoplastic (genitourinary cancer) (0.537) Antimetastatic (0.514) | Antiprotozoal (0.955) Antiprotozoal (Plasmodium) (0.950) | |
| 99 | Antineoplastic (0.875) Chemopreventive (0.648) Antineoplastic (sarcoma) (0.633) Apoptosis agonist (0.630) Proliferative diseases treatment (0.566) Antimetastatic (0.523) Antineoplastic (lymphocytic leukemia) (0.512) Anticarcinogenic (0.506) | Anti-ischemic, cerebral (0.770) Immunosuppressant (0.747) | |
| 100 | Antineoplastic (0.875) Chemopreventive (0.648) Antineoplastic (sarcoma) (0.633) Apoptosis agonist (0.630) Proliferative diseases treatment (0.566) Antimetastatic (0.523) Anticarcinogenic (0.506) | Anti-ischemic, cerebral (0.770) Immunosuppressant (0.747) | |
| 101 | Antineoplastic (0.869) Anticarcinogenic (0.823) Proliferative diseases treatment (0.781) Chemopreventive (0.717) Apoptosis agonist (0.667) Antineoplastic (sarcoma) (0.560) Antimetastatic (0.516) | Anti-ischemic, cerebral (0.702) | |
| 102 | Cytoprotectant (0.758) Antineoplastic (0.720) Chemopreventive (0.591) Apoptosis agonist (0.564) | Hypolipemic (0.679) Anti-hypercholesterolemic (0.599) Atherosclerosis treatment (0.541) Cholesterol synthesis inhibitor (0.533) | Choleretic (0.733) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 103 | Antineoplastic (0.911) Apoptosis agonist (0.677) Chemopreventive (0.658) Cytoprotectant (0.630) Antineoplastic (sarcoma) (0.558) | Anti-hypercholesterolemic (0.791) Hypolipemic (0.789) | Choleretic (0.885) |
| 104 | Antineoplastic (0.822) Proliferative diseases treatment (0.668) Cytoprotectant (0.635) Chemopreventive (0.557) Apoptosis agonist (0.536) Antineoplastic (sarcoma) (0.530) Antimetastatic (0.518) | Anti-hypercholesterolemic (0.862) Hypolipemic (0.757) Cholesterol synthesis inhibitor (0.517) | Anti-ischemic, cerebral (0.952) Choleretic (0.935) |
| 105 | Antineoplastic (0.934) Proliferative diseases treatment (0.644) Prostate cancer treatment (0.585) Antineoplastic (sarcoma) (0.575) Cytoprotectant (0.544) Antineoplastic (renal cancer) (0.520) Apoptosis agonist (0.517) | Anti-hypercholesterolemic (0.828) Hypolipemic (0.709) | Choleretic (0.879) Anti-ischemic, cerebral (0.674) |
| 106 | Antineoplastic (0.839) Chemopreventive (0.697) Cytoprotectant (0.670) Proliferative diseases treatment (0.642) Apoptosis agonist (0.607) Prostatic (benign) hyperplasia treatment (0.520) Antimetastatic (0.515) Antineoplastic (renal cancer) (0.513) | Anti-hypercholesterolemic (0.850) Hypolipemic (0.728) | Choleretic (0.910) |
| 107 | Antineoplastic (0.849) Chemopreventive (0.789) Proliferative diseases treatment (0.785) Apoptosis agonist (0.750) Cytoprotectant (0.717) Anticarcinogenic (0.658) Prostate cancer treatment (0.601) Antimetastatic (0.584) Antineoplastic (sarcoma) (0.578) Antineoplastic (pancreatic cancer) (0.547) | Anti-hypercholesterolemic (0.964) Hypolipemic (0.849) Anti-hyperlipoproteinemic (0.801) Cholesterol synthesis inhibitor (0.671) Atherosclerosis treatment (0.610) | Respiratory analeptic (0.964) Choleretic (0.856) |
| 108 | Antineoplastic (0.861) Antimetastatic (0.552) | Angiogenesis inhibitor (0.928) | |
| 109 | Antineoplastic (0.821) Chemopreventive (0.743) Prostatic (benign) hyperplasia treatment (0.663) Cytoprotectant (0.660) Proliferative diseases treatment (0.648) Apoptosis agonist (0.594) Antimetastatic (0.550) Prostate cancer treatment (0.538) | Anti-hypercholesterolemic (0.923) Hypolipemic (0.732) Atherosclerosis treatment (0.643) Cholesterol synthesis inhibitor (0.640) | Respiratory analeptic (0.844) Anesthetic general (0.834) |
| 110 | Antineoplastic (0.821) Chemopreventive (0.743) Prostatic (benign) hyperplasia treatment (0.663) Cytoprotectant (0.660) Proliferative diseases treatment (0.648) Apoptosis agonist (0.594) Antimetastatic (0.550) Prostate cancer treatment (0.538) | Anti-hypercholesterolemic (0.923) Hypolipemic (0.732) Atherosclerosis treatment (0.643) Cholesterol synthesis inhibitor (0.640) | |
| 111 | Antineoplastic (0.898) Apoptosis agonist (0.586) Cytoprotectant (0.553) Antineoplastic (sarcoma) (0.516) | Hypolipemic (0.778) Anti-hypercholesterolemic (0.520) | Choleretic (0.711) Antiprotozoal (Plasmodium) (0.640) |
| 112 | Antineoplastic (0.922) Prostate disorders treatment (0.553) Proliferative diseases treatment (0.545) Antineoplastic (sarcoma) (0.536) | Hypolipemic (0.692) Anti-hypercholesterolemic (0.578) | Choleretic (0.707) |
| 113 | Antineoplastic (0.845) Chemopreventive (0.734) Cytoprotectant (0.730) Proliferative diseases treatment (0.700) Antimetastatic (0.634) Anticarcinogenic (0.607) Prostate cancer treatment (0.533) | Anti-hypercholesterolemic (0.909) Hypolipemic (0.872) Atherosclerosis treatment (0.639) Cholesterol synthesis inhibitor (0.584) | Choleretic (0.962) |
| 114 | Antineoplastic (0.832) Cytoprotectant (0.668) Proliferative diseases treatment (0.659) Chemopreventive (0.611) Antineoplastic (sarcoma) (0.555) Prostatic (benign) hyperplasia treatment (0.500) | Anti-hypercholesterolemic (0.865) Hypolipemic (0.743) Atherosclerosis treatment (0.553) Cholesterol synthesis inhibitor (0.514) | Choleretic (0.934) Respiratory analeptic (0.897) |
