Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production
Abstract
:1. Introduction
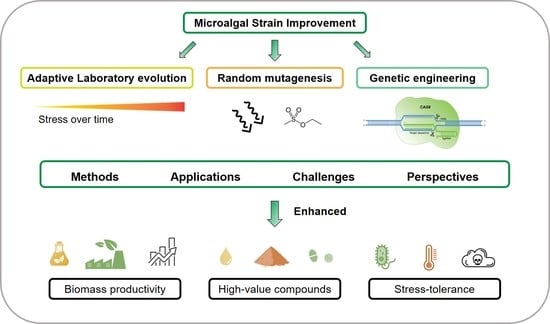
2. Strategies for Microalgal Strain Improvement
2.1. Random Mutagenesis
2.1.1. Historical Perspective
2.1.2. Physical and Chemical Mutagenesis
2.1.3. Mutant Selection Methods
2.1.4. Random Mutagenesis Applications
2.2. Adaptive Laboratory Evolution
2.3. Genetic Engineering
2.4. Regulatory Frameworks on Genetically Modified Organisms (GMOs) Applied to Microalgae
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Austic, R.E.; Mustafa, A.; Jung, B.; Gatrell, S.; Lei, X.G. Potential and limitation of a new defatted diatom microalgal biomass in replacing soybean meal and corn in diets for broiler chickens. J. Agric. Food Chem. 2013, 61, 7341–7348. [Google Scholar] [CrossRef]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.S.; Chang, J.S. Genetic engineering of microalgae for enhanced biorefinery capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H.; Burke, J. Water Pollution from Agriculture: A Global Review; FAO: Rome, Italy, 2017. [Google Scholar]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Schulze, P.S.C.; Schüler, L.M.; Santos, T.; Barreira, L.; Varela, J. Fluorescence activated cell-sorting principles and applications in microalgal biotechnology. Algal Res. 2018, 30, 113–120. [Google Scholar] [CrossRef]
- Gerashchenko, B.I. Fluorescence-Activated Cell Sorting (FACS)-Based Characterization of Microalgae. Mar. Ecol. Curr. Future Dev. 2020, 2, 148–160. [Google Scholar]
- Zhao, Q.; Huang, H. Adaptive Evolution Improves Algal Strains for Environmental Remediation. Trends Biotechnol. 2021, 39, 112–115. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef]
- Schüler, L.; Morais, E.; Dos Santos, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.B.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and characterization of novel Chlorella vulgaris mutants with low chlorophyll and improved protein contents for food applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef]
- Kselíková, V.; Singh, A.; Bialevich, V.; Čížková, M.; Bišová, K. Improving microalgae for biotechnology—From genetics to synthetic biology—Moving forward but not there yet. Biotechnol. Adv. 2022, 58, 107885. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of Microalgae: Recent Advances, Perspectives, and Regulatory Challenges for Industrial Application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Trovão, M.; Pereira, H.; Silva, J.; Páramo, J.; Quelhas, P.; Santos, T.; Silva, J.T.; Machado, A.; Gouveia, L.; Barreira, L.; et al. Growth performance, biochemical composition and sedimentation velocity of Tetraselmis sp. CTP4 under different salinities using low-cost lab- and pilot-scale systems. Heliyon 2019, 5, e01553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Lowrey, J.; Armenta, R.E.; Brooks, M.S. Nutrient and media recycling in heterotrophic microalgae cultures. Appl. Microbiol. Biotechnol. 2016, 100, 1061–1075. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Vaidyanathan, S. Microalgae: A robust ‘green bio-bridge’ between energy and environment. Crit. Rev. Biotechnol. 2018, 38, 351–368. [Google Scholar] [CrossRef] [Green Version]
- Cunha, P.; Pereira, H.; Costa, M.; Pereira, J.; Silva, J.T.; Fernandes, N.; Varela, J.; Simões, M. Nannochloropsis oceanica cultivation in pilot-scale raceway ponds-from design to cultivation. Appl. Sci. 2020, 10, 1725. [Google Scholar] [CrossRef] [Green Version]
- Tredici, M.R. Mass Production of Microalgae: Photobioreactors. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science: Cornwall, UK, 2004; Volume 9, pp. 178–213. [Google Scholar]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L.; Tang, M.S.Y.; Nagarajan, D.; Ling, T.C.; Ooi, C.W.; Chang, J.S. A holistic approach to managing microalgae for biofuel applications. Int. J. Mol. Sci. 2017, 18, 215. [Google Scholar] [CrossRef] [Green Version]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Yen, H.W.; Hu, I.C.; Chen, C.Y.; Ho, S.H.; Lee, D.J.; Chang, J.S. Microalgae-based biorefinery—From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Chang, C.H.; Chen, C.Y.; Chang, J.S.; Ng, I.S. Towards protein production and application by using Chlorella species as circular economy. Bioresour. Technol. 2019, 289, 121625. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Yen, H.W.; Philippidis, G.P. Harnessing the power of mutagenesis and adaptive laboratory evolution for high lipid production by Oleaginous Microalgae and yeasts. Sustainability 2020, 12, 5125. [Google Scholar] [CrossRef]
- Song, M.; Pei, H. The growth and lipid accumulation of Scenedesmus quadricauda during batch mixotrophic/heterotrophic cultivation using xylose as a carbon source. Bioresour. Technol. 2018, 263, 525–531. [Google Scholar] [CrossRef]
- Doan, T.T.Y.; Obbard, J.P. Enhanced lipid production in Nannochloropsis sp. using fluorescence-activated cell sorting. GCB Bioenergy 2011, 3, 264–270. [Google Scholar] [CrossRef]
