Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-Destructive Extraction
Abstract
:1. Introduction
2. Marine Algae—A Vital Source of Lectins
3. Challenges in Algal Lectin Extraction
4. Lectin Production from Algal Cell Suspension
5. Recent Advances in Algal Lectin Isolation
6. Non-Destructive Extracellular Lectin Production in Bioreactors
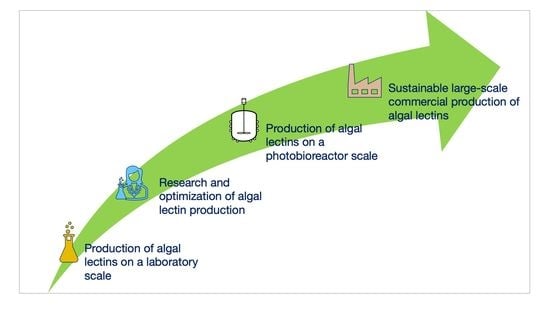
7. Prospects for Algal Lectin Production
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rorrer, G.L.; Cheney, D.P. Bioprocess Engineering of Cell and Tissue Cultures for Marine Seaweeds. Aquac. Eng. 2004, 32, 11–41. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Current Knowledge and Challenges in Extraction, Characterization and Bioactivity of Seaweed Protein and Seaweed-Derived Proteins. Adv. Bot. Res. 2020, 95, 289–326. [Google Scholar]
- Lam, S.; Ng, T. Lectins: Production and Practical Applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsaneva, M.; Van Damme, E.J.M. 130 Years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef]
- Boyd, W.C.; Almodovar, L.R.; Boyd, L.G. Agglutinins in Marine Algae for Human Erythrocytes. Transfusion 1966, 6, 82–83. [Google Scholar] [CrossRef]
- Molchanova, V.; Chernikov, O.; Chikalovets, I.; Lukyanov, P. Purification and Partial Characterization of the Lectin from the Marine Red Alga Tichocarpus Crinitus. Rupr. Bot. Mar. 2010, 53, 69–78. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [Green Version]
- Terme, N.; Hardouin, K.; Cortès, H.P.; Peñuela, A.; Freile-Pelegrín, Y.; Robledo, D.; Bedoux, G.; Bourgougnon, N. Chapter 10—Emerging Seaweed Extraction Techniques: Enzyme-Assisted Extraction a Key Step of Seaweed Biorefinery ? In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–256. [Google Scholar]
- Puspita, M.; Deniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Bedoux, G.; Bourgougnon, N. Antioxidant and Antibacterial Activity of Solid-Liquid and Enzyme-Assisted Extraction of Phenolic Compound from Three Species of Tropical Sargassum. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012057. [Google Scholar] [CrossRef]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef] [PubMed]
- Djabayan-Djibeyan, P.; Carpenter, B.; Medina-Ramírez, G.; Andueza-Leal, F.; León-Leal, A.; Djabayan-Russo, A.; Jaramillo-Abril, D.; Valarezo-García, C.; Araujo-Baptista, L. Cold Steeping Infusion, a Novel Lectin Extraction Technique for the Isolation, Purification and Partial Characterization of Lectins from the Green Venezuelan Marine Alga Caulerpa serrulata. Nat. Prod. Commun. 2018, 13, 1715–1719. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Chojnacka, K. Algae as Production Systems of Bioactive Compounds. Eng. Life Sci. 2014, 15, 160–176. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine Macroalgae as Sources of Protein and Bioactive Compounds in Feed for Monogastric Animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential Application of Microalga Spirulina platensis as a Protein Source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Silva, P. A Concise Review of the Red Macroalgae Chondracanthus teedei (Mertens Ex Roth) Kützing and Chondracanthus teedei var. lusitanicus (J.E. De Mesquita Rodrigues) Bárbara & Cremades. J. Appl. Phycol. 2021, 33, 111–131. [Google Scholar]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in Common Norwegian Seaweeds and Evaluation of Their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Marsham, S.; Scott, G.W.; Tobin, M.L. Comparison of Nutritive Chemistry of a Range of Temperate Seaweeds. Food Chem. 2007, 100, 1331–1336. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of Different Extractive Procedures for Proteins from the Edible Seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Masarin, F.; Cedeno, F.R.P.; Chavez, E.G.S.; de Oliveira, L.E.; Gelli, V.C.; Monti, R. Chemical Analysis and Biorefinery of Red Algae Kappaphycus alvarezii for Efficient Production of Glucose from Residue of Carrageenan Extraction Process. Biotechnol. Biofuels 2016, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Admassu, H.; Abera, T.; Abraha, B.; Yang, R.; Zhao, W. Proximate, Mineral and Amino Acid Composition of Dried Laver (Porphyra Spp.) Seaweed. J. Acad. Ind. Res. 2018, 6, 149. [Google Scholar]
- Barbarino, E.; Lourenço, S.O. An Evaluation of Methods for Extraction and Quantification of Protein from Marine Macro- and Microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Praseptiangga, D. Algal Lectins and Their Potential Uses. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2015, 10, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.; Dwek, M.; Schumacher, U. Functional and Molecular Glycobiology; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Ludwig, A.-K.; Kaltner, H.; Kopitz, J.; Gabius, H.-J. Lectinology 4.0: Altering Modular (Ga) Lectin Display for Functional Analysis and Biomedical Applications. Biochim. Biophys. Acta. Gen. Subj. 2019, 1863, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.S. Chapter One—Lectin Biosensors in Cancer Glycan Biomarker Detection. Adv. Clin. Chem. 2019, 93, 1–61. [Google Scholar]
- Stillmark, H. Über Ricin ein Giftiges Ferment aus den Samen von Ricinus communis L. und Einigen Anderen Euphorbiaceen. Inaugural Dissertation, University of Dorpat, Dorpat, Estonia, 1888. [Google Scholar]
- Singh, R.S.; Thakur, S.R.; Bansa, P. Algal Lectins as Promising Biomolecules for Biomedical Research. Crit. Rev. Microbiol. 2015, 41, 77–88. [Google Scholar] [CrossRef]
- Teixeira, E.H.; Arruda, F.V.S.; do Nascimento, K.S.; Carneiro, V.A.; Nagano, C.S.; da Silva, B.R.; Sampaio, A.H.; Cavada, B.S. Biological Applications of Plants and Algae Lectins: An Overview. In Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology; IntechOpen: London, UK, 2012. [Google Scholar]
- Catanzaro, E.; Calcabrini, C.; Bishayee, A.; Fimognari, C. Antitumor Potential of Marine and Freshwater Lectins. Mar. Drugs 2020, 18, 11. [Google Scholar] [CrossRef] [Green Version]
- Chaves, R.P.; da Silva, S.R.; Neto, L.G.N.; Carneiro, R.F.; da Silva, A.L.C.; Sampaio, A.H.; de Sousa, B.L.; Cabral, M.G.; Videira, P.A.; Teixeira, E.H.; et al. Structural Characterization of Two Isolectins from the Marine Red Alga Solieria filiformis (Kützing) P.W. Gabrielson and Their Anticancer Effect on MCF-7 Breast Cancer Cells. Int. J. Biol. Macromol. 2018, 107, 1320–1329. [Google Scholar] [CrossRef]
- Sato, Y.; Morimoto, K.; Hirayama, M.; Hori, K. High Mannose-Specific Lectin (KAA-2) from the Red Alga Kappaphycus alvarezii Potently Inhibits Influenza Virus Infection in a Strain-Independent Manner. Biochem. Biophys. Res. Commun. 2011, 405, 291–296. [Google Scholar] [CrossRef]