| 115 | Antineoplastic (0.858) Cytoprotectant (0.699) Antineoplastic (sarcoma) (0.685) Antimetastatic (0.591) Antineoplastic (renal cancer) (0.585) Prostate disorders treatment (0.578) Proliferative diseases treatment (0.554) Apoptosis agonist (0.549) Antineoplastic (pancreatic cancer) (0.531) Chemopreventive (0.522) Antineoplastic (genitourinary cancer) (0.506) | Hypolipemic (0.713) | Immunosuppressant (0.780) |
| 116 | Antineoplastic (0.682) Prostate disorders treatment (0.670) Apoptosis agonist (0.613) Chemopreventive (0.604) Cytoprotectant (0.566) Prostatic (benign) hyperplasia treatment (0.532) Antimetastatic (0.527) | Anti-hypercholesterolemic (0.836) Cholesterol synthesis inhibitor (0.587) Hypolipemic (0.563) | |
| 117 | Antineoplastic (0.706) Prostate disorders treatment (0.630) Cytoprotectant (0.600) Antimetastatic (0.555) Prostatic (benign) hyperplasia treatment (0.510) | Hypolipemic (0.587) Cholesterol synthesis inhibitor (0.509) | Immunosuppressant (0.720) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 118 | Chemopreventive (0.963) Proliferative diseases treatment (0.931) Antineoplastic (0.885) Anticarcinogenic (0.861) Apoptosis agonist (0.790) Antineoplastic (sarcoma) (0.624) Antimetastatic (0.569) Antineoplastic (liver cancer) (0.529) Antineoplastic (lymphocytic leukemia) (0.516) Antineoplastic (pancreatic cancer) (0.502) | Anti-hypercholesterolemic (0.953) Hypolipemic (0.758) Lipid metabolism regulator (0.674) Atherosclerosis treatment (0.513) | Respiratory analeptic (0.982) Hepatoprotectant (0.979) |
| 119 | Chemopreventive (0.960) Proliferative diseases treatment (0.921) Antineoplastic (0.904) Anticarcinogenic (0.851) Apoptosis agonist (0.824) Antineoplastic (sarcoma) (0.633) Antimetastatic (0.569) Prostate disorders treatment (0.548) Antineoplastic (liver cancer) (0.543) | Anti-hypercholesterolemic (0.939) Hypolipemic (0.746) Lipid metabolism regulator (0.599) | Respiratory analeptic (0.987) Hepatoprotectant (0.984) Antiprotozoal (Leishmania) (0.880) |
| 120 | Apoptosis agonist (0.975) Chemopreventive (0.916) Antineoplastic (0.845) Prostate disorders treatment (0.615) Cytoprotectant (0.611) Antimetastatic (0.543) | Atherosclerosis treatment (0.731) Hypolipemic (0.632) | Antiprotozoal (Plasmodium) (0.768) |
| 121 | Antineoplastic (0.845) Chemopreventive (0.832) Apoptosis agonist (0.822) Proliferative diseases treatment (0.818) Prostate cancer treatment (0.584) Antimetastatic (0.537) Antineoplastic (sarcoma) (0.531) | Anti-hypercholesterolemic (0.969) Hypolipemic (0.810) Lipid metabolism regulator (0.716) Cholesterol synthesis inhibitor (0.707) Atherosclerosis treatment (0.586) | Wound healing agent (0.916) Respiratory analeptic (0.902) |
| 122 | Antineoplastic (0.818) Chemopreventive (0.742) Apoptosis agonist (0.690) Prostatic (benign) hyperplasia treatment (0.660) Cytoprotectant (0.642) Proliferative diseases treatment (0.622) Antimetastatic (0.556) Prostate cancer treatment (0.541) | Anti-hypercholesterolemic (0.903) Hypolipemic (0.709) Atherosclerosis treatment (0.613) Cholesterol synthesis inhibitor (0.595) | Anesthetic general (0.884) Respiratory analeptic (0.876) |
| 123 | Chemopreventive (0.857) Antineoplastic (0.850) Apoptosis agonist (0.759) Cytoprotectant (0.723) Prostatic (benign) hyperplasia treatment (0.685) Proliferative diseases treatment (0.671) Antimetastatic (0.568) Prostate cancer treatment (0.557) Antineoplastic (pancreatic cancer) (0.530) Anticarcinogenic (0.517) Antineoplastic (breast cancer) (0.516) | Anti-hypercholesterolemic (0.961) Hypolipemic (0.755) Atherosclerosis treatment (0.690) Cholesterol synthesis inhibitor (0.652) Anti-hyperlipoproteinemic (0.607) Lipid metabolism regulator (0.572) | Respiratory analeptic (0.901) |
| 124 | Antineoplastic (0.753) Apoptosis agonist (0.677) Prostate disorders treatment (0.584) | ||
| 125 | Antineoplastic (0.791) Prostate disorders treatment (0.613) Proliferative diseases treatment (0.556) | Anti-hypercholesterolemic (0.704) Hypolipemic (0.556) Cholesterol synthesis inhibitor (0.530) | Anti-inflammatory (0.833) |
| 126 | Antineoplastic (0.697) | Anti-hypercholesterolemic (0.555) Cholesterol synthesis inhibitor (0.504) | |
| 127 | Apoptosis agonist (0.756) Antineoplastic (0.660) | Antiprotozoal (Plasmodium) (0.687) | |
| 128 | Antineoplastic (0.731) Apoptosis agonist (0.599) | Anti-hypercholesterolemic (0.571) Hypolipemic (0.546) | |
| 129 | Antineoplastic (0.824) Chemopreventive (0.726) Proliferative diseases treatment (0.657) Prostatic (benign) hyperplasia treatment (0.656) Cytoprotectant (0.654) Apoptosis agonist (0.637) Antimetastatic (0.539) Prostate cancer treatment (0.538) Antineoplastic (sarcoma) (0.537) Antineoplastic (breast cancer) (0.507) | Anti-hypercholesterolemic (0.935) Hypolipemic (0.731) Anti-hyperlipoproteinemic (0.689) Cholesterol synthesis inhibitor (0.600) | Anti-eczematic (0.961) Respiratory analeptic (0.904) |
| 130 | Antineoplastic (0.813) Chemopreventive (0.717) Proliferative diseases treatment (0.695) Cytoprotectant (0.670) Prostatic (benign) hyperplasia treatment (0.649) Antineoplastic (sarcoma) (0.628) Apoptosis agonist (0.608) Prostate cancer treatment (0.559) Anticarcinogenic (0.556) Antineoplastic (pancreatic cancer) (0.550) Antineoplastic (breast cancer) (0.528) Antimetastatic (0.524) Antineoplastic (renal cancer) (0.514) | Anti-hypercholesterolemic (0.908) Hypolipemic (0.726) Cholesterol synthesis inhibitor (0.589) Anti-hyperlipoproteinemic (0.587) | Anti-eczematic (0.960) Respiratory analeptic (0.905) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 131 | Antineoplastic (0.780) Apoptosis agonist (0.559) Antimetastatic (0.549) Antineoplastic (myeloid leukemia) (0.537) | Hypolipemic (0.577) Lipid metabolism regulator (0.567) | |