- Pereira, H.; Falchier, A.; Yan, C.G.; Yeagle, E.M.; Linn, G.S.; Megevand, P.; Thielscher, A.; Deborah, A.R.; Milham, M.P.; Mehta, A.D.; et al. Isolation of a euryhaline microalgal strain, Tetraselmis sp. CTP4, as a robust feedstock for biodiesel production. Sci. Rep. 2016, 6, 31236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Sá, M.; Cabanelas, I.T.D.; Wijffels, R.H.; Barbosa, M.J. Improved fucoxanthin and docosahexaenoic acid productivities of a sorted self-settling Tisochrysis lutea phenotype at pilot scale. Bioresour. Technol. 2021, 325, 124725. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Mayfield, S.P. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 2012, 488, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, W.; Chen, J. Recent advances of microbial breeding via heavy-ion mutagenesis at IMP. Lett. Appl. Microbiol. 2017, 65, 274–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spicer, A.; Molnar, A. Gene Editing of Microalgae: Scientific Progress and Regulatory Challenges in Europe. Biology 2018, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Cagnon, C.; Mirabella, B.; Nguyen, H.M.; Beyly-Adriano, A.; Bouvet, S.; Cuiné, S.; Beisson, F.; Peltier, G.; Li-Beisson, Y. Development of a forward genetic screen to isolate oil mutants in the green microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2013, 6, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aklilu, E. Review on forward and reverse genetics in plant breeding. All Life 2021, 14, 127–135. [Google Scholar] [CrossRef]
- Hlavova, M.; Turoczy, Z.; Bisova, K. Improving microalgae for biotechnology—From genetics to synthetic biology. Biotechnol. Adv. 2015, 33, 1194–1203. [Google Scholar] [CrossRef]
- Lopez-Rodas, V.; Agrelo, M.A.R.; Carrillo, E.; Ferrero, L.M.; Larrauri, A.; Martín-Otero, L.; Costas, E. Resistance of microalgae to modern water contaminants as the result of rare spontaneous mutations. Eur. J. Phycol. 2001, 36, 179–190. [Google Scholar] [CrossRef]
- Eyre-Walker, A.; Keightley, P.D. The distribution of fitness effects of new mutations. Nat. Rev. Genet. 2007, 8, 610–618. [Google Scholar] [CrossRef] [PubMed]
- González, R.; García-Balboa, C.; Rouco, M.; Lopez-Rodas, V.; Costas, E. Adaptation of microalgae to lindane: A new approach for bioremediation. Aquat. Toxicol. 2012, 109, 25–32. [Google Scholar] [CrossRef]
- Krasovec, M.; Sanchez-Brosseau, S.; Grimsley, N.; Piganeau, G. Spontaneous mutation rate as a source of diversity for improving desirable traits in cultured microalgae. Algal Res. 2018, 35, 85–90. [Google Scholar] [CrossRef]
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive laboratory evolution principles and applications in industrial biotechnology. Biotechnol. Adv. 2022, 54, 107795. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, J.; Meng, F. Adaptive Laboratory Evolution of Microalgae: A Review of the Regulation of Growth, Stress Resistance, Metabolic Processes, and Biodegradation of Pollutants. Front. Microbiol. 2021, 12, 2401. [Google Scholar] [CrossRef]
- Chakdar, H.; Hasan, M.; Pabbi, S.; Nevalainen, H.; Shukla, P. High-throughput proteomics and metabolomic studies guide re-engineering of metabolic pathways in eukaryotic microalgae: A review. Bioresour. Technol. 2020, 321, 124495. [Google Scholar] [CrossRef]
- Zakhrabekova, S.M.; Gough, S.; Lundh, L.; Hansson, M. Functional Genomics and Forward and Reverse Genetics Approaches for Identification of Important QTLs in Plants. Proc. Azerbaijan Natl. Acad. Sci. 2013, 68, 23–28. [Google Scholar]
- Rohr, J.; Sarkar, N.; Balenger, S.; Jeong, B.R.; Cerutti, H. Tandem inverted repeat system for selection of effective transgenic RNAi strains in Chlamydomonas. Plant J. 2004, 40, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Su, Y.; Xu, M.; Bergmann, A.; Ingthorsson, S.; Rolfsson, O.; Salehi-Ashtiani, K.; Brynjolfsson, S.; Fu, W. Chemical mutagenesis and fluorescence-based high-throughput screening for enhanced accumulation of carotenoids in a model marine diatom Phaeodactylum tricornutum. Mar. Drugs 2018, 16, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.D.; Sojin, K.; Santhanam, P.; Dhanalakshmi, B.; Latha, S.; Park, M.S.; Kim, M.K. Triggering of fatty acids on Tetraselmis sp. by ethyl methanesulfonate mutagenic treatment. Bioresour. Technol. Rep. 2018, 2, 21–28. [Google Scholar] [CrossRef]
- Manandhar-Shrestha, K.; Hildebrand, M. Development of flow cytometric procedures for the efficient isolation of improved lipid accumulation mutants in a Chlorella sp. microalga. J. Appl. Phycol. 2013, 25, 1643–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichuk, K.; Brynjólfsson, S.; Fu, W. Biotechnological production of value-added carotenoids from microalgae: Emerging technology and prospects. Bioengineered 2014, 5, 204–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillich, U.M.; Wolter, N.; Franke, P.; Dühring, U.; Frohme, M. Screening and genetic characterization of thermo-tolerant Synechocystis sp. PCC6803 strains created by adaptive evolution. BMC Biotechnol. 2014, 14, 66. [Google Scholar] [CrossRef] [Green Version]