- Sugahara, T.; Ohama, Y.; Fukuda, A.; Hayashi, M.; Kawakubo, A.; Kato, K. The Cytotoxic Effect of Eucheuma serra Agglutinin (ESA) on Cancer Cells and Its Application to Molecular Probe for Drug Delivery System Using Lipid Vesicles. Cytotechnology 2001, 36, 93–99. [Google Scholar] [CrossRef]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.J.; Shirakura, M. Antiviral Lectins from Red and Blue-Green Algae Show Potent in Vitro and in Vivo Activity against Hepatitis C Virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.A.; Ramessar, K.; O’Keefe, B.R. Antiviral Lectins: Selective Inhibitors of Viral Entry. Antiviral Res. 2017, 142, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B. Isolation and Characterization of Griffithsin, a Novel HIV-Inactivating Protein from the Red Alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, J.; Hirayama, M.; Sato, Y.; Morimoto, K.; Hori, K. A Novel High-Mannose Specific Lectin from the Green Alga Halimeda renschii Exhibits a Potent Anti-Influenza Virus Activity through High-Affinity Binding to the Viral Hemagglutinin. Mar. Drugs 2017, 15, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, K.; Matsuda, H.; Miyazawa, K.; Ito, K. A Mitogenic Agglutinin from the Red Alga Carpopeltis flabellata. Phytochemistry 1987, 26, 1335–1338. [Google Scholar] [CrossRef]
- Barre, A.; Van Damme, E.J.; Simplicien, M.; Le Poder, S.; Klonjkowski, B.; Benoist, H.; Peyrade, D.; Rougé, P. Man-Specific Lectins from Plants, Fungi, Algae and Cyanobacteria, as Potential Blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) Coronaviruses: Biomedical Perspectives. Cells 2021, 10, 1619. [Google Scholar] [CrossRef]
- Alam, M.; Parra-Saldivar, R.; Bilal, M.; Afroze, C.A.; Ahmed, M.; Iqbal, H.; Xu, J. Algae-Derived Bioactive Molecules for the Potential Treatment of Sars-Cov-2. Molecules 2021, 26, 2134. [Google Scholar] [CrossRef]
- Thompson, A.J.; Cao, L.; Ma, Y.; Wang, X.; Diedrich, J.K.; Kikuchi, C.; Willis, S.; Worth, C.; McBride, R.; Yates, J.R., III. Human Influenza Virus Hemagglutinins Contain Conserved Oligomannose N-Linked Glycans Allowing Potent Neutralization by Lectins. Cell Host Microbe 2020, 27, 725–735. [Google Scholar] [CrossRef]
- Hayashi, K.; Walde, P.; Miyazaki, T.; Sakayama, K.; Nakamura, A.; Kameda, K.; Masuda, S.; Umakoshi, H.; Kato, K. Active Targeting to Osteosarcoma Cells and Apoptotic Cell Death Induction by the Novel Lectin Eucheuma serra Agglutinin Isolated from a Marine Red Alga. J. Drug Deliv. 2012, 2012, 842785. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Lin, J.; Shieh, W.; Jeng, W.; Huang, R. Antibiotic Activity of Lectins from Marine Algae against Marine Vibrios. J. Ind. Microbiol. Biotechnol. 2003, 30, 433–439. [Google Scholar] [CrossRef]
- Monteiro Abreu, T.; Maria Castelo Melo Silva, L.; Sousa Oliveira Vanderlei, E.; Moutinho Lagos de Melo, C.; Rego Alves Pereira, V.; Maria Barros Benevides, N. Cytokine Production Induced by Marine Algae Lectins in BALB/c Mice Splenocytes. Protein Pept. Lett. 2012, 19, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Gondim, A.C.S.; da Silva, S.R.; Mathys, L.; Noppen, S.; Liekens, S.; Sampaio, A.H.; Nagano, C.S.; Rocha, C.R.C.; Nascimento, K.S.; Cavada, B.S.; et al. Potent Antiviral Activity of Carbohydrate-Specific Algal and Leguminous Lectins from the Brazilian Biodiversity. Med. Chem. Commun. 