Chitosan-Based Films with 2-Aminothiophene Derivative: Formulation, Characterization and Potential Antifungal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
2.1.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.1.2. Differential Scanning Calorimetry (DSC)
2.1.3. Thermogravimetric Analysis (TGA)
2.1.4. X-ray Diffraction (XRD)
2.1.5. Scanning Electron Microscopy (SEM)
2.2. Film Thickness
2.3. Drug Content in 6CN-Chitosan Films
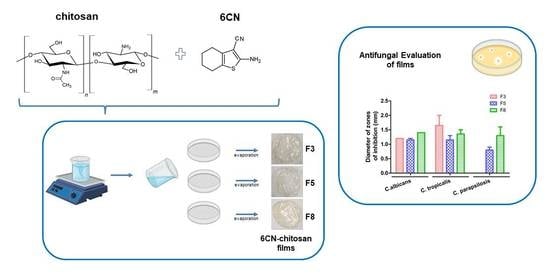
2.4. Antifungal Activity of 6CN-Chitosan Films
3. Materials and Methods
3.1. Materials
3.2. Development of Chitosan-Based Films
3.3. Physicochemical Analyses
3.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3.2. Differential Scanning Calorimetry (DSC)
3.3.3. Thermogravimetric Analysis (TGA)
3.3.4. X-ray Diffraction (XRD)
3.3.5. Scanning Electronic Microscopy (SEM)
3.4. Film Thickness
3.5. Drug Content of Films
3.6. Antifungal Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thamban Chandrika, N.; Shrestha, S.K.; Ngo, H.X.; Howard, K.C.; Garneau-Tsodikova, S. Novel fluconazole derivatives with promising antifungal activity. Bioorg. Med. Chem. 2018, 26, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kummari, L.K.; Butler, M.S.; Furlong, E.; Blundell, R.; Nouwens, A.; Silva, A.B.; Kappler, U.; Fraser, J.A.; Kobe, B.; Cooper, M.A.; et al. Antifungal benzo[b]thiophene 1,1-dioxide IMPDH inhibitors exhibit pan- assay interference (PAINS) profiles. Bioorg. Med. Chem. 2018, 26, 5408–5419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotti, L.; Scotti, M.T.; De Oliveira Lima, E.; Da Silva, M.S.; Do Carmo Alves De Lima, M.; Da Rocha Pitta, I.; De Moura, R.O.; De Oliveira, J.G.B.; Da Cruz, R.M.D.; Mendonça, F.J.B. Experimental methodologies and evaluations of computer-aided drug design methodologies applied to a series of 2-aminothiophene derivatives with antifungal activities. Molecules 2012, 17, 2298–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonça, F.J.B.; Lima-Neto, R.G.; de Oliveira, T.B.; de Lima, M.C.A.; Pitta, I.R.; Galdino, S.L.; da Cruz, R.M.D.; de Araújo, R.S.A.; Neves, R.P. Synthesis and evaluation of the antifungal activity of 2-(substituted-amino)-4,5-dialkyl-thiophene-3-carbonitrile derivatives. Lat. Am. J. Pharm. 2011, 30, 1492–1499. [Google Scholar]
- de Araújo Neto, L.N.; do Carmo Alves de Lima, M.; de Oliveira, J.F.; de Souza, E.R.; Buonafina, M.D.S.; Vitor Anjos, M.N.; Brayner, F.A.; Alves, L.C.; Neves, R.P.; Mendonça-Junior, F.J.B. Synthesis, cytotoxicity and antifungal activity of 5-nitro-thiophene-thiosemicarbazones derivatives. Chem. Biol. Interact. 2017, 272, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.P.; de Freitas Araújo Reis, M.Y.; da Silva, D.T.C.; Mendonça Junior, F.J.B.; Converti, A.; Pessoa, A.; de Lima Damasceno, B.P.G.; da Silva, J.A. Antifungal activity of topical microemulsion containing a thiophene derivative. Braz. J. Microbiol. 2014, 45, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Faria, D.R.; Sakita, K.M.; Capoci, I.R.G.; Arita, G.S.; Rodrigues-Vendramini, F.A.V.; de Oliveira, A.G.; Felipe, M.S.S.; de Souza Bonfim de Mendonça, P.; Svidzinski, T.I.E.; Kioshima, E.S. Promising antifungal activity of new oxadiazole against Candida krusei. PLoS ONE 2020, 15, e0227876. [Google Scholar] [CrossRef]
- Meotti, F.C.; Silva, D.O.; Dos Santos, A.R.S.; Zeni, G.; Rocha, J.B.T.; Nogueira, C.W. Thiophenes and furans derivatives: A new class of potential pharmacological agents. Environ. Toxicol. Pharmacol. 2003, 15, 37–44. [Google Scholar] [CrossRef]
