Sonneradon A Extends Lifespan of Caenorhabditis elegans by Modulating Mitochondrial and IIS Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Capacity of SDA in Wild-Type Worms
2.2. SDA Delay of the Progression of Aging-Related Diseases in C. elegans Models of Alzheimer’s Disease (AD) and Upregulation of the Innate Immune Response
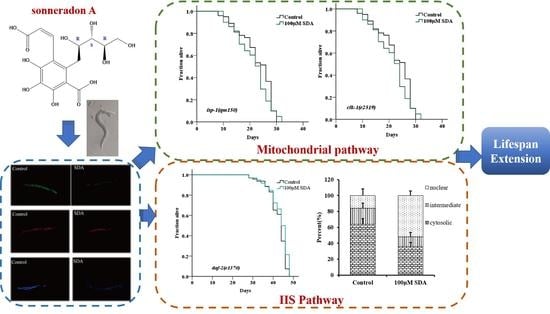
2.3. Potential of SDA to Extend C. elegans’ Lifespan through Insulin/Insulin-like Growth Factor-1 Signaling Pathways
3. Discussion
3.1. SDA Reduces Free Radical Production and Enhances Nematode Immunity
3.2. SDA Prolongs the Lifespan of C. elegans by Regulating Mitochondrial and IIS Signaling Pathways, but Not DR
3.3. Key Points of Experimental Operation
4. Materials and Methods
4.1. Reagents and C. elegans Strains
4.2. C. elegans Culture
4.3. Lifespan Assays
4.4. Assay for Paraquat-Induced Oxidative Stress
4.5. Measurement of Reactive Oxygen Species Production
4.6. Lipid-Staining Assay by Nile Red
4.7. Autofluorescence Assay
4.8. Paralysis Assays
4.9. Pseudomonas Aeruginosa Infection Assay
4.10. Reproduction
4.11. Intracellular Localization of DAF-16::GFP
4.12. RNA Extraction and Quantitative Polymerase Chain Reaction
4.13. Western Blot
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros, A.G.D.A.; Liu, J.; Lemieux, G.A.; Mullaney, B.C.; Ashrafi, K. Analyses of C. elegans Fat Metabolic Pathways. Methods Cell Biol. 2012, 107, 383–407. [Google Scholar] [CrossRef]
- Fei, T.; Fei, J.; Huang, F.; Xie, T.; Xu, J.; Zhou, Y.; Yang, P. The anti-aging and anti-oxidation effects of tea water extract in Caenorhabditis elegans. Exp. Gerontol. 2017, 97, 89–96. [Google Scholar] [CrossRef]
- Senchuk, M.M.; Dues, D.J.; Van Raamsdonk, J.M. Measuring Oxidative Stress in Caenorhabditis elegans: Paraquat and Juglone Sensitivity Assays. Bio-Protocol 2017, 7, e2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, X.; Jiang, S.; Qin, M.; Liu, K.; Cao, P.; Chen, S.; Deng, J.; Gao, C. Compounds from the fruits of mangrove Sonneratia apetala: Isolation, molecular docking and antiaging effects using a Caenorhabditis elegans model. Bioorganic Chem. 2020, 99, 103813. [Google Scholar] [CrossRef]
- Liu, J.; Luo, D.; Wu, Y.; Gao, C.; Lin, G.; Chen, J.; Wu, X.; Zhang, Q.; Cai, J.; Su, Z. The Protective Effect of Sonneratia apetala Fruit Extract on Acetaminophen-Induced Liver Injury in Mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 6919834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, S.K.; Thatoi, H. Phytochemical Profiling and Bioactivity of A Mangrove Plant, Sonneratia apetala, from Odisha Coast of India. Chin. J. Integr. Med. 2015, 21, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.J.; Basar, M.H.; Rokeya, B.; Arif, K.M.T.; Sultana, M.S.; Rahman, M.H. Evaluation of antioxidant, antidiabetic and antibacterial activities of the fruit of Sonneratia apetala (Buch.-Ham.). Orient. Pharm. Exp. Med. 2013, 13, 95–102. [Google Scholar] [CrossRef]