- Jakhwal, P.; Biswas, J.K.; Tiwari, A.; Kwon, E.E.; Bhatnagar, A. Genetic and non-genetic tailoring of microalgae for the enhanced production of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—A review. Bioresour. Technol. 2022, 344, 126250. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Lenski, R.E.; Ebert, D.; Hollis, B.; Olivieri, I.; Whitlock, M.C. Experimental evolution. Trends Ecol. Evol. 2012, 27, 547–560. [Google Scholar] [CrossRef] [Green Version]
- Gregory, T.R. Understanding Natural Selection: Essential Concepts and Common Misconceptions. Evol. Educ. Outreach 2009, 2, 156–175. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-Wide Insertional Mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Patena, W.; Armbruster, U.; Gang, S.S.; Blum, S.R.; Jonikas, M.C. High-throughput genotyping of green algal mutants reveals random distribution of mutagenic insertion sites and endonucleolytic cleavage of transforming DNA. Plant Cell 2014, 26, 1398–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, W.S.; Lee, H.; Sung, M.G.; Hwang, K.T.; Jung, S.M.G.; Kwon, J.H. Enrichment as a screening method for a high-growth-rate microalgal strain under continuous cultivation system. Biotechnol. Bioprocess. Eng. 2016, 21, 268–273. [Google Scholar] [CrossRef]
- Henley, W.J.; Litaker, R.W.; Novoveská, L.; Duke, C.S.; Quemada, H.D.; Sayre, R.T. Initial risk assessment of genetically modified (GM) microalgae for commodity-scale biofuel cultivation. Algal Res. 2013, 2, 66–77. [Google Scholar] [CrossRef]
- Zimny, T.; Sowa, S.; Tyczewska, A.; Twardowski, T. Certain new plant breeding techniques and their marketability in the context of EU GMO legislation—Recent developments. New Biotechnol. 2019, 51, 49–56. [Google Scholar] [CrossRef]
- De Riso, V.; Raniello, R.; Maumus, F.; Rogato, A.; Bowler, C.; Falciatore, A. Gene silencing in the marine diatom Phaeodactylum tricornutum. Nucleic Acids Res. 2009, 37, 14. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.G.; Cha, J.; Chandrasegaran, S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Lim, J.-M.; Lee, H.-G.; Shin, S.-E.; Kang, N.K.; Park, Y.-I.; Oh, H.-M.; Jeong, W.-J.; Jeong, B.-R.; Chang, Y.K. Current status and perspectives of genome editing technology for microalgae. Biotechnol. Biofuels 2017, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.T.; Jiang, J.-Y.; Shi, T.-Q.; Sun, X.-M.; Zhao, Q.-Y.; Huang, H.; Ren, L.-J. Application of the CRISPR/Cas system for genome editing in microalgae. Appl. Microbiol. Biotechnol. 2019, 103, 3239–3248. [Google Scholar] [CrossRef]
- Ghribi, M.; Nouemssi, S.B.; Meddeb-Mouelhi, F.; Desgagné-Penix, I. Genome editing by CRISPR-Cas: A game change in the genetic manipulation of Chlamydomonas. Life 2020, 10, 295. [Google Scholar] [CrossRef]
- Naduthodi, M.I.S.; Claassens, N.J.; D’Adamo, S.; van der Oost, J.; Barbosa, M.J. Synthetic Biology Approaches to Enhance Microalgal Productivity. Trends Biotechnol. 2021, 39, 1019–1036. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.S.; Tan, S.I.; Kao, P.H.; Chang, Y.K.; Chang, J.S. Recent Developments on Genetic Engineering of Microalgae for Biofuels and Bio-Based Chemicals. Biotechnol. J. 2017, 12, 1600644. [Google Scholar] [CrossRef]
- Henikoff, S.; Till, B.J.; Comai, L.; Division, B.S.; Hutchinson, F.; Washington, S.H. Perspectives on Translational Biology TILLING. Traditional Mutagenesis Meets Functional Genomics. Cancer Res. 2004, 135, 630–636. [Google Scholar]
- Gilchrist, E.J.; O’Neil, N.J.; Rose, A.M.; Zetka, M.C.; Haughn, G.W. TILLING is an effective reverse genetics technique for Caenorhabditis elegans. BMC Genom. 2006, 7, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillich, U.M.; Lehmann, S.; Schulze, K.; Dühring, U.; Frohme, M. The Optimal Mutagen Dosage to Induce Point-Mutations in Synechocystis sp. PCC6803 and Its Application to Promote Temperature Tolerance. PLoS ONE 2012, 7, e49467. [Google Scholar] [CrossRef]
- Muller, H.J. Artificial Transmutation of the Gene. Science 1927, 66, 84–87. [Google Scholar] [CrossRef]
- Mavor, J.W. On the elimination of the x-chromosome from the egg of Drosophila melanogaster by x-rays. Sci. Proc. 1921, 18, 301–302. [Google Scholar]
- Little, C.C.; Bagg, H.J. The occurrence of four inheritable morphological variations in mice and their possible relation to treatment with x-rays. J. Exp. Zool. 1924, 41, 45–91. [Google Scholar] [CrossRef]
- Botstein, D.; Shortle, D. Strategies and applications of in vitro mutagenesis. Science 1985, 229, 1193–1201. [Google Scholar] [CrossRef]
- Claes, H. Analyse der biochemischen Synthesekette für Carotinoide mit Hilfe von Chlorella-Mutanten. J. Chem. Sci. 1954, 9, 461–469. [Google Scholar] [CrossRef]
- Flibotte, S.; Edgley, M.L.; Chaudhry, I.; Taylor, J.; Neil, S.E.; Rogula, A.; Zapf, R.; Hirst, M.; Butterfield, Y.; Jones, S.J.; et al. Whole-genome profiling of mutagenesis in Caenorhabditis elegans. Genetics 2010, 185, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarze, N.O.; Frandsen, P. Herstellung von Chlorella-Farbmutanten mit Hilfe von radioaktiven Isotopen. Kurze Orig. 1960, 47, 29–34. [Google Scholar] [CrossRef]