- Véras of Aguiar, A.C.; of Moura, R.O.; Bezerra Mendonça, J.F.; de Oliveira Rocha, H.A.; Gomes Câmara, R.B.; dos Santos Carvalho Schiavon, M. Evaluation of the antiproliferative activity of 2-amino thiophene derivatives against human cancer cells lines. Biomed. Pharmacother. 2016, 84, 403–414. [Google Scholar] [CrossRef]
- Félix, M.B.; de Souza, E.R.; do C.A. de Lima, M.; Frade, D.K.G.; de L. Serafim, V.; da F. Rodrigues, K.A.; do N. Néris, P.L.; Ribeiro, F.F.; Scotti, L.; Scotti, M.T.; et al. Antileishmanial activity of new thiophene–indole hybrids: Design, synthesis, biological and cytotoxic evaluation, and chemometric studies. Bioorg. Med. Chem. 2016, 24, 3972–3977. [Google Scholar] [CrossRef]
- Desai, N.C.; Dodiya, A.M.; Rajpara, K.M.; Rupala, Y.M. Synthesis and antimicrobial screening of 1,3,4-oxadiazole and clubbed thiophene derivatives. J. Saudi Chem. Soc. 2014, 18, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Isloor, A.M.; Kalluraya, B.; Sridhar Pai, K. Synthesis, characterization and biological activities of some new benzo[b]thiophene derivatives. Eur. J. Med. Chem. 2010, 45, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Eleamen, G.R.A.; Da Costa, S.C.; Lima-Neto, R.G.; Neves, R.P.; Rolim, L.A.; Rolim-Neto, P.J.; Moura, R.O.; De Aquino, T.M.; Bento, E.S.; Scotti, M.T.; et al. Improvement of solubility and antifungal activity of a new aminothiophene derivative by complexation with 2-hydroxypropyl-β-cyclodextrin. J. Braz. Chem. Soc. 2017, 28, 116–125. [Google Scholar] [CrossRef]
- Ferreira, E.B.; da Silva Júnior, W.F.; de Oliveira Pinheiro, J.G.; da Fonseca, A.G.; Moura Lemos, T.M.A.; de Oliveira Rocha, H.A.; de Azevedo, E.P.; Mendonça Junior, F.J.B.; De Lima, Á.A.N. Characterization and antiproliferative activity of a novel 2-aminothiophene derivative-β-cyclodextrin binary system. Molecules 2018, 23, 3130. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, S.B.; Duarte, F.Í.C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; De Lima, Á.A.N. Cyclodextrin-drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [Green Version]
- da Silva Oliveira, V.; de Almeida, A.S.; da Silva Albuquerque, I.; Duarte, F.Í.C.; Queiroz, B.C.S.H.; Converti, A.; de Lima, Á.A.N. Therapeutic applications of solid dispersions for drugs and new molecules: In vitro and in vivo activities. Pharmaceutics 2020, 12, 933. [Google Scholar] [CrossRef]
- da Silva Oliveira, V.; Dantas, E.D.; de Sousa Queiroz, A.T.; de Freitas Oliveira, J.W.; de Sousa da Silva, M.; Ferreira, P.G.; de Carvalho da Siva, F.; Ferreira, V.F.; de Lima, Á.A.N. Novel Solid Dispersions of Naphthoquinone Using Different Polymers for Improvement of Antichagasic Activity. Pharmaceutics 2020, 12, 1136. [Google Scholar] [CrossRef]
- Pereira, J.R.; Bezerra, G.S.; Furtado, A.A.; de Carvalho, T.G.; da Silva, V.C.; Monteiro, A.L.B.; Guerra, G.C.B.; de Araújo Júnior, R.F.; Sant’ana, A.E.G.; de Freitas Fernandes-Pedrosa, M.; et al. Chitosan film containing mansoa hirsuta fraction for wound healing. Pharmaceutics 2020, 12, 484. [Google Scholar] [CrossRef]
- Laranjeira, M.C.M.; De Fávere, V.T. Quitosana: Biopolimero funcional com potencial industrial biomedico. Quim. Nova 2009, 32, 672–678. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Y.; Zhang, Y.; Yu, C.; Cao, S. Preparation and structural analysis of chitosan films with and without sorbitol. Food Hydrocoll. 2013, 33, 186–191. [Google Scholar] [CrossRef]