| 132 | Antineoplastic (0.780) Apoptosis agonist (0.559) Antimetastatic (0.549) Antineoplastic (myeloid leukemia) (0.537) | Hypolipemic (0.577) Lipid metabolism regulator (0.567) | |
| 133 | Antineoplastic (0.769) Apoptosis agonist (0.576) Antimetastatic (0.547) | Hypolipemic (0.660) Lipid metabolism regulator (0.604) | |
| 134 | Antineoplastic (0.769) Apoptosis agonist (0.576) Antimetastatic (0.547) | Hypolipemic (0.660) Lipid metabolism regulator (0.604) | |
| 135 | Antineoplastic (0.811) Apoptosis agonist (0.639) Chemopreventive (0.560) Antineoplastic (myeloid leukemia) (0.545) Antimetastatic (0.562) | Hypolipemic (0.629) Lipid metabolism regulator (0.544) | |
| 136 | Antineoplastic (0.795) Apoptosis agonist (0.625) Prostate disorders treatment (0.605) Antineoplastic (sarcoma) (0.574) Chemopreventive (0.573) Antineoplastic (myeloid leukemia) (0.538) | Hypolipemic (0.597) Lipid metabolism regulator (0.537) | Hepatoprotectant (0.791) |
| 137 | Antineoplastic (0.758) Chemopreventive (0.661) Prostate disorders treatment (0.654) Apoptosis agonist (0.643) Cytoprotectant (0.621) Proliferative diseases treatment (0.590) Antimetastatic (0.588) Prostatic (benign) hyperplasia treatment (0.512) | Anti-hypercholesterolemic (0.895) Hypolipemic (0.707) Cholesterol synthesis inhibitor (0.549) Atherosclerosis treatment (0.533) | Anti-eczematic (0.849) Anti-psoriatic (0.691) |
| 138 | Antineoplastic (0.758) Chemopreventive (0.661) Prostate disorders treatment (0.654) Apoptosis agonist (0.643) Cytoprotectant (0.621) Proliferative diseases treatment (0.590) Antimetastatic (0.588) | Anti-hypercholesterolemic (0.895) Cholesterol synthesis inhibitor (0.549) Atherosclerosis treatment (0.533) | Anti-eczematic (0.849) Anti-psoriatic (0.691) |
| 139 | Antineoplastic (0.809) Cytoprotectant (0.681) Chemopreventive (0.670) Apoptosis agonist (0.647) Antimetastatic (0.635) Proliferative diseases treatment (0.635) Prostate disorders treatment (0.632) Antineoplastic (pancreatic cancer) (0.509) | Anti-hypercholesterolemic (0.797) Hypolipemic (0.709) Cholesterol synthesis inhibitor (0.557) | Anti-eczematic (0.921) Anti-psoriatic (0.780) |
| 140 | Antineoplastic (0.724) Antimetastatic (0.695) Apoptosis agonist (0.626) | ||
| 141 | Antineoplastic (0.855) Apoptosis agonist (0.637) Antimetastatic (0.504) | ||
| 142 | Antineoplastic (0.688) Antineoplastic (renal cancer) (0.524) | ||
| 143 | Apoptosis agonist (0.908) Antineoplastic (0.857) Chemopreventive (0.804) Antineoplastic (liver cancer) (0.797) Proliferative diseases treatment (0.587) Prostate cancer treatment (0.507) | Hypolipemic (0.788) Atherosclerosis treatment (0.625) Cholesterol synthesis inhibitor (0.548) | Anti-eczematic (0.828) |
| 144 | Antineoplastic (0.812) Chemopreventive (0.619) Cytoprotectant (0.558) Antimetastatic (0.521) | Hypolipemic (0.701) | Anti-inflammatory (0.862) |
| 145 | Apoptosis agonist (0.870) Antineoplastic (0.824) Chemopreventive (0.647) | Hypolipemic (0.710) | Anti-inflammatory (0.801) |
| 146 | Chemopreventive (0.987) Antineoplastic (0.858) Anticarcinogenic (0.815) Apoptosis agonist (0.802) Proliferative diseases treatment (0.660) | Atherosclerosis treatment (0.640) Anti-hypercholesterolemic (0.635) Hypolipemic (0.511) | Hepatoprotectant (0.993) Wound healing agent (0.872) |
| 147 | Chemopreventive (0.980) Antineoplastic (0.852) Anticarcinogenic (0.792) Apoptosis agonist (0.787) Proliferative diseases treatment (0.631) | Atherosclerosis treatment (0.645) Anti-hypercholesterolemic (0.640) | Hepatoprotectant (0.988) Wound healing agent (0.925) |
| 148 | Chemopreventive (0.969) Antineoplastic (0.867) Apoptosis agonist (0.801) Anticarcinogenic (0.775) Proliferative diseases treatment (0.625) | Atherosclerosis treatment (0.663) Anti-hypercholesterolemic (0.611) Hypolipemic (0.539) | Hepatoprotectant (0.987) Wound healing agent (0.949) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 149 | Antineoplastic (0.891) Apoptosis agonist (0.665) | Antidepressant (0.954) Psychotropic (0.919) | |
| 150 | Antineoplastic (0.871) Apoptosis agonist (0.814) Prostate disorders treatment (0.699) Cytoprotectant (0.670) | Antidepressant (0.961) Psychotropic (0.953) | |
| 151 | Antineoplastic (0.845) | Atherosclerosis treatment (0.600) | Cardiovascular analeptic (0.828) |
| 152 | Antineoplastic (0.827) Prostate disorders treatment (0.723) Prostatic (benign) hyperplasia treatment (0.619) | Anti-hypercholesterolemic (0.642) | Anti-seborrheic (0.905) |
| 153 | Antineoplastic (0.877) Apoptosis agonist (0.611) | Anti-seborrheic (0.849) | |
| 154 | Antineoplastic (0.864) Prostate disorders treatment (0.731) Prostatic (benign) hyperplasia treatment (0.652) Prostate cancer treatment (0.564) | Anti-seborrheic (0.844) | |
| 155 | Antineoplastic (0.905) Prostate disorders treatment (0.742) Prostatic (benign) hyperplasia treatment (0.621) | Anti-seborrheic (0.823) | |
| 156 | Antineoplastic (0.791) Cytoprotectant (0.713) Proliferative diseases treatment (0.662) | Anti-hypercholesterolemic (0.881) Hypolipemic (0.735) Cholesterol synthesis inhibitor (0.641) | Anti-eczematic (0.850) |