- Schmid, G.H.; Schwarze, P. Blue Light Enhanced Respiration in a Colorless Chlorella Mutant. Hoppe-Seyler’s Z. Physiol. Chem. 1969, 350, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Gavrilã, G.; Mihaescu, L. Mutagenesis in Blue-Green Algae (Cyano-Bacteria) and Some Evolutionary Considerations. Caryologia 1982, 35, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Loppes, R. Effect of the selective medium on the manisfestation of mutations induced with mono-alkylating agents in Chlamydomonas reinhardi. Mutat. Res. 1969, 7, 25–34. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, Y.; Zhang, Z.; Yang, W. Modification and improvement of microalgae strains for strengthening CO2 fixation from coal-fired flue gas in power plants. Bioresour. Technol. 2019, 291, 121850. [Google Scholar] [CrossRef]
- Mba, C.; Afza, R.; Bado, S.; Jain, S.M. Induced Mutagenesis in Plants Using Physical and Chemical Agents. Plant Cell Cult. Essent. Methods 2010, 3, 111–130. [Google Scholar]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Bökel, C. EMS Screens from Mutagenesis to Screening and Mapping. In Drosophila Methods and Protocols; Dahmann, C., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 420, pp. 119–138. [Google Scholar]
- Guénet, J.L. Chemical mutagenesis of the mouse genome: An overview. Genetica 2004, 122, 9–24. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Zhu, M.; Yu, C.; Cao, Y.; Zhang, D.; Zhou, G. Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis. Bioresour. Technol. 2013, 136, 360–367. [Google Scholar] [CrossRef]
- Wang, W.; Wei, T.; Fan, J.; Yi, J.; Li, Y.; Wan, M.; Wang, J.; Bai, W. Repeated mutagenic effects of 60Co-γ irradiation coupled with high-throughput screening improves lipid accumulation in mutant strains of the microalgae Chlorella pyrenoidosa as a feedstock for bioenergy. Algal Res. 2018, 33, 71–77. [Google Scholar] [CrossRef]
- Xing, W.; Zhang, R.; Shao, Q.; Meng, C.; Wang, X.; Wei, Z.; Sun, F.; Wang, C.; Cao, K.; Zhu, B.; et al. Effects of laser mutagenesis on microalgae production and lipid accumulation in two economically important fresh Chlorella strains under heterotrophic conditions. Agronomy 2021, 11, 961. [Google Scholar] [CrossRef]
- Ottenheim, C.; Nawrath, M.; Wu, J.C. Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): The latest development. Bioresour. Bioprocess. 2018, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Kazama, Y.; Hirano, T.; Saito, H.; Liu, Y.; Ohbu, S.; Hayashi, Y.; Abe, T. Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 161. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Ding, X.T.; Wang, L.J.; Jiang, E.Y.; Van, P.N.; Li, F.L. Rapid sorting of fucoxanthin-producing Phaeodactylum tricornutum mutants by flow cytometry. Mar. Drugs 2021, 19, 228. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Liu, L.; Ao, X.; Ma, L.; Wu, M.; Ma, F. Improving Cell Growth and Lipid Accumulation in Green Microalgae Chlorella sp. via UV Irradiation. Appl. Biochem. Biotechnol. 2015, 175, 3507–3518. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Altenburg, E. The Artificial Production of Mutations by Ultra-Violet Light. Am. Nat. 1934, 68, 491–507. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [Green Version]
- Sheik, S.A.; Wani, M.R.; Kozgar, M.I.; Ahmad, P. Wheat Improvement: Historical Perspective and Mutational Approach—A Review. In Improvement of Crops in the Era of Climatic Changes; Ahmad, P., Wani, M.R., Azooz, M.M., Tran, L.P., Eds.; Springer: New York, NY, USA, 2014; Volume 2, pp. 297–322. [Google Scholar]
- Ikehata, H.; Ono, T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Helena, J.M.; Joubert, A.M.; Grobbelaar, S.; Nolte, E.M.; Nel, M.; Pepper, M.S.; Coetzee, M.; Mercier, A.E. Deoxyribonucleic acid damage and repair: Capitalizing on our understanding of the mechanisms of maintaining genomic integrity for therapeutic purposes. Int. J. Mol. Sci. 2018, 19, 1148. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, G.P.; You, Y.H.; Besaratinia, A. Mutations induced by ultraviolet light. Mutat. Res. 2005, 571, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, M.; Zou, S.; Fei, C.; Yan, Y.; Zheng, S.; Rajper, A.A.; Wang, C. Breeding of high biomass and lipid producing Desmodesmus sp. by Ethylmethane sulfonate-induced mutation. Bioresour. Technol. 2016, 207, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kamath, B.S.; Vidhyavathi, R.; Sarada, R.; Ravishankar, G.A. Enhancement of carotenoids by mutation and stress induced carotenogenic genes in Haematococcus pluvialis mutants. Bioresour. Technol. 2008, 99, 8667–8673. [Google Scholar] [CrossRef]
- Patel, V.K.; Maji, D.; Pandey, S.S.; Rout, P.K.; Sundaram, S.; Kalra, A. Rapid budding EMS mutants of Synechocystis PCC 6803 producing carbohydrate or lipid enriched biomass. Algal Res. 2016, 16, 36–45. [Google Scholar] [CrossRef]
- Singer, J.T.; Kusmierek, B. Chemical Mutagenesis. Ann. Rev. Biochem. 1982, 52, 93. [Google Scholar] [CrossRef]