- Valenta, C.; Auner, B.G. The use of polymers for dermal and transdermal delivery. Eur. J. Pharm. Biopharm. 2004, 58, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Harris, R.; Navarro-García, F.; Heras, A.; Acosta, N. Chitosan based films as supports for dual antimicrobial release. Carbohydr. Polym. 2016, 146, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.L.G.D.; Brandão, H.M.; De Oliveira, L.F.C. Spectroscopic and thermogravimetric study of chitosan after incubation in bovine rumen. J. Mol. Struct. 2011, 1005, 186–191. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Angelici, F.; Ricci, M.; Giovagnoli, S.; Capuccella, M.; Rossi, C. Development of mucoadhesive patches for buccal administration of ibuprofen. J. Control. Release 2004, 99, 73–82. [Google Scholar] [CrossRef]
- Zarandona, I.; Estupiñán, M.; Pérez, C.; Alonso-Sáez, L.; Guerrero, P.; Caba, K. de la Chitosan Films Incorporated with Exopolysaccharides from Deep Seawater Alteromonas sp. Mar. Drugs 2020, 18, 447. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Leite, H.F.; Yoshida, M.I.; Saliba, J.B.; Junior, A.S.C.; Faraco, A.A.G. In vitro release and characterization of chitosan films as dexamethasone carrier. Int. J. Pharm. 2009, 368, 1–6. [Google Scholar] [CrossRef]
- Wanderley, D.M.S.; Melo, D.F.; Silva, L.M.; Silva, W.C.; Correia, L.P.; Oshiro-Junior, J.A.; Fook, M.V.L.; Moura, R.O.; Lima, R.S.C.; Damasceno, B.P.G.L. Physical–chemical characterization of N-acylhydrazone derivative chitosan films using spectroscopic and thermoanalytical techniques. J. Therm. Anal. Calorim. 2019, 138, 3789–3796. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, P.K.; Sen, P. Preparation and characterization of optical property of crosslinkable film of chitosan with 2-thiophenecarboxaldehyde. Carbohydr. Polym. 2010, 80, 563–569. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Shahbazi, M.; Rajabzadeh, G.; Ahmadi, S.J. Characterization of nanocomposite film based on chitosan intercalated in clay platelets by electron beam irradiation. Carbohydr. Polym. 2017, 157, 226–235. [Google Scholar] [CrossRef]
- Park, S.-I.; Zhao, Y. Incorporation of a High Concentration of Mineral or Vitamin into Chitosan-Based Films. J. Agric. Food Chem. 2004, 52, 1933–1939. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Zhang, J.; Xu, J. Effect of sorbitol content on microstructure and thermal properties of chitosan films. Int. J. Biol. Macromol. 2018, 119, 1294–1297. [Google Scholar] [CrossRef]
- Mathew, S.; Brahmakumar, M.; Abraham, T.E. Microstructural imaging and characterization of the mechanical, chemical, thermal, and swelling properties of starch-chitosan blend films. Biopolymers 2006, 82, 176–187. [Google Scholar] [CrossRef]
- do Nascimento, E.G.; de Azevedo, E.P.; Alves-Silva, M.F.; Aragão, C.F.S.; Fernandes-Pedrosa, M.F.; da Silva-Junior, A.A. Supramolecular aggregates of cyclodextrins with co-solvent modulate drug dispersion and release behavior of poorly soluble corticosteroid from chitosan membranes. Carbohydr. Polym. 2020, 248, 116724. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Parrilha, G.L.; Da Silva, J.G.; Gouveia, L.F.; Gasparoto, A.K.; Dias, R.P.; Rocha, W.R.; Santos, D.A.; Speziali, N.L.; Beraldo, H. Pyridine-derived thiosemicarbazones and their tin(IV) complexes with antifungal activity against Candida spp. Eur. J. Med. Chem. 2011, 46, 1473–1482. [Google Scholar] [CrossRef]