2019, 10, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, Y.; Sugahara, T.; Ueno, M.; Fukuta, Y.; Ochi, Y.; Akiyama, K.; Miyazaki, T.; Masuda, S.; Kawakubo, A.; Kato, K. The Anti-Tumor Effect of Euchema serra Agglutinin on Colon Cancer Cells in Vitro and in Vivo. Anticancer Drugs 2006, 17, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, J.X.; de Brito, T.V.; Fontenelle, T.P.C.; Damasceno, R.O.S.; de Souza, M.H.L.P.; de Souza Lopes, J.L.; Beltramini, L.M.; Barbosa, A.L.R.; Freitas, A.L.P. Lectin from Red Algae Amansia multifida Lamouroux: Extraction, Characterization and Anti-Inflammatory Activity. Int. J. Biol. Macromol. 2021, 170, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, A.; Makino, H.; Ohnishi, J.; Hirohara, H.; Hori, K. Occurrence of Highly Yielded Lectins Homologous within the Genus Eucheuma. J. Appl. Phycol. 1999, 11, 149–156. [Google Scholar] [CrossRef]
- Millet, J.K.; Séron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East Respiratory Syndrome Coronavirus Infection is Inhibited by Griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L. Broad-Spectrum in Vitro Activity and in Vivo Efficacy of the Antiviral Protein Griffithsin against Emerging Viruses of the Family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef] [Green Version]
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [Green Version]
- Ishag, H.Z.A.; Li, C.; Wang, F.; Mao, X. Griffithsin Binds to the Glycosylated Proteins (E and prM) of Japanese Encephalitis Virus and Inhibit Its Infection. Virus Res. 2016, 215, 50–54. [Google Scholar] [CrossRef]
- Nixon, B.; Stefanidou, M.; Mesquita, P.M.M.; Fakioglu, E.; Segarra, T.; Rohan, L.; Halford, W.; Palmer, K.E.; Herold, B.C. Griffithsin Protects Mice from Genital Herpes by Preventing Cell-to-Cell Spread. J. Virol. 2013, 87, 6257–6269. [Google Scholar] [CrossRef] [Green Version]
- Barre, A.; Simplicien, M.; Benoist, H.; Van Damme, E.J.M.; Rougé, P. Mannose-Specific Lectins from Marine Algae: Diverse Structural Scaffolds Associated to Common Virucidal and Anti-Cancer Properties. Mar. Drugs 2019, 17, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, V.P.T.; Debray, H.; Dus, D.; Teixeira, E.H.; de Oliveira, T.M.; Carneiro, V.A.; Teixeira, A.H.; Filho, G.C.; Nagano, C.S.; Nascimento, K.S.; et al. Lectins from the Red Marine Algal Species Bryothamnion seaforthii and Bryothamnion triquetrum as Tools to Differentiate Human Colon Carcinoma Cells. Adv. Pharmacol. Pharm. Sci. 2009, 2009, 862162. [Google Scholar]
- Calvete, J.J.; Costa, F.H.F.; Saker-Sampaio, S.; Murciano, M.P.M.; Nagano, C.S.; Cavada, B.S.; Grangeiro, T.B.; Ramos, M.V.; Bloch, C., Jr.; Silveira, S.B.; et al. The Amino Acid Sequence of the Agglutinin Isolated from the Red Marine Alga Bryothamnion triquetrum Defines a Novel Lectin Structure. Cell. Mol. Life Sci. 2000, 57, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of Protein from the Macroalga Palmaria palmata. LWT—Food. Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Gordalina, M.; Pinheiro, H.M.; Mateus, M.; da Fonseca, M.M.R.; Cesário, M.T. Macroalgae as Protein Sources—A Review on Protein Bioactivity, Extraction, Purification and Characterization. Appl. Sci. 2021, 11, 7969. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction and Characterization of Protein from Irish Brown Seaweed Ascophyllum nodosum. Food Res. Int. 2017, 99, 1021–1027. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B.; Teissié, J. High Yield Electroextraction of Proteins from Yeast by a Flow Process. Anal. Biochem. 2003, 315, 77–84. [Google Scholar] [CrossRef]