- Mobini-Dehkordi, M.; Nahvi, I.; Zarkesh-Esfahani, H.; Ghaedi, K.; Tavassoli, M.; Akada, R. Isolation of a novel mutant strain of Saccharomyces cerevisiae by an ethyl methane sulfonate-induced mutagenesis approach as a high producer of bioethanol. J. Biosci. Bioeng. 2008, 105, 403–408. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Dall’Osto, L.; Szaub, J.; Scibilia, L.; Ballottari, M.; Purton, S.; Bassi, R. Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light-use efficiency in a photobioreactor. Biotechnol. Biofuels 2014, 7, 157. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.C.; Kao, C.Y.; Chiu, S.Y.; Tsai, M.T.; Lin, C.S. Characterization of the thermal-tolerant mutants of Chlorella sp. with high growth rate and application in outdoor photobioreactor cultivation. Bioresour. Technol. 2010, 101, 2880–2883. [Google Scholar] [CrossRef]
- Nakanishi, K.; Deuchi, K. Culture of a high-chlorophyll-producing and halotolerant Chlorella vulgaris. J. Biosci. Bioeng. 2014, 117, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.M.; Lin, T.H.; Yang, Y.C.; Zhang, W.X.; Lai, J.T.; Wu, H.T.; Chang, J.S.; Lin, C.S. Ability of an alkali-tolerant mutant strain of the microalga Chlorella sp. AT1 to capture carbon dioxide for increasing carbon dioxide utilization efficiency. Bioresour. Technol. 2017, 244, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Tseng, Y.F.; Cheng, C.L.; Chen, Y.C.; Lin, C.S.; Su, H.Y.; Chow, T.J.; Chen, C.Y.; Chang, J.S. Characterization of a heat-tolerant Chlorella sp. GD mutant with enhanced photosynthetic CO2 fixation efficiency and its implication as lactic acid fermentation feedstock. Biotechnol. Biofuels 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, H.H.; Su, H.Y.; Song, X.D.; Chow, T.J.; Chen, C.Y.; Chang, J.S.; Lee, T.M. Isolation and characterization of Chlorella sp. mutants with enhanced thermo- and CO2 tolerances for CO2 sequestration and utilization of flue gases. Biotechnol. Biofuels 2019, 12, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, M.; Suh, W.I.; Oh, Y.T.; Ryu, A.J.; Jeong, K.J.; Kim, M.; Mohapatra, R.K.; Lee, B.; Chang, Y.K. Directed evolution of Chlorella sp. HS2 towards enhanced lipid accumulation by ethyl methanesulfonate mutagenesis in conjunction with fluorescence-activated cell sorting based screening. Fuel 2022, 316, 123410. [Google Scholar] [CrossRef]
- Tharek, A.; Yahya, A.; Salleh, M.M.; Jamaluddin, H.; Yoshizaki, S.; Hara, H.; Iwamoto, K.; Suzuki, I.; Mohamad, S.E. Improvement and screening of astaxanthin producing mutants of newly isolated Coelastrum sp. using ethyl methane sulfonate induced mutagenesis technique. Biotechnol. Rep. 2021, 32, e00673. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Bellan, A.; Segalla, A.; Meneghesso, A.; Alboresi, A.; Morosinotto, T. Generation of random mutants to improve light-use efficiency of Nannochloropsis gaditana cultures for biofuel production. Biotechnol. Biofuels 2015, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Potijun, S.; Jaingam, S.; Sanevas, N.; Vajrodaya, S.; Sirikhachornkit, A. Green microalgae strain improvement for the production of sterols and squalene. Plants 2021, 10, 1673. [Google Scholar] [CrossRef]
- Senthamilselvi, D.; Kalaiselvi, T. Gamma ray mutants of oleaginous microalga Chlorella sp. KM504965 with enhanced biomass and lipid for biofuel production. Biomass Convers. Biorefinery 2022, 1–17. [Google Scholar] [CrossRef]
- de Jaeger, L.; Verbeek, R.E.M.; Draaisme, R.B.; Martens, D.E.; Springer, J.; Eggink, G.; Wijffels, R.H. Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (I) mutant generation and characterization. Biotechnol. Biofuels 2014, 7, 69. [Google Scholar] [CrossRef]
- Sarayloo, E.; Simsek, S.; Unlu, Y.S.; Cevahir, G.; Erkey, C.; Kavakli, I.H. Enhancement of the lipid productivity and fatty acid methyl ester profile of Chlorella vulgaris by two rounds of mutagenesis. Bioresour. Technol. 2018, 250, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Schüler, L.M.; Bombo, G.; Duarte, P.; Santos, T.F.; Maia, I.B.; Pinheiro, F.; Marques, J.; Jacinto, R.; Schulze, P.S.; Pereira, H.; et al. Carotenoid biosynthetic gene expression, pigment and n-3 fatty acid contents in carotenoid-rich Tetraselmis striata CTP4 strains under heat stress combined with high light. Bioresour. Technol. 2021, 337, 125385. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.P.G.; Watson, C.W.; Url, S. Inhibition of Carotenoid Synthesis by Fluridone and Norflurazon. Weed Sci. 1978, 26, 198–203. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Yang, X.; Chen, J.; Qi, Z. Astaxanthin accumulation in Haematococcus pluvialis observed through Fourier-transform infrared microspectroscopy imaging. J. Mol. Struct. 2019, 1182, 119–122. [Google Scholar] [CrossRef]
- Harker, M.; Young, A.J. Inhibition of astaxanthin synthesis in the green alga, Haematococcus pluvialis. Eur. J. Phycol. 1995, 30, 179–187. [Google Scholar] [CrossRef]
- Britton, G.; Singh, R.K.; Malhotra, H.C.; Goodwin, T.W.; Ben-Aziz, A. Biosynthesis of 1,2-dihydrocarotenoids in Rhodopseudomonas viridis: Experiments with inhibitors. Phytochemistry 1977, 16, 1561–1566. [Google Scholar] [CrossRef]