| 157 | Antineoplastic (0.744) Prostate disorders treatment (0.677) Cytoprotectant (0.653) Prostatic (benign) hyperplasia treatment (0.589) | Anti-hypercholesterolemic (0.873) Hypolipemic (0.789) Cholesterol synthesis inhibitor (0.619) | Respiratory analeptic (0.898) |
| 158 | Antineoplastic (0.851) Apoptosis agonist (0.634) Prostate cancer treatment (0.613) Prostatic (benign) hyperplasia treatment (0.592) | Aldosterone antagonist (0.842) Anti-hyperaldosteronism (0.842) | Diuretic (0.973) Mineralocorticoid antagonist (0.956) Antihypertensive (0.802) |
| 159 | Antineoplastic (0.841) Prostatic (benign) hyperplasia treatment (0.636) Cytoprotectant (0.620) | Anti-seborrheic (0.892) | |
| 160 | Antineoplastic (0.749) Prostate disorders treatment (0.737) Prostatic (benign) hyperplasia treatment (0.603) | Anti-hypercholesterolemic (0.580) | Respiratory analeptic (0.765) Cardiovascular analeptic (0.745) |
| 161 | Antineoplastic (0.792) Prostate disorders treatment (0.742) Prostatic (benign) hyperplasia treatment (0.657) | Anti-hypercholesterolemic (0.909) Hypolipemic (0.602) | |
| 162 | Antineoplastic (0.849) Prostate disorders treatment (0.733) Prostatic (benign) hyperplasia treatment (0.665) | Anti-hypercholesterolemic (0.666) | Erythropoiesis stimulant (0.816) |
| 163 | Antineoplastic (0.849) Apoptosis agonist (0.750) Prostate disorders treatment (0.744) Prostate cancer treatment (0.601) | Anti-hypercholesterolemic (0.964) Atherosclerosis treatment (0.610) | Respiratory analeptic (0.964) Anesthetic general (0.898) |
| 164 | Antineoplastic (0.714) Cytoprotectant (0.710) Prostate disorders treatment (0.619) | Hypolipemic (0.689) Anti-hypercholesterolemic (0.625) | Respiratory analeptic (0.863) Erythropoiesis stimulant (0.784) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 165 | Antineoplastic (0.754) Chemopreventive (0.703) Cytoprotectant (0.609) Apoptosis agonist (0.602) Antineoplastic (pancreatic cancer) (0.532) Antimetastatic (0.523) Prostate disorders treatment (0.505) | Hypolipemic (0.541) | Anti-eczematic (0.905) Anti-psoriatic (0.650) |
| 166 | Antineoplastic (0.730) Chemopreventive (0.693) Cytoprotectant (0.608) Apoptosis agonist (0.572) Antimetastatic (0.517) Antineoplastic (pancreatic cancer) (0.512) | Hypolipemic (0.571) | Anti-eczematic (0.899) Anti-psoriatic (0.650) |
| 167 | Antineoplastic (0.744) Chemopreventive (0.706) Cytoprotectant (0.627) Apoptosis agonist (0.526) Antimetastatic (0.510) Antineoplastic (pancreatic cancer) (0.503) | Hypolipemic (0.515) | Anti-eczematic (0.895) Anti-psoriatic (0.656) |
| 168 | Antineoplastic (0.796) Apoptosis agonist (0.667) Cytoprotectant (0.621) Chemopreventive (0.599) | Hypolipemic (0.588) Atherosclerosis treatment (0.528) | |
| 169 | Antineoplastic (0.768) Chemopreventive (0.628) Apoptosis agonist (0.574) | Hypolipemic (0.638) | |
| 170 | Antineoplastic (0.780) Apoptosis agonist (0.675) Cytoprotectant (0.602) Chemopreventive (0.599) | Hypolipemic (0.560) | |
| 171 | Antineoplastic (0.821) Apoptosis agonist (0.740) Chemopreventive (0.726) Cytoprotectant (0.707) Proliferative diseases treatment (0.553) Prostate cancer treatment (0.551) Antineoplastic (pancreatic cancer) (0.538) | Lipid metabolism regulator (0.794) Anti-hypercholesterolemic (0.738) Hypolipemic (0.709) Cholesterol synthesis inhibitor (0.574) | Anti-secretoric (0.823) |
| 172 | Antineoplastic (0.847) Antineoplastic (myeloid leukemia) (0.624) | ||
| 173 | Antineoplastic (0.786) Apoptosis agonist (0.725) Antineoplastic (sarcoma) (0.643) Antimetastatic (0.580) Antineoplastic (renal cancer) (0.500) | Hypolipemic (0.543) | |
| 174 | Antineoplastic (0.781) Apoptosis agonist (0.722) Antineoplastic (sarcoma) (0.635) Antimetastatic (0.572) | Hypolipemic (0.534) | |
| 175 | Antineoplastic (0.897) Chemopreventive (0.718) Apoptosis agonist (0.658) Antimetastatic (0.649) Antineoplastic (renal cancer) (0.611) Prostate cancer treatment (0.595) Antineoplastic (pancreatic cancer) (0.547) | Hypolipemic (0.663) | |
| 176 | Antineoplastic (0.850) Chemopreventive (0.847) Apoptosis agonist (0.829) Cytoprotectant (0.665) Antimetastatic (0.604) Antineoplastic (pancreatic cancer) (0.539) | Hypolipemic (0.567) Cholesterol synthesis inhibitor (0.529) | Anti-inflammatory (0.902) Choleretic (0.726) |
| 177 | Antineoplastic (0.819) Apoptosis agonist (0.746) | Antiviral (Influenza) (0.647) | |
| 178 | Antineoplastic (0.820) Apoptosis agonist (0.795) Chemopreventive (0.601) Cytoprotectant (0.594) Antimetastatic (0.533) | Hypolipemic (0.592) | Anti-inflammatory (0.826) |
| 179 | Antineoplastic (0.820) Apoptosis agonist (0.795) Chemopreventive (0.601) Cytoprotectant (0.594) Antimetastatic (0.533) | Hypolipemic (0.592) | Anti-inflammatory (0.826) |
| 180 | Antineoplastic (0.853) Apoptosis agonist (0.848) Chemopreventive (0.717) Cytoprotectant (0.636) Antimetastatic (0.543) Antineoplastic (myeloid leukemia) (0.523) | Hypolipemic (0.616) | Anti-inflammatory (0.757) |
| 181 | Antineoplastic (0.853) Apoptosis agonist (0.848) Chemopreventive (0.717) Cytoprotectant (0.636) Antimetastatic (0.543) Antineoplastic (myeloid leukemia) (0.523) | Hypolipemic (0.616) | Anti-inflammatory (0.757) |