- Srivastava, S.; Sonkar, R.; Mishra, S.K.; Tiwari, A.; Balramnavar, V.; Mir, S.; Bhatia, G.; Saxena, A.K.; Lakshmi, V. Erratum to: Antidyslipidemic and Antioxidant Effects of Novel Lupeol-Derived Chalcones. Lipids 2013, 48, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Link, P.; Roth, K.; Sporer, F.; Wink, M. Carlina acaulis Exhibits Antioxidant Activity and Counteracts Abeta Toxicity in Caenorhabditis elegans. Molecules 2016, 21, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, W.; Tang, Y.; Lin, C.; Cao, Y.; Chen, Y. Mechanism of pentagalloyl glucose in alleviating fat accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2019, 67, 14110–14120. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Komura, T.; Yamanaka, M.; Nishimura, K.; Hara, K.; Nishikawa, Y. Autofluorescence as a noninvasive biomarker of senescence and advanced glycation end products in Caenorhabditis elegans. NPJ Aging Mech. Dis. 2021, 7, 12. [Google Scholar] [CrossRef]
- Teuscher, A.C.; Ewald, C.Y. Caenorhabditis elegansOvercoming Autofluorescence to Assess GFP Expression During Normal Physiology and Aging in Caenorhabditis elegans. Bio Protoc. 2018, 8, e2940. [Google Scholar] [CrossRef] [Green Version]
- Shintani, T.; Sakoguchi, H.; Yoshihara, A.; Izumori, K.; Sato, M. d-Allulose, a stereoisomer of d-fructose, extends Caenorhabditis elegans lifespan through a dietary restriction mechanism: A new candidate dietary restriction mimetic. Biochem. Biophys. Res. Commun. 2017, 493, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Tepper, R.G.; Ashraf, J.; Kaletsky, R.; Kleemann, G.; Murphy, C.T.; Bussemaker, H.J. PQM-1 Complements DAF-16 as a Key Transcriptional Regulator of DAF-2-Mediated Development and Longevity. Cell 2013, 154, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Matsunami, K. Frailty and Caenorhabditis elegans as a Benchtop Animal Model for Screening Drugs Including Natural Herbs. Front. Nutr. 2018, 5, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhi, L.; Yu, Y.; Li, X.; Wang, D. Molecular Control of Innate Immune Response to Pseudomonas aeruginosa Infection by Intestinal let-7 in Caenorhabditis elegans. PLoS Pathog. 2017, 13, e1006152. [Google Scholar] [CrossRef]
- Gerstbrein, B.; Stamatas, G.; Kollias, N.; Driscoll, M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell 2005, 4, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Coburn, C.; Allman, E.; Mahanti, P.; Benedetto, A.; Cabreiro, F.; Pincus, Z.; Matthijssens, F.; Araiz, C.; Mandel, A.; Vlachos, M.; et al. Anthranilate Fluorescence Marks a Calcium-Propagated Necrotic Wave That Promotes Organismal Death in C. elegans. PLoS Biol. 2013, 11, e1001613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sforzini, S.; Moore, M.N.; Mou, Z.; Boeri, M.; Banni, M.; Viarengo, A. Mode of action of Cr(VI) in immunocytes of earthworms: Implications for animal health. Ecotoxicol. Environ. Saf. 2017, 138, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Viña, J. The free radical theory of frailty: Mechanisms and opportunities for interventions to promote successful aging. Free Radic. Biol. Med. 2019, 134, 690–694. [Google Scholar] [CrossRef]
- Kritsiligkou, P.; Rand, J.D.; Weids, A.J.; Wang, X.; Kershaw, C.; Grant, C.M. Endoplasmic reticulum (ER) stress–induced reactive oxygen species (ROS) are detrimental for the fitness of a thioredoxin reductase mutant. J. Biol. Chem. 2018, 293, 11984–11995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandakumar, R.; Tschismarov, R.; Meissner, F.; Prabakaran, T.; Krissanaprasit, A.; Farahani, E.; Zhang, B.C.; Assil, S.; Martin, A.; Bertrams, W.; et al. Intracellular bacteria engage a STING-TBK1-MVB12b pathway to enable paracrine cGAS-STING signaling. Nat. Microbiol. 2019, 4, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, W.-B.; Zhang, D.-D.; Cao, X.-F.; Shi, H.-J.; Li, X.-F. Interactions between dietary carbohydrate and metformin: Implications on energy sensing, insulin signaling pathway, glycolipid metabolism and glucose tolerance in blunt snout bream Megalobrama amblycephala. Aquaculture 2018, 483, 183–195. [Google Scholar] [CrossRef]