- Walsh, M.C.; Klopfenstein, W.E.; Harwood, J.L. The short chain condensing enzyme has a widespread occurrence in the fatty acid synthetases from higher plants. Phytochemistry 1990, 29, 3797–3799. [Google Scholar] [CrossRef]
- Chaturvedi, Y.; Uppalapati, R.S.; Alamsjah, M.A.; Fujita, Y. Isolation of quizalofop-resistant mutants of Nannochloropsis oculata (Eustigmatophyceae) with high eicosapentaenoic acid following N-methyl-N-nitrosourea-induced random mutagenesis. J. Appl. Phycol. 2004, 16, 135–144. [Google Scholar] [CrossRef]
- Sendra, M.; Moreno-Garrido, I.; Blasco, J.; Araújo, C.V.M. Effect of erythromycin and modulating effect of CeO2 NPs on the toxicity exerted by the antibiotic on the microalgae Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Environ. Pollut. 2018, 242, 357–366. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Fujita, Y. Isolation of enhanced eicosapentaenoic acid producing mutants of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycol. Res. 2006, 54, 208–219. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, C.Y.; Chang, J.S. Lutein production with wild-type and mutant strains of Chlorella sorokiniana MB-1 under mixotrophic growth. J. Taiwan Inst. Chem. Eng. 2017, 79, 66–73. [Google Scholar] [CrossRef]
- Kleinegris, D.M.M.; van Es, M.A.; Janssen, M.; Brandenburg, W.A.; Wijffels, R.H. Carotenoid fluorescence in Dunaliella salina. J. Appl. Phycol. 2010, 22, 645–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, Y.K.; Ryu, H.G.; Jeon, A.J.; Jeong, S.; Jeong, K.J.; Chang, B. Photosynthetic Improvement of Industrial Microalgae for Biomass and Biofuel Production. In Microbial Photosynthesis; Wang, Q., Ed.; Springer: Singapore, 2020; pp. 285–317. [Google Scholar]
- Huesemann, M.H.; Hausmann, T.S.; Bartha, R.; Aksoy, M.; Weissman, J.C.; Benemann, J.R. Biomass productivities in wild type and pigment mutant of Cyclotella sp. (Diatom). Appl. Biochem. Biotechnol. 2009, 157, 507–526. [Google Scholar] [CrossRef] [PubMed]
- de Mooij, T.; Janssen, M.; Cerezo-Chinarro, O.; Mussgnug, J.H.; Kruse, O.; Ballottari, M.; Bassi, R.; Bujaldon, S.; Wollman, F.A.; Wijffels, R.H. Antenna size reduction as a strategy to increase biomass productivity: A great potential not yet realized. J. Appl. Phycol. 2015, 27, 1063–1077. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Cazzaniga, S.; Guardini, Z.; Barera, S.; Benedetti, M.; Mannino, G.; Maffei, M.E.; Bassi, R. Combined resistance to oxidative stress and reduced antenna size enhance light-to-biomass conversion efficiency in Chlorella vulgaris cultures. Biotechnol. Biofuels 2019, 12, 221. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Prakash, G.; Lali, A.M. Reduced chlorophyll antenna mutants of Chlorella saccharophila for higher photosynthetic efficiency and biomass productivity under high light intensities. J. Appl. Phycol. 2020, 32, 1559–1567. [Google Scholar] [CrossRef]
- Lee, B.; Choi, G.G.; Choi, Y.E.; Sung, M.; Park, M.S.; Yang, J.W. Enhancement of lipid productivity by ethyl methane sulfonate-mediated random mutagenesis and proteomic analysis in Chlamydomonas reinhardtii. Korean J. Chem. Eng. 2014, 31, 1036–1042. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Yang, G.; Han, J.; Thomsen, L.; Pan, K. Breeding 3 elite strains of Nannochloropsis oceanica by nitrosoguanidine mutagenesis and robust screening. Algal Res. 2016, 19, 104–108. [Google Scholar] [CrossRef]
- Cecchin, M.; Berteotti, S.; Paltrinieri, S.; Vigliante, I.; Iadarola, B.; Giovannone, B.; Maffei, M.; Delledonne, M.; Ballottari, M. Improved lipid productivity in Nannochloropsis gaditana in nitrogen-replete conditions by selection of pale green mutants. Biotechnol. Biofuels 2020, 13, 78. [Google Scholar] [CrossRef]
- Beacham, T.A.; Macia, V.M.; Rooks, P.; White, D.A.; Ali, S.T. Altered lipid accumulation in Nannochloropsis salina CCAP849/3 following EMS and UV induced mutagenesis. Biotechnol. Rep. 2015, 7, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, N.; Gupta, R.P.; Mathur, A.S.; Tuli, D.K. Enhanced lipid production in thermo-tolerant mutants of Chlorella pyrenoidosa NCIM 2738. Bioresour. Technol. 2016, 221, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Hyka, P.; Lickova, S.; Přibyl, P.; Melzoch, K.; Kovar, K. Flow cytometry for the development of biotechnological processes with microalgae. Biotechnol. Adv. 2013, 31, 2–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schüler, L.M.; Schulze, P.S.C.; Pereira, H.; Barreira, L.; León, R.; Varela, J. Trends and strategies to enhance triacylglycerols and high-value compounds in microalgae. Algal Res. 2017, 25, 263–273. [Google Scholar] [CrossRef]
- Dettman, J.R.; Rodrigue, N.; Melnyk, A.H.; Wong, A.; Bailey, S.F.; Kassen, R. Evolutionary insight from whole-genome sequencing of experimentally evolved microbes. Mol. Ecol. 2012, 21, 2058–2077. [Google Scholar] [CrossRef]
- Reboud, X.; Bell, G. Experimental evolution in Chlamydomonas III. Evolution of specialist and generalist types in environments that vary in space and time. Heredity 1997, 78, 507–514. [Google Scholar] [CrossRef]