| 182 | Antineoplastic (0.772) Apoptosis agonist (0.764) Cytoprotectant (0.684) Antineoplastic (multiple myeloma) (0.631) Antineoplastic (pancreatic cancer) (0.589) Antineoplastic (carcinoma) (0.571) Antineoplastic (squamous cell carcinoma) (0.571) Antimetastatic (0.565) | Hypolipemic (0.765) | Anti-inflammatory (0.855) |
| 183 | Antineoplastic (0.774) Apoptosis agonist (0.730) Cytoprotectant (0.597) Antineoplastic (pancreatic cancer) (0.573) Antineoplastic (multiple myeloma) (0.565) Antineoplastic (carcinoma) (0.559) Antineoplastic (squamous cell carcinoma) (0.559) Antimetastatic (0.510) | Hypolipemic (0.797) Lipid metabolism regulator (0.571) | Anti-inflammatory (0.851) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 184 | Antineoplastic (0.805) Apoptosis agonist (0.787) Chemopreventive (0.603) Cytoprotectant (0.586) Antimetastatic (0.533) | Hypolipemic (0.615) Lipid metabolism regulator (0.511) Anti-hypercholesterolemic (0.503) | Anti-inflammatory (0.817) Choleretic (0.771) |
| 185 | Antineoplastic (0.805) Apoptosis agonist (0.787) Chemopreventive (0.603) Cytoprotectant (0.586) Antimetastatic (0.533) | Hypolipemic (0.615) Lipid metabolism regulator (0.511) Anti-hypercholesterolemic (0.503) | Anti-inflammatory (0.817) Choleretic (0.771) |
| 186 | Antineoplastic (0.802) Apoptosis agonist (0.782) Chemopreventive (0.636) Antimetastatic (0.547) | Hypolipemic (0.598) Anti-hypercholesterolemic (0.515) | Anti-inflammatory (0.803) Choleretic (0.706) |
| 187 | Antineoplastic (0.802) Apoptosis agonist (0.782) Chemopreventive (0.636) Antimetastatic (0.547) | Hypolipemic (0.598) Anti-hypercholesterolemic (0.515) | Anti-inflammatory (0.803) Choleretic (0.706) |
| 188 | Antineoplastic (0.866) Apoptosis agonist (0.671) | Genital warts treatment (0.744) | |
| 189 | Antineoplastic (0.863) Apoptosis agonist (0.584) | Genital warts treatment (0.736) | |
| 190 | Antineoplastic (0.846) Apoptosis agonist (0.553) | Genital warts treatment (0.745) | |
| 191 | Antineoplastic (0.850) Apoptosis agonist (0.577) | Genital warts treatment (0.675) | |
| 192 | Antineoplastic (0.847) | Genital warts treatment (0.671) | |
| 193 | Antineoplastic (0.844) | Genital warts treatment (0.664) | |
| 194 | Apoptosis agonist (0.684) | Genital warts treatment (0.707) | |
| 195 | Antineoplastic (0.845) | Genital warts treatment (0.682) | |
| 196 | Antineoplastic (0.863) | Genital warts treatment (0.736) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 197 | Antineoplastic (0.929) Prostatic (benign) hyperplasia treatment (0.663) Prostate cancer treatment (0.570) | Anti-hypercholesterolemic (0.696) Immunosuppressant (0.672) Lipid metabolism regulator (0.604) | Anti-seborrheic (0.907) |
| 198 | Antineoplastic (0.784) Apoptosis agonist (0.627) Cytoprotectant (0.558) Chemopreventive (0.542) | Anti-hypercholesterolemic (0.724) Hypolipemic (0.645) | Anesthetic (0.921) Neuroprotector (0.880) Psychostimulant (0.675) |
| 199 | Antineoplastic (0.889) Proliferative diseases treatment (0.676) Prostate disorders treatment (0.628) Cytoprotectant (0.627) Antimetastatic (0.617) Apoptosis agonist (0.614) Chemopreventive (0.606) Antineoplastic (pancreatic cancer) (0.530) | Anti-hypercholesterolemic (0.902) Hypolipemic (0.721) Cholesterol synthesis inhibitor (0.534) | Anti-eczematic (0.911) Choleretic (0.839) |
| 200 | Antineoplastic (0.801) Apoptosis agonist (0.706) Proliferative diseases treatment (0.667) Chemopreventive (0.665) Cytoprotectant (0.616) Antimetastatic (0.598) Prostatic (benign) hyperplasia treatment (0.528) | Anti-hypercholesterolemic (0.932) Hypolipemic (0.695) Cholesterol synthesis inhibitor (0.588) | Anti-eczematic (0.871) Choleretic (0.791) |
| 201 | Antineoplastic (0.865) Cytoprotectant (0.669) Antineoplastic (breast cancer) (0.662) Antineoplastic (renal cancer) (0.602) Apoptosis agonist (0.602) Antineoplastic (sarcoma) (0.588) Prostate cancer treatment (0.557) Proliferative diseases treatment (0.548) | Anti-hypercholesterolemic (0.740) Lipid metabolism regulator (0.643) Hypolipemic (0.613) | Anti-seborrheic (0.946) Anti-eczematic (0.723) |
| 202 | Antineoplastic (0.757) Prostate disorders treatment (0.652) Antineoplastic (breast cancer) (0.637) Apoptosis agonist (0.541) | Anti-seborrheic (0.841) Cardiotonic (0.654) Psychosexual dysfunction treatment (0.575) | |
| 203 | Antineoplastic (0.719) Antineoplastic (breast cancer) (0.540) | Hypolipemic (0.810) | Anti-seborrheic (0.818) Cardiotonic (0.691) |
| 204 | Antineoplastic (0.872) Antineoplastic (sarcoma) (0.683) Antineoplastic (breast cancer) (0.625) Apoptosis agonist (0.621) Antineoplastic (renal cancer) (0.605) Prostate cancer treatment (0.548) Antineoplastic (pancreatic cancer) (0.546) | Anti-hypercholesterolemic (0.616) Lipid metabolism regulator (0.565) Hypolipemic (0.546) | Anti-seborrheic (0.917) Anti-secretoric (0.908) |
| 205 | Antineoplastic (0.778) Prostate disorders treatment (0.737) Prostatic (benign) hyperplasia treatment (0.617) Cytoprotectant (0.616) Antimetastatic (0.571) Proliferative diseases treatment (0.527) | Anti-hypercholesterolemic (0.638) Hypolipemic (0.542) Cholesterol synthesis inhibitor (0.535) | Anti-eczematic (0.831) Anti-osteoporotic (0.799) |