- Boisson, A.-M.; Gout, E.; Bligny, R.; Rivasseau, C. A Simple and Efficient Method for the Long-Term Preservation of Plant Cell Suspension Cultures. Plant Methods 2012, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.; Jha, B.; Fujita, Y.; Ohno, M. Seaweed Micropropagation Techniques and Their Potentials: An Overview. J. Appl. Phycol. 2008, 20, 609–617. [Google Scholar] [CrossRef]
- Schnell, D.J.; Hori, K.; Herrmann, S.M.; Gegg, C.V.; Etzler, M.E. Biosynthesis of DB58 Lectin in Cell Suspension Cultures of Dolichos biflorus. Arch. Biochem. Biophys. 1994, 310, 229–235. [Google Scholar] [CrossRef]
- D’Silva, I.; Kumar Podder, S. Peanut Agglutinin from Callus and Cell Suspension Cultures of Arachis hypogaea L. Plant Cell Rep. 1994, 14, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, M.; Mujin, T.; Shibamoto, N.; Tashiro, F.; Ikuta, A. Production of Aralin, a Selective Cytotoxic Lectin against Human Transformed Cells, in Callus Culture of Aralia elata. Planta Med. 2004, 70, 469–471. [Google Scholar] [PubMed]
- Ramakrishna, W.; Kumari, A.; Rahman, N.; Mandave, P. Anticancer Activities of Plant Secondary Metabolites: Rice Callus Suspension Culture as a New Paradigm. Rice Sci. 2021, 28, 13–30. [Google Scholar] [CrossRef]
- Macharoen, K.; Li, Q.; Márquez-Escobar, V.A.; Corbin, J.M.; Lebrilla, C.B.; Nandi, S.; McDonald, K.A. Effects of Kifunensine on Production and N-Glycosylation Modification of Butyrylcholinesterase in a Transgenic Rice Cell Culture Bioreactor. Int. J. Mol. Sci. 2020, 21, 6896. [Google Scholar] [CrossRef] [PubMed]
- Rorrer, G.L.; Modrell, J.; Zhi, C.; Yoo, H.-D.; Nagle, D.N.; Gerwick, W.H. Bioreactor Seaweed Cell Culture for Production of Bioactive Oxylipins. J. Appl. Phycol. 1995, 7, 187–198. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Mullikin, R.; Huang, B.; Gerwick, W.H.; Maliakal, S.; Cheney, D.P. Production of Bioactive Metabolites by Cell and Tissue Cultures of Marine Macroalgae in Bioreactor Systems. In Plant Cell and Tissue Culture for the Production of Food Ingredients; Fu, T.-J., Singh, G., Curtis, W.R., Eds.; Springer: Boston, MA, USA, 1999; pp. 165–184. [Google Scholar]
- Chen, L.C.M. Cell Suspension Culture from Porphyra linearis (Rhodophyta) a Multicellular Marine Red Alga. J. Appl. Phycol. 1989, 1, 153. [Google Scholar] [CrossRef]
- Rorrer, G.L. Seaweeds: Cell and Tissue Suspension Cultures. In Encyclopedia of Cell Technology; American Cancer Society: Atlanta, GA, USA, 2003. [Google Scholar]
- Polzin, J.; Rorrer, G.L. Selective Production of the Acyclic Monoterpene β-Myrcene by Microplantlet Suspension Cultures of the Macrophytic Marine Red Alga Ochtodes secundiramea under Nutrient Perfusion Cultivation with Bromide-Free Medium. Algal Res. 2018, 36, 159–166. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Tucker, M.P.; Cheney, D.P.; Maliakal, S. Bromoperoxidase Activity in Microplantlet Suspension Cultures of the Macrophytic Red Alga Ochtodes secundiramea. Biotechnol. Bioeng. 2001, 74, 389–395. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Mendiola, J.; Ibáñez, E. Subcritical Water Extraction of Bioactive Components from Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Shaston, UK, 2013; pp. 534–560. [Google Scholar]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and Characterization of Bioactive Compounds with Health Benefits from Marine Resources: Macro and Micro Algae, Cyanobacteria and Invertebrates. In Marine Bioactive Compounds; Springer: Boston, MA, USA, 2012; pp. 55–98. [Google Scholar]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave Assisted Extraction of Phenolic Compounds from Four Economic Brown Macroalgae Species and Evaluation of Their Antioxidant Activities and Inhibitory Effects on α-Amylase, α-Glucosidase, Pancreatic Lipase and Tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification and Applications. Mar. Drugs 2021, 19, 500. [Google Scholar] [CrossRef]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of Ultrasound-Assisted Extraction Conditions for Phenolic Content and Antioxidant Activities of the Alga Hormosira banksii Using Response Surface Methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable Natural Products from Marine and Freshwater Macroalgae Obtained from Supercritical Fluid Extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) Activity of Carrageenans from the Red Seaweed Solieria chordalis (Rhodophyta, Gigartinales) Extracted by Microwave-Assisted Extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Fayad, S.; Nehmé, R.; Tannoury, M.; Lesellier, E.; Pichon, C.; Morin, P. Macroalga Padina pavonica Water Extracts Obtained by Pressurized Liquid Extraction and Microwave-Assisted Extraction Inhibit Hyaluronidase Activity as Shown by Capillary Electrophoresis. J. Chromatogr. A 2017, 1497, 19–27. [Google Scholar] [CrossRef]
- Sousa, G.; Trifunovska, M.; Antunes, M.; Miranda, I.; Moldão, M.; Alves, V.; Vidrih, R.; Lopes, P.A.; Aparicio, L.; Neves, M.; et al. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Pelvetia canaliculata to Sunflower Oil. Foods 2021, 10, 1732. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of Different Non-Conventional Extraction Methods on the Antibacterial and Antiviral Activity of Fucoidans Extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef]
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; MacKenzie, A.D. Enzyme-Assisted Extraction of Fucoxanthin and Lipids Containing Polyunsaturated Fatty Acids from Undaria pinnatifida Using Dimethyl Ether and Ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Fujii, K. Process Integration of Supercritical Carbon Dioxide Extraction and Acid Treatment for Astaxanthin Extraction from a Vegetative Microalga. Food Bioprod. Process. 2012, 90, 762–766. [Google Scholar] [CrossRef]
- Anaëlle, T.; Serrano Leon, E.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.-P. Green Improved Processes to Extract Bioactive Phenolic Compounds from Brown Macroalgae Using Sargassum muticum as Model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A. Supercritical Fluid Extraction of Algae Enhances Levels of Biologically Active Compounds Promoting Plant Growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.-J.; Park, Y.-B.; Woo, H.-C.; Chun, B.-S. Structural, Antioxidant, and Emulsifying Activities of Fucoidan from Saccharina japonica Using Pressurized Liquid Extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of Pressurised Liquid Extraction and Solid–Liquid Extraction Methods on the Phenolic Content and Antioxidant Activities of Irish Macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Etzler, M. Distribution and Function of Plant Lectins. In The Lectins: Properties, Functions, and Applications in Biology and Medicine; Academic Press, Inc.: Cambridge, MA, USA, 1986; pp. 371–435. [Google Scholar]