- Wang, L.; Xue, C.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Strain improvement of Chlorella sp. for phenol biodegradation by adaptive laboratory evolution. Bioresour. Technol. 2016, 205, 264–268. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, M.; Magnusdottir, M.; Zhang, Y.; Brynjolfsson, S.; Fu, W. Photo-oxidative stress-driven mutagenesis and adaptive evolution on the marine diatom Phaeodactylum tricornutum for enhanced carotenoid accumulation. Mar. Drugs 2015, 13, 6138–6151. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Luo, S.W.; Luo, W.; Yang, W.D.; Liu, J.S.; Li, H.Y. Adaptive evolution of microalgal strains empowered by fulvic acid for enhanced polyunsaturated fatty acid production. Bioresour. Technol. 2019, 277, 204–210. [Google Scholar] [CrossRef]
- Barten, R.; Peeters, T.; Navalho, S.; Fontowicz, L.; Wijffels, R.H.; Barbosa, M. Expanding the upper-temperature boundary for the microalga Picochlorum sp. (BPE23) by adaptive laboratory evolution. Biotechnol. J. 2022, 17, 2100659. [Google Scholar] [CrossRef]
- Manis, J.P. Knock Out, Knock In, Knock Down—Genetically Manipulated Mice and the Nobel Prize. N. Engl. J. Med. 2007, 357, 2426–2429. [Google Scholar] [CrossRef]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 1, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malla, A.; Rosales-Mendoza, S.; Phoolcharoen, W.; Vimolmangkang, S. Efficient Transient Expression of Recombinant Proteins Using DNA Viral Vectors in Freshwater Microalgal Species. Front. Plant Sci. 2021, 12, 650820. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 6213. [Google Scholar] [CrossRef] [PubMed]
- Thurtle-Schmidt, D.M.; Lo, T.W. Molecular biology at the cutting edge: A review on CRISPR/CAS9 gene editing for undergraduates. Biochem. Mol. Biol. Educ. 2018, 46, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA—Guided. Science 2012, 337, 816–822. [Google Scholar] [CrossRef]
- Kindle, K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1990, 87, 1228–1232. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.F.; Zhang, M.H.; Li, D.W.; Yang, W.D.; Liu, J.S.; Bai, W.B.; Li, H.Y. Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum. Mar. Drugs 2013, 11, 4558–4569. [Google Scholar] [CrossRef] [Green Version]
- Li, D.W.; Cen, S.-Y.; Liu, Y.-H.; Balamurugan, S.; Zheng, X.-Y.; Alimujiang, A.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. A type 2 diacylglycerol acyltransferase accelerates the triacylglycerol biosynthesis in heterokont oleaginous microalga Nannochloropsis oceanica. J. Biotechnol. 2016, 229, 65–71. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.-F.; Li, R.-Y.; Yang, W.-D.; Liu, J.-S.; Lin, C.S.K.; Balamurugan, S.; Li, H.-Y. TAG pathway engineering via GPAT2 concurrently potentiates abiotic stress tolerance and oleaginicity in Phaeodactylum tricornutum. Biotechnol. Biofuels 2020, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Ikeda, K.; Shimojima, M.; Ohta, H. Enhancement of extraplastidic oil synthesis in Chlamydomonas reinhardtii using a type-2 diacylglycerol acyltransferase with a phosphorus starvation-inducible promoter. Plant Biotechnol. J. 2014, 12, 808–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengel, R.; Smith, R.T.; Haslam, R.P.; Sayanova, O.; Vila, M.; León, R. Overexpression of acetyl-CoA synthetase (ACS) enhances the biosynthesis of neutral lipids and starch in the green microalga Chlamydomonas reinhardtii. Algal Res. 2018, 31, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Südfeld, C.; Hubáček, M.; Figueiredo, D.; Naduthodi, M.I.; Van Der Oost, J.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. High-throughput insertional mutagenesis reveals novel targets for enhancing lipid accumulation in Nannochloropsis oceanica. Metab. Eng. 2021, 66, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Niu, Y.F.; Huang, T.; Yang, W.D.; Liu, J.S.; Li, H.Y. Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metab. Eng. 2015, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.G.; Tong, L. Structure and Function of Malic Enzymes, A New Class of Oxidative Decarboxylases. Biochemistry 2003, 42, 12721–12733. [Google Scholar] [CrossRef]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.R.; Ng, I.S. Development of CRISPR/Cas9 system in Chlorella vulgaris FSP-E to enhance lipid accumulation. Enzyme Microb. Technol. 2020, 133, 109458. [Google Scholar] [CrossRef]
- Galarza, J.I.; Gimpel, J.A.; Rojas, V.; Arredondo-Vega, B.O.; Henríquez, V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res. 2018, 31, 291–297. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Q.; Xin, Y.; Lu, Y.; Xu, J. Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase. Algal Res. 2017, 27, 366–375. [Google Scholar] [CrossRef]
- Groot, M.J.A.; Bundock, P.; Hooykaas, P.J.J.; Beijersbergen, A.G.M. Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nat. Biotechnol. 1998, 16, 839–842. [Google Scholar] [CrossRef]
- Statement by the Group of Chief Scientific Advisors: A Scientific Perspective on the Regulatory Status of Products Derived from Gene Editing and the Implications for the GMO Directive. Available online: https://data.europa.eu/doi/10.2777/407732 (accessed on 4 November 2021).