- de Oliveira Paiva, R.; Kneipp, L.F.; Goular, C.M.; Albuquerque, M.A.; Echevarria, A. Antifungal activities of thiosemicarbazones and semicarbazones against mycotoxigenic fungi. Ciência e Agrotecnologia 2014, 38, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Singh, J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J Pharmacol. Pharmacother. 2015, 6, 185–187. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Diekema, D.J. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J. Clin. Microbiol. 2012, 50, 2846–2856. [Google Scholar] [CrossRef] [Green Version]








| Sample | Loss Stage | Temperature (°C) | Mass Loss (%) | Process |
|---|---|---|---|---|
| 6CN | First | 146–250 | 98.27 | Degradation 6CN |
| CF | First | 35–120 | 26.23 | Dehydration |
| Second | 250–355 | 32.10 | Degradation/depolymerization | |
| Third | 355–700 | 38.00 | Degradation | |
| F3 | First | 35–120 | 15.35 | Dehydration |
| Second | 146–240 | 4.25 | 6CN degradation | |
| Third | 240–350 | 32.43 | Degradation/depolymerization | |
| Fourth | 350–640 | 43.62 | Degradation | |
| F5 | First | 35–120 | 12.10 | Dehydration |
| Second | 146–240 | 6.21 | 6CN degradation | |
| Third | 240–350 | 36.87 | Degradation/depolymerization | |
| Fourth | 350–640 | 43.90 | Degradation | |
| F8 | First | 35–120 | 11.10 | Dehydration |
| Second | 146–240 | 10.77 | 6CN degradation | |
| Third | 240–350 | 35.62 | Degradation/depolymerization | |
| Fourth | 350–640 | 41.25 | Degradation |
| Parameter | F3 | F5 | F8 |
|---|---|---|---|
| Film mass (size 15 mm × 15 mm) (mg) | 19.07 ± 1.21 | 19.50 ± 1.90 | 19.70 ± 1.87 |
| * mass of 6CN (mg) | 1.01 ± 0.07 | 2.03 ± 0.09 | 3.35 ± 0.60 |
| 6CN content (% w/w) | 5.30 ± 0.36 | 10.52 ± 1.40 | 16.91 ± 1.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, V.d.S.; Cruz, M.M.d.; Bezerra, G.S.; Silva, N.E.S.e.; Nogueira, F.H.A.; Chaves, G.M.; Sobrinho, J.L.S.; Mendonça-Junior, F.J.B.; Damasceno, B.P.G.d.L.; Converti, A.; et al. Chitosan-Based Films with 2-Aminothiophene Derivative: Formulation, Characterization and Potential Antifungal Activity. Mar. Drugs 2022, 20, 103. https://doi.org/10.3390/md20020103
Oliveira VdS, Cruz MMd, Bezerra GS, Silva NESe, Nogueira FHA, Chaves GM, Sobrinho JLS, Mendonça-Junior FJB, Damasceno BPGdL, Converti A, et al. Chitosan-Based Films with 2-Aminothiophene Derivative: Formulation, Characterization and Potential Antifungal Activity. Marine Drugs. 2022; 20(2):103. https://doi.org/10.3390/md20020103
Chicago/Turabian StyleOliveira, Verônica da Silva, Meriângela Miranda da Cruz, Gabriela Suassuna Bezerra, Natan Emanuell Sobral e Silva, Fernando Henrique Andrade Nogueira, Guilherme Maranhão Chaves, José Lamartine Soares Sobrinho, Francisco Jaime Bezerra Mendonça-Junior, Bolívar Ponciano Goulart de Lima Damasceno, Attilio Converti, and et al. 2022. "Chitosan-Based Films with 2-Aminothiophene Derivative: Formulation, Characterization and Potential Antifungal Activity" Marine Drugs 20, no. 2: 103. https://doi.org/10.3390/md20020103
APA StyleOliveira, V. d. S., Cruz, M. M. d., Bezerra, G. S., Silva, N. E. S. e., Nogueira, F. H. A., Chaves, G. M., Sobrinho, J. L. S., Mendonça-Junior, F. J. B., Damasceno, B. P. G. d. L., Converti, A., & Lima, Á. A. N. d. (2022). Chitosan-Based Films with 2-Aminothiophene Derivative: Formulation, Characterization and Potential Antifungal Activity. Marine Drugs, 20(2), 103. https://doi.org/10.3390/md20020103