- Djabayan-Djibeyan, P.; Gibbs, R.; Carpenter, B. In Vivo Release of Lectins from the Green Alga Ulva fasciata. Nat. Prod. Commun. 2010, 5, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.H.; Bomsel, M.; de Paillerets, C.; Weintraub, H.; Alfsen, A. Biochemical Characterization of Algal Coated Vesicles. Biochimie 1990, 72, 41–49. [Google Scholar] [CrossRef]
- Georgiev, M.; Weber, J.; Maciuk, A. Bioprocessing of Plant Cell Cultures for Mass Production of Targeted Compounds. Appl. Microbiol. Biotechnol. 2009, 83, 809–823. [Google Scholar] [CrossRef]
- Haas, C.; Weber, J.; Ludwig-Mueller, J.; Deponte, T.; Bley, T.; Georgiev, M. Flow Cytometry and Phytochemical Analysis of a Sunflower Cell Suspension Culture in a 5-L Bioreactor. Z. Naturforsch. C. J. Bio. Sci. 2008, 63, 699–705. [Google Scholar] [CrossRef]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the Production of Plant Secondary Metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Rao, R.; Ravishankar, G.A. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [PubMed]
- Castro, A.H.F.; da Silva Tavares, H.; Pereira, S.R.F.; Granjeiro, P.A.; da Silva, J.A.; Galdino, A.S. Production and Characterization of Lectin from Bauhinia holophylla (Fabaceae: Cercideae) Calli. Plant Cell Tissue Organ Cult. 2018, 134, 423–432. [Google Scholar] [CrossRef]
- Harrison, P.J.; Hurd, C.L. Nutrient Physiology of Seaweeds: Application of Concepts to Aquaculture. Cah. Biol. Mar. 2001, 42, 71–82. [Google Scholar]
- Oliveira, S.R.M.; Nascimento, A.E.; Lima, M.E.P.; Leite, Y.F.M.M.; Benevides, N.M.B. Purification and Characterisation of a Lectin from the Red Marine Alga Pterocladiella capillacea (S.G. Gmel.) Santel. & Hommers. Rev. Bras. Bot. 2002, 25, 397–403. [Google Scholar]
- Oliveira, A.S.; Lossio, C.F.; Rangel, A.J.; Martins, M.G.Q.; do Nascimento, F.E.P.; de Andrade, M.L.L.; Cavada, B.S.; Lacerda, S.R.; do Nascimento, K. Detection, Purification and Characterization of a Lectin from Freshwater Green Algae Spirogyra spp. An. Acad. Bras. Ciênc. 2016, 89, 2113–2117. [Google Scholar] [CrossRef] [Green Version]

| Species | Protein Content (Dry Matter Basis) | Reference |
|---|---|---|
| Laminaria hyperborea2 | 50.2 ± 2.8 | [18] |
| Laminaria digitata1 | 15.9 | [19] |
| Ulva lactuca2 | 86.5 ± 3.3 | [18] |
| Ulva rigida2 | 112.0 ± 5.8 | [20] |
| Ulva rotundata2 | 100.1 ± 4.9 | [20] |
| Palmaria palmata2 | 122.6 ± 3.1 | [18] |
| Kappaphycus sp. 2 | 25−38 | [21] |
| Porphyra spp. 2 | 429.9 | [22] |
| Porphyra acanthophora1 | 16.5 | [23] |
| Species | Lectin | Specificity | Extraction | Purification | Applications | Reference |
|---|---|---|---|---|---|---|
| Eucheuma serra | ESA | Mannose | Phosphate buffer | Ethanol precipitation, fast protein liquid chromatography (FPLC) | Anticancer (apoptosis on cancer cell lines such as OST, LM8, Colo201 and HeLa); antibacterial | [31,33,34,43,44] |
| Solieria filiformis | SfL-1 SfL-2 | Mannose | Grinding with liquid nitrogen, phosphate buffer | Ammonium sulfate precipitation, ion-exchange chromatography | Anticancer (apoptosis on cell lines Colo201, LM8 and mouse colon26 adenocarcinoma); induce Th2 immune responses in mouse splenocytes; anti-depressant | [32,43,45,46,47] |
| Amansia multifida | AmL | Avidin, fetuin, mannose | Grinding with liquid nitrogen, sodium phosphate | Ammonium sulfate precipitation, ion-exchange chromatography | Anti-inflammatory action (reducing edema formation, leukocyte migration, and reducing level of proinflammatory cytokines) | [46,48] |