- Press Release 111/18: Organisms Obtained by Mutagenesis Are GMOs and Are, in Principle, Subject to the Obligations Laid Down by the GMO Directive. Available online: https://curia.europa.eu/jcms/upload/docs/application/pdf/2018-07/cp180111en.pdf (accessed on 4 November 2021).
- How GMOs Are Regulated for Food and Plant Safety in the United States. Available online: https://www.fda.gov/food/agricultural-biotechnology/how-gmos-are-regulated-food-and-plant-safety-united-states (accessed on 12 April 2022).
- Coordinated Framework for Regulation of Biotechnology, 51 FR 23302. Available online: https://www.aphis.usda.gov/brs/fedregister/coordinated_framework.pdf (accessed on 26 May 2022).






| Species | Method | Target | Screening | Improvement | References |
|---|---|---|---|---|---|
| Chemical mutagenesis | |||||
| Chlorella sp. | EMS 100 mM, 30 min | Lipid content | FACS using BODIPY 505/515 staining | 1.4-fold increased lipid content | [109] |
| Chlorella sp. | EMS 100 mM, 60 min | Thermotolerance | Incubation at 40 °C; size | Increase of 1.8-fold at 25 °C and 6.7-fold at 40 °C for growth rate | [104] |
| Chlorella sp. | NTG 5 μg mL−1 for 60 min | Alkali tolerance | pH 11.5; size | CO2 utilization efficiency | [106] |
| Chlorella vulgaris | EMS 300 mM, 60 min | Chlorophyll deficiency | Color and norflurazon | Up to 99% lower chlorophyll and 60% higher protein content | [11] |
| Coelastrum sp. | EMS 400 mM, 60 min | Carotenoid content | Glufosinate 25 μM and size | 2-fold higher astaxanthin content | [110] |
| Desmodesmus sp. | EMS 600–800 mM, 30–60 min | Lipid content | Nile red fluorescence | Increased lipid productivity of up to 74% | [98] |
| Nannochloropsis gaditana | EMS 70 mM, 60 min | Chlorophyll deficiency | In vivo fluorescence imaging | Photosynthetic activity and biomass productivity | [111] |
| Physical mutagenesis | |||||
| Chlamydomonas reinhardtii | UV, 30 min | Sterols | On 0.1–1.0 mM terbinafine | 50% overproduction of sterols and squalene, higher resistance to oxidative stress | [112] |
| Chlorella sp. | Gamma ray, 800 Gy | Lipid content | Nile red fluorescence | Increased lipid content and productivity | [113] |
| Phaeodactylum tricornutum | Heavy-ion irradiation | Carotenoid content | FACS (chlorophyll autofluorescence) | 25% higher fucoxanthin content | [88] |
| Tetradesmus (Scenedesmus) obliquus | UV 254 nm (40,000 µJ cm−1) | Starchless mutants | Iodine vapor staining to screen for starch | 41% increased total fatty acid productivity | [114] |
| Hybrid mutagenesis | |||||
| Chlorella vulgaris | UV 254 nm (0.5–10 min) + EMS 25 mM 60 min | Lipid content | Growth and Nilered staining; | Lipid content and biomass were, respectively, 67% and 35% higher than those of the wildtype | [115] |
| Species | Method | Target | Improvement | References |
|---|---|---|---|---|
| Chlorella sp. | 31 cycles under 500 mg/L of phenol | Phenol wastewater removal | 100% phenol removal in 7 days; maximum biomass concentration increased 2-fold | [141] |
| Chlorella sp. | 46 cycles with flue gas | Tolerance to flue gas | Growth under 10% CO2, 200 ppm NOx, and 100 ppm SOx | [78] |
| Phaeodactylum tricornutum | 11 cycles, 5 days each, light-induced oxidative stress supplied by LED | Carotenoid content | 2-fold higher biomass production and fucoxanthin content | [142] |
| Phaeodactylum tricornutum | 35 cycles, 7 days each, of hyposaline treatment | Fatty acid content | EPA content increased up to 139 µg/mg biomass; improved growth | [143] |
| Picochlorum sp. | 390 days under temperature stress | Thermotolerance | 1.5 °C increase in the maximum tolerable temperature | [144] |
| Tisochrysis lutea | 2 rounds of direct evolution + FACS | Carotenoid and fatty acid content | 3.1-fold fucoxanthin and 1.6-fold DHA higher productivities | [29] |
| Species | Method | Target | Improvement | References |
|---|---|---|---|---|
| Chlamydomonas reinhardtii | Heterologous overexpression of phytoene synthase (PSY) | Carotenoid content | 2.0- and 2.2-fold higher in violaxanthin and lutein content | [161] |
| Chlamydomonas reinhardtii | Overexpression of acetyl-CoA synthetase (ACS) | Lipid content | 2.4-fold more TAG in N depletion media | [157] |
| Chlamydomonas reinhardtii | Overexpression of type-2 diacylglycerol acyl-CoA acyltransferase (DGTT4) | Lipid content | 2.5-fold increased TAG content in P depletion media | [156] |
| Chlorella vulgaris | Heterologous overexpression of mGFP | Lipid content | 46% (w/w) higher lipid accumulation | [162] |
| Haematococcus pluvialis | Overexpression of phytoene desaturase (PDS) gene | Carotenoid content | 67% increase in astaxanthin accumulation | [163] |
| Nannochloropsis oceanica | Knockout of NO06G03670 | Lipid content | Increase in neutral lipids