- Jia, K.; Levine, B. Autophagy is Required for Dietary Restriction-Mediated Life Span Extension in C. elegans. Autophagy 2007, 3, 597–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelino, S.; Chang, J.T.; Kumsta, C.; She, X.; Davis, A.; Nguyen, C.; Panowski, S.; Hansen, M. Intestinal Autophagy Improves Healthspan and Longevity in C. elegans during Dietary Restriction. PLoS Genet. 2016, 12, e1006135. [Google Scholar]
- Aparicio, R.; Hansen, M.; Walker, D.W.; Kumsta, C. The selective autophagy receptor SQSTM1/p62 improves lifespan and proteostasis in an evolutionarily conserved manner. Autophagy 2020, 16, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Galimov, E.R.; Gems, D. Shorter life and reduced fecundity can increase colony fitness in virtual Caenorhabditis elegans. Aging Cell 2020, 19, e13141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Qu, Y.; Zhou, X.-G.; Chen, J.-N.; Luo, H.-R.; Wu, G.-S. A Dihydroflavonoid Naringin Extends the Lifespan of C. elegans and Delays the Progression of Aging-Related Diseases in PD/AD Models via DAF-16. Oxidative Med. Cell. Longev. 2020, 2020, 6069354. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Kwon, E.-S.; Conte, D.; Liu, H.; Gilchrist, M.J.; MacNeil, L.T.; Tissenbaum, H.A. Transcriptional regulation of Caenorhabditis elegans FOXO/DAF-16 modulates lifespan. Longev. Healthspan 2014, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Ewald, C.Y.; Marfil, V.; Li, C. Alzheimer-related protein APL -1 modulates lifespan through heterochronic gene regulation in Caenorhabditis elegans. Aging Cell 2016, 15, 1051–1062. [Google Scholar] [CrossRef] [Green Version]
- Dhondt, I.; Petyuk, V.A.; Cai, H.; Vandemeulebroucke, L.; Vierstraete, A.; Smith, R.D.; Depuydt, G.; Braeckman, B.P. FOXO/DAF-16 Activation Slows Down Turnover of the Majority of Proteins in C. elegans. Cell Rep. 2016, 16, 3028–3040. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Murphy, C.T.; Kenyon, C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009, 10, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Gerke, P.; Keshet, A.; Mertenskötter, A.; Paul, R.J. The JNK-Like MAPK KGB-1 of Caenorhabditis elegans Promotes Reproduction, Lifespan, and Gene Expressions for Protein Biosynthesis and Germline Homeostasis but Interferes with Hyperosmotic Stress Tolerance. Cell. Physiol. Biochem. 2014, 34, 1951–1973. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, B.-K.; Park, S.; Park, S.-K. Phosphatidylcholine Extends Lifespan via DAF-16 and Reduces Amyloid-Beta-Induced Toxicity in Caenorhabditis elegans. Oxidative Med. Cell. Longev. 2019, 2019, 2860642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.-K.; Kim, S.-A.; Baek, S.-M.; Lee, E.Y.; Lee, E.S.; Chung, C.H.; Ahn, C.M.; Park, S.-K. Cur2004-8, a synthetic curcumin derivative, extends lifespan and modulates age-related physiological changes in Caenorhabditis elegans. Drug Discov. Ther. 2019, 13, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Choe, K. Characterization of skn-1/wdr-23 phenotypes in Caenorhabditis elegans; pleiotrophy, aging, glutathione, and interactions with other longevity pathways. Mech. Ageing Dev. 2015, 149, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zheng, B.; Li, T.; Liu, R.H. Raspberry extract ameliorates oxidative stress in Caenorhabditis elegans via the SKN-1/Nrf2 pathway. J. Funct. Foods 2020, 70, 103977. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; Brenkman, A.B. Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing Res. Rev. 2013, 12, 918–930. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Stuhr, N.L.; Curran, S.P. Bacterial diets differentially alter lifespan and healthspan trajectories in C. elegans. Commun. Biol. 2020, 3, 653. [Google Scholar] [CrossRef]