| 206 | Antineoplastic (0.908) Prostate disorders treatment (0.703) Antineoplastic (breast cancer) (0.635) Antineoplastic (renal cancer) (0.596) Antineoplastic (sarcoma) (0.567) Prostate cancer treatment (0.553) Apoptosis agonist (0.536) | Anti-seborrheic (0.884) Anti-osteoporotic (0.848) | |
| 207 | Antineoplastic (0.785) Prostate disorders treatment (0.758) Prostatic (benign) hyperplasia treatment (0.673) Cytoprotectant (0.656) Antineoplastic (sarcoma) (0.568) Antimetastatic (0.565) Apoptosis agonist (0.563) Proliferative diseases treatment (0.540) Antineoplastic (pancreatic cancer) (0.520) Antineoplastic (breast cancer) (0.518) | Anti-hypercholesterolemic (0.813) Hypolipemic (0.648) Cholesterol synthesis inhibitor (0.578) | Anesthetic general (0.901) Choleretic (0.725) |
| 208 | Antineoplastic (0.832) Prostate disorders treatment (0.740) Apoptosis agonist (0.711) Cytoprotectant (0.697) Chemopreventive (0.677) Proliferative diseases treatment (0.651) Prostate cancer treatment (0.613) Antineoplastic (breast cancer) (0.608) Antineoplastic (renal cancer) (0.552) Antineoplastic (pancreatic cancer) (0.525) | Anti-hypercholesterolemic (0.886) Lipid metabolism regulator (0.837) Hypolipemic (0.709) Cholesterol synthesis inhibitor (0.605) Atherosclerosis treatment (0.523) | Respiratory analeptic (0.969) Neuroprotector (0.924) Psychostimulant (0.707) |
| 209 | Antineoplastic (0.839) Chemopreventive (0.781) Apoptosis agonist (0.722) Proliferative diseases treatment (0.714) Cytoprotectant (0.654) Prostate disorders treatment (0.636) Antimetastatic (0.591) | Anti-hypercholesterolemic (0.782) Hypolipemic (0.702) Cholesterol synthesis inhibitor (0.604) | Respiratory analeptic (0.949) |
| 210 | Antineoplastic (0.878) Prostate disorders treatment (0.807) Prostate cancer treatment (0.721) Antineoplastic (sarcoma) (0.719) Antineoplastic (breast cancer) (0.701) Cytoprotectant (0.631) Apoptosis agonist (0.599) | Anti-hypercholesterolemic (0.538) | Cardiovascular analeptic (0.862) |
| 211 | Antineoplastic (0.845) Prostate disorders treatment (0.648) Antineoplastic (myeloid leukemia) (0.645) Antineoplastic (sarcoma) (0.626) Cytoprotectant (0.585) Antineoplastic (breast cancer) (0.580) Antineoplastic (renal cancer) (0.561) Antineoplastic (carcinoma) (0.521) Antineoplastic (squamous cell carcinoma) (0.521) | Hypolipemic (0.929) Lipoprotein disorders treatment (0.687) | Anti-seborrheic (0.902) |
| 212 | Antineoplastic (0.804) Cytoprotectant (0.719) Chemopreventive (0.678) Proliferative diseases treatment (0.622) Prostate disorders treatment (0.614) Antimetastatic (0.596) | Anti-hypercholesterolemic (0.832) Hypolipemic (0.820) Cholesterol synthesis inhibitor (0.627) | Anesthetic general (0.931) Respiratory analeptic (0.888) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 213 | Antineoplastic (0.891) | Male reproductive disfunction treatment (0.923) Aromatase inhibitor (0.717) | |
| 214 | Antineoplastic (0.909) Prostatic (benign) hyperplasia treatment (0.663) Prostate cancer treatment (0.570) | Anti-hypercholesterolemic (0.696) Lipid metabolism regulator (0.604) | Anti-seborrheic (0.914) Respiratory analeptic (0.756) |
| 215 | Antineoplastic (0.860) Prostate disorders treatment (0.717) Prostatic (benign) hyperplasia treatment (0.621) | Ovulation inhibitor (0.794) Neuroprotector (0.716) | |
| 216 | Antineoplastic (0.805) Prostatic (benign) hyperplasia treatment (0.591) | Hepatic disorders treatment (0.601) Anti-hypercholesterolemic (0.589) | Respiratory analeptic (0.871) Anti-inflammatory (0.837) |
| 217 | Antineoplastic (0.805) Prostatic (benign) hyperplasia treatment (0.591) | Anti-hypercholesterolemic (0.592) | Respiratory analeptic (0.874) Anti-inflammatory (0.839) |
| 218 | Antineoplastic (0.736) Prostate disorders treatment (0.589) | Anti-hypercholesterolemic (0.582) Atherosclerosis treatment (0.534) | Anti-seborrheic (0.915) Alopecia treatment (0.893) |
| 219 | Antineoplastic (0.750) Prostate disorders treatment (0.713) Prostatic (benign) hyperplasia treatment (0.501) | Anti-seborrheic (0.917) Anti-osteoporotic (0.904) | |
| 220 | Antineoplastic (0.786) Apoptosis agonist (0.567) | Anti-seborrheic (0.924) Anti-osteoporotic (0.752) | |
| 221 | Antineoplastic (0.854) Proliferative diseases treatment (0.588) Antimetastatic (0.552) | Hypolipemic (0.832) Anti-hypercholesterolemic (0.635) Cholesterol synthesis inhibitor (0.612) | Anti-eczematic (0.814) Anti-osteoporotic (0.657) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 222 | Antineoplastic (0.783) Prostate disorders treatment (0.679) Cytoprotectant (0.622) Apoptosis agonist (0.607) Antineoplastic (sarcoma) (0.603) Prostatic (benign) hyperplasia treatment (0.519) Antimetastatic (0.514) Antineoplastic (pancreatic cancer) (0.509) | Hypolipemic (0.551) | Anti-inflammatory (0.778) |
| 223 | Antineoplastic (0.813) Apoptosis agonist (0.683) Prostate disorders treatment (0.654) Antineoplastic (sarcoma) (0.593) Antineoplastic (pancreatic cancer) (0.541) | Anti-inflammatory (0.775) Antiprotozoal (Plasmodium) (0.622) | |
| 224 | Antineoplastic (0.787) Prostate disorders treatment (0.685) Apoptosis agonist (0.629) Antineoplastic (sarcoma) (0.589) Prostatic (benign) hyperplasia treatment (0.550) Antineoplastic (pancreatic cancer) (0.506) | Anti-inflammatory (0.829) Antiprotozoal (Plasmodium) (0.625) | |
| 225 | Antineoplastic (0.931) Apoptosis agonist (0.899) Antineoplastic enhancer (0.537) Cytostatic (0.519) Antineoplastic (genitourinary cancer) (0.512) | Cardiotonic (0.763) Immunosuppressant (0.683) | |