| Kappaphycus alvarezii | KAA-2 | High mannose glycan | Homogenization, ethanol | Ethanol precipitation, size exclusion chromatography, ion exchange chromatography | Anti-influenza (inhibits influenza virus propagation by directly binding to high mannose glycans on the envelope glycoprotein hemagglutinin) | [33,49] |
| Griffithsia sp. | Griffithsin | Mannose | Freeze drying, distilled water | Ammonium sulfate precipitation, hydrophobic interaction chromatography | Antiviral (targeting high mannose arrays present on pathogenic enveloped virus such as HIV, coronaviruses, hepatitis C viruses and Japanese encephalitis virus | [35,37,50,51,52,53,54] |
| Bryothamnion triquetrum Bryothamnion seaforthii | BTL BSL | Mucins | Grinding with liquid nitrogen, sodium phosphate | Ammonium sulfate precipitation, ion exchange chromatography | Cancer biomarkers, drug delivery | [55,56,57] |
| Marine Alga Species | Extraction Method | Isolated Compounds | References |
|---|---|---|---|
| Ascophyllym nodosum | Microwave-assisted extraction, ultrasound-assisted extraction | Fucose-sulfated polysaccharides | [9] |
| Sargassum aquifolium, Sargassum ilicifolium, Sargassum polycystum | Enzyme-assisted extraction | Phenolic compound | [11] |
| Fucus vesiculosus | Microwave-assisted extraction | Phlorotannins Polysaccharides | [12] |
| Ascophyllum nodosum, Laminaria japonica, Lessonia trabeculate, Lessonia nigrescens | Microwave-assisted extraction | Phenolic compounds | [77] |
| Solieria chordalis | Microwave-assisted extraction | Carrageenan | [83] |
| Ulva pertusa | Microwave-assisted extraction | Polysaccharides | [84] |
| Padina pavonica | Pressurized liquid extraction, Microwave-assisted extraction | Water extract | [85] |
| Pelvetia canaliculate | Ultrasound-assisted extraction | Antioxidants | [86] |
| Nizamuddinia zanardinii | Ultrasound-assisted extraction | Fucoidans | [87] |
| Undaria pinnatifida | Enzyme-assisted extraction | Fucoxanthin | [88] |
| Haematococcus pluvialis | Supercritical fluid extraction | Astaxanthin | [89] |
| Sargassum muticum | Supercritical fluid extraction | Polyphenols | [90] |
| Polysiphonia nigrescens, Ulva clathrata, Cladophora sp. | Supercritical fluid extraction with CO2 |
Auxins Cytokinins Polyphenols Microelements Macroelements | [91] |
| Saccharina japonica | Subcritical water extraction | Fucoidan | [92] |
| Ascophyllum nodosum, Pelvetia canaliculata, Fucus spiralis, Ulva intestinalis | Subcritical water extraction | Polyphenols | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliki, I.M.; Misson, M.; Teoh, P.L.; Rodrigues, K.F.; Yong, W.T.L. Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-Destructive Extraction. Mar. Drugs 2022, 20, 102. https://doi.org/10.3390/md20020102
Maliki IM, Misson M, Teoh PL, Rodrigues KF, Yong WTL. Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-Destructive Extraction. Marine Drugs. 2022; 20(2):102. https://doi.org/10.3390/md20020102
Chicago/Turabian StyleMaliki, Intan Mariana, Mailin Misson, Peik Lin Teoh, Kenneth Francis Rodrigues, and Wilson Thau Lym Yong. 2022. "Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-Destructive Extraction" Marine Drugs 20, no. 2: 102. https://doi.org/10.3390/md20020102
APA StyleMaliki, I. M., Misson, M., Teoh, P. L., Rodrigues, K. F., & Yong, W. T. L. (2022). Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-Destructive Extraction. Marine Drugs, 20(2), 102. https://doi.org/10.3390/md20020102