- Zheng, S.; Liao, S.; Zou, Y.; Qu, Z.; Shen, W.; Shi, Y. Mulberry leaf polyphenols delay aging and regulate fat metabolism via the germline signaling pathway in Caenorhabditis elegans. Age 2014, 36, 9719. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Li, J.; Rao, Y.; Wang, W.; Fu, Y. A Chinese Herbal Formula, Gengnianchun, Ameliorates beta-Amyloid Peptide Toxicity in a Caenorhabditis elegans Model of Alzheimer’s Disease. Evid.-Based Complementary Altern. Med. 2017, 2017, 7480980. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, S.; Pandey, R. 5′-Hydroxy-6, 7, 8, 3′, 4′-pentamethoxyflavone extends longevity mediated by DR-induced autophagy and oxidative stress resistance in C. elegans. Geroscience 2021, 43, 759–772. [Google Scholar] [CrossRef]
- Ogawa, T.; Kodera, Y.; Hirata, D.; Blackwell, T.K.; Mizunuma, M. Natural thioallyl compounds increase oxidative stress resistance and lifespan in Caenorhabditis elegans by modulating SKN-1/Nrf. Sci. Rep. 2016, 6, 21611. [Google Scholar] [CrossRef] [Green Version]
- Fischer, N.; Büchter, C.; Koch, K.; Albert, S.; Csuk, R.; Wätjen, W. The resveratrol derivatives trans-3,5-dimethoxy-4-fluoro-4′-hydroxystilbene and trans-2,4′,5-trihydroxystilbene decrease oxidative stress and prolong lifespan in Caenorhabditis elegans. J. Pharm. Pharmacol. 2016, 69, 73–81. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, X.; Xing, T.; Ding, A.; Wu, G.; Luo, H. Chlorogenic Acid Extends the Lifespan of Caenorhabditis elegans via Insulin/IGF-1 Signaling Pathway. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2017, 72, 464–472. [Google Scholar]
- Rebolledo, D.L.; Aldunate, R.; Kohn, R.; Neira, I.; Minniti, A.N.; Inestrosa, N.C. Copper reduces Abeta oligomeric species and ameliorates neuromuscular synaptic defects in a C. elegans model of inclusion body myositis. J. Neurosci. 2011, 31, 10149–10158. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Jiang, C.-P.; Cao, P.; Liu, Y.-H.; Gao, C.-H.; Yi, X.-X. Sonneradon A Extends Lifespan of Caenorhabditis elegans by Modulating Mitochondrial and IIS Signaling Pathways. Mar. Drugs 2022, 20, 59. https://doi.org/10.3390/md20010059
Jiang S, Jiang C-P, Cao P, Liu Y-H, Gao C-H, Yi X-X. Sonneradon A Extends Lifespan of Caenorhabditis elegans by Modulating Mitochondrial and IIS Signaling Pathways. Marine Drugs. 2022; 20(1):59. https://doi.org/10.3390/md20010059
Chicago/Turabian StyleJiang, Shu, Cui-Ping Jiang, Pei Cao, Yong-Hong Liu, Cheng-Hai Gao, and Xiang-Xi Yi. 2022. "Sonneradon A Extends Lifespan of Caenorhabditis elegans by Modulating Mitochondrial and IIS Signaling Pathways" Marine Drugs 20, no. 1: 59. https://doi.org/10.3390/md20010059
APA StyleJiang, S., Jiang, C. -P., Cao, P., Liu, Y. -H., Gao, C. -H., & Yi, X. -X. (2022). Sonneradon A Extends Lifespan of Caenorhabditis elegans by Modulating Mitochondrial and IIS Signaling Pathways. Marine Drugs, 20(1), 59. https://doi.org/10.3390/md20010059