| 226 | Apoptosis agonist (0.876) Antineoplastic (0.873) Antineoplastic (genitourinary cancer) (0.530) | Inflammatory Bowel disease treatment (0.704) Immunosuppressant (0.681) | |
| 227 | Antineoplastic (0.885) Apoptosis agonist (0.824) Antineoplastic (genitourinary cancer) (0.550) Antimetastatic (0.513) | Cardiotonic (0.698) | |
| 228 | Antineoplastic (0.878) Apoptosis agonist (0.861) Chemopreventive (0.717) Proliferative diseases treatment (0.581) | Anti-hypercholesterolemic (0.808) Hypolipemic (0.788) Atherosclerosis treatment (0.534) | Immunosuppressant (0.813) |
| 229 | Antineoplastic (0.668) | Respiratory analeptic (0.874) | |
| 230 | Antineoplastic (0.735) Apoptosis agonist (0.545) | Anti-inflammatory (0.604) | |
| 231 | Antineoplastic (0.846) Cytostatic (0.771) Apoptosis agonist (0.613) Antineoplastic (sarcoma) (0.526) | Hepatic disorders treatment (0.977) Macular degeneration treatment (0.882) | |
| 232 | Antineoplastic (0.788) Apoptosis agonist (0.645) | Hepatic disorders treatment (0.937) Antiprotozoal (Plasmodium) (0.820) | |
| 233 | Antineoplastic (0.709) Apoptosis agonist (0.632) | Anti-eczematic (0.636) | |
| 234 | Antineoplastic (0.840) Apoptosis agonist (0.749) | Cardiotonic (0.572) | |
| 235 | Antineoplastic (0.840) Apoptosis agonist (0.749) | Anti-inflammatory (0.637) | |
| 236 | Apoptosis agonist (0.814) Antineoplastic (0.647) Cytoprotectant (0.613) Chemopreventive (0.564) Prostate disorders treatment (0.564) | Anti-hypercholesterolemic (0.578) Hypolipemic (0.546) Cholesterol synthesis inhibitor (0.534) | Anti-inflammatory (0.716) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 237 | Antineoplastic (0.761) Prostate disorders treatment (0.755) Prostatic (benign) hyperplasia treatment (0.683) | Anti-hypercholesterolemic (0.829) Hypolipemic (0.756) Atherosclerosis treatment (0.632) | Anesthetic general (0.901) Respiratory analeptic (0.884) |
| 238 | Antineoplastic (0.830) Prostatic (benign) hyperplasia treatment (0.532) | Antiprotozoal (0.781) Cardiotonic (0.773) | |
| 239 | Antineoplastic (0.820) Prostate disorders treatment (0.784) Prostatic (benign) hyperplasia treatment (0.684) Prostate cancer treatment (0.627) | Cardiovascular analeptic (0.913) | |
| 240 | Antineoplastic (0.910) Apoptosis agonist (0.765) Cytoprotectant (0.593) Prostate cancer treatment (0.538) | Cardiovascular analeptic (0.888) | |
| 241 | Antineoplastic (0.765) Prostatic (benign) hyperplasia treatment (0.653) Cytoprotectant (0.641) | Anti-hypercholesterolemic (0.824) Hypolipemic (0.686) Atherosclerosis treatment (0.629) | Anti-eczematic (0.862) Anti-osteoporotic (0.826) Antiparkinsonian, rigidity relieving (0.625) |
| 242 | Antineoplastic (0.803) Prostatic (benign) hyperplasia treatment (0.617) Prostate cancer treatment (0.518) | Neurodegenerative diseases treatment (0.642) | Anti-osteoporotic (0.972) Anti-psoriatic (0.884) |
| 243 | Antineoplastic (0.797) Prostate disorders treatment (0.680) Prostatic (benign) hyperplasia treatment (0.551) | Alzheimer’s disease treatment (0.750) | Anti-osteoporotic (0.965) Anti-seborrheic (0.891) Anti-psoriatic (0.864) |
| 244 | Antineoplastic (0.775) Prostate disorders treatment (0.706) Cytoprotectant (0.638) Prostatic (benign) hyperplasia treatment (0.624) Apoptosis agonist (0.620) | Anti-hypercholesterolemic (0.772) Hypolipemic (0.617) | Anti-eczematic (0.846) Anti-osteoporotic (0.787) |
| 245 | Antineoplastic (0.777) Cytoprotectant (0.689) Prostate disorders treatment (0.677) Prostatic (benign) hyperplasia treatment (0.581) | Anti-hypercholesterolemic (0.866) Hypolipemic (0.705) Cholesterol synthesis inhibitor (0.588) | Anti-eczematic (0.840) Anti-osteoporotic (0.792) |
| 246 | Antineoplastic (0.918) Aromatase inhibitor (0.903) Apoptosis agonist (0.894) Prostate disorders treatment (0.699) Prostatic (benign) hyperplasia treatment (0.597) Cytoprotectant (0.597) | Anti-hypercholesterolemic (0.674) Hypolipemic (0.622) | Anti-eczematic (0.907) |
| 247 | Antineoplastic (0.943) Prostate disorders treatment (0.705) Prostatic (benign) hyperplasia treatment (0.601) Apoptosis agonist (0.596) | Neuroprotector (0.734) Immunosuppressant (0.650) | |
| 248 | Antineoplastic (0.937) Aromatase inhibitor (0.903) Prostate disorders treatment (0.697) Prostatic (benign) hyperplasia treatment (0.591) | Neuroprotector (0.735) Immunosuppressant (0.654) | |
| 249 | Antineoplastic (0.902) Prostate disorders treatment (0.740) Prostatic (benign) hyperplasia treatment (0.662) Prostate cancer treatment (0.569) | Cardiovascular analeptic (0.854) Anesthetic (0.698) Cardiotonic (0.605) | |
| 250 | Antineoplastic (0.892) Apoptosis agonist (0.710) Prostate disorders treatment (0.662) Prostatic (benign) hyperplasia treatment (0.541) | Anti-osteoporotic (0.972) | |
| 251 | Antineoplastic (0.742) Prostate disorders treatment (0.726) Prostatic (benign) hyperplasia treatment (0.662) | Anti-hypercholesterolemic (0.622) | Neuroprotector (0.734) Immunosuppressant (0.705) |
| 252 | Antineoplastic (0.769) Prostate disorders treatment (0.753) Prostatic (benign) hyperplasia treatment (0.663) | Anticonvulsant (0.733) Neuroprotector (0.727) | |
| 253 | Antineoplastic (0.810) Prostate disorders treatment (0.726) Prostatic (benign) hyperplasia treatment (0.647) | Anti-hypercholesterolemic (0.705) | Immunosuppressant (0.764) Neuroprotector (0.749) |
| 254 | Antineoplastic (0.754) | Antiprotozoal (Plasmodium) (0.648) | |
| 255 | Antineoplastic (0.774) Cytoprotectant (0.633) Prostate disorders treatment (0.572) | Hypolipemic (0.766) Anti-hypercholesterolemic (0.652) Cholesterol synthesis inhibitor (0.615) | |
| 256 | Antineoplastic (0.858) Proliferative diseases treatment (0.604) Apoptosis agonist (0.583) Cytoprotectant (0.561) Antimetastatic (0.549) Prostate disorders treatment (0.535) | Hypolipemic (0.838) Anti-hypercholesterolemic (0.611) Cholesterol synthesis inhibitor (0.601) | |
| 257 | Antineoplastic (0.694) Prostate disorders treatment (0.621) Antineoplastic (breast cancer) (0.572) | Anti-seborrheic (0.928) Cardiovascular analeptic (0.674) | |
| 258 | Antineoplastic (0.854) Prostatic (benign) hyperplasia treatment (0.621) | Anti-hypercholesterolemic (0.682) | Neuroprotector (0.756) Acute neurologic disorders treatment (0.741) |
| 259 | Antineoplastic (0.845) Apoptosis agonist (0.654) Prostatic (benign) hyperplasia treatment (0.585) | Hypolipemic (0.548) | Cardiotonic (0.917) Antiarrhythmic (0.809) |
| 260 | Antineoplastic (0.823) Prostate disorders treatment (0.746) Prostatic (benign) hyperplasia treatment (0.615) | Anesthetic general (0.841) | |
| 261 | Antineoplastic (0.715) Prostate disorders treatment (0.701) Prostatic (benign) hyperplasia treatment (0.619) | Anesthetic general (0.712) | |
| 262 | Antineoplastic (0.834) | Anti-hypercholesterolemic (0.794) | Anesthetic general (0.805) |
| 263 | Antineoplastic (0.796) Apoptosis agonist (0.723) Prostate disorders treatment (0.676) | Anti-hypercholesterolemic (0.527) | Anti-osteoporotic (0.934) Anti-seborrheic (0.918) |
| 264 | Antineoplastic (0.757) Prostate disorders treatment (0.658) Apoptosis agonist (0.550) Prostatic (benign) hyperplasia treatment (0.503) | Spasmolytic, urinary (0.961) |
| No. | Antitumor & Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 265 | Antineoplastic (0.933) Apoptosis agonist (0.667) | Antimitotic (0.843) | |
| 266 | Antineoplastic (0.942) Apoptosis agonist (0.619) Antineoplastic (sarcoma) (0.510) | Antimitotic (0.848) | |
| 267 | Antineoplastic (0.934) Apoptosis agonist (0.890) Cytostatic (0.688) Antineoplastic (sarcoma) (0.647) T cell inhibitor (0.608) Prostate disorders treatment (0.606) Antineoplastic (pancreatic cancer) (0.573) | Antimitotic (0.829) Antiprotozoal (Plasmodium) (0.650) | |
| 268 | Antineoplastic (0.936) Apoptosis agonist (0.720) Antimetastatic (0.515) Antineoplastic (pancreatic cancer) (0.504) | Antimitotic (0.849) | |
| 269 | Antineoplastic (0.922) Apoptosis agonist (0.641) Antimetastatic (0.515) | Antimitotic (0.819) Antiprotozoal (Plasmodium) (0.694) | |
| 270 | Antineoplastic (0.929) Apoptosis agonist (0.669) Antineoplastic (renal cancer) (0.570) | Antimitotic (0.853) | |
| 271 | Antineoplastic (0.930) Apoptosis agonist (0.753) Cytostatic (0.735) Antineoplastic (renal cancer) (0.603) Antineoplastic (sarcoma) (0.602) Antineoplastic (pancreatic cancer) (0.551) Antineoplastic (lymphocytic leukemia) (0.548) Antineoplastic (myeloid leukemia) (0.529) Antineoplastic (genitourinary cancer) (0.523) | Antimitotic (0.776) Immunosuppressant (0.665) | |
| 272 | Antineoplastic (0.933) Apoptosis agonist (0.805) Antimetastatic (0.535) Antineoplastic (pancreatic cancer) (0.524) | Antimitotic (0.808) Immunosuppressant (0.745) | |
| 273 | Antineoplastic (0.934) Apoptosis agonist (0.805) Antineoplastic (sarcoma) (0.530) Antineoplastic (pancreatic cancer) (0.524) | Antimitotic (0.804) Immunosuppressant (0.731) Antiprotozoal (Plasmodium) (0.668) | |
| 274 | Antineoplastic (0.875) Apoptosis agonist (0.728) Chemopreventive (0.693) Prostate disorders treatment (0.670) Proliferative diseases treatment (0.659) Anticarcinogenic (0.630) Antineoplastic (breast cancer) (0.551) Antineoplastic (pancreatic cancer) (0.540) Prostatic (benign) hyperplasia treatment (0.526) Antineoplastic (sarcoma) (0.517) | Anti-hypercholesterolemic (0.858) Hypolipemic (0.767) Cholesterol synthesis inhibitor (0.608) Atherosclerosis treatment (0.600) Lipid metabolism regulator (0.590) | Anti-ischemic, cerebral (0.932) Antiprotozoal (Leishmania) (0.559) |
| 275 | Chemopreventive (0.966) Apoptosis agonist (0.896) Antineoplastic (0.866) | Hypolipemic (0.575) | |
| 276 | Chemopreventive (0.958) Apoptosis agonist (0.842) T cell inhibitor (0.620) | Hypolipemic (0.540) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Antitumor Profile of Carbon-Bridged Steroids (CBS) and Triterpenoids. Mar. Drugs 2021, 19, 324. https://doi.org/10.3390/md19060324
Dembitsky VM, Gloriozova TA, Poroikov VV. Antitumor Profile of Carbon-Bridged Steroids (CBS) and Triterpenoids. Marine Drugs. 2021; 19(6):324. https://doi.org/10.3390/md19060324
Chicago/Turabian StyleDembitsky, Valery M., Tatyana A. Gloriozova, and Vladimir V. Poroikov. 2021. "Antitumor Profile of Carbon-Bridged Steroids (CBS) and Triterpenoids" Marine Drugs 19, no. 6: 324. https://doi.org/10.3390/md19060324
APA StyleDembitsky, V. M., Gloriozova, T. A., & Poroikov, V. V. (2021). Antitumor Profile of Carbon-Bridged Steroids (CBS) and Triterpenoids. Marine Drugs, 19(6), 324. https://doi.org/10.3390/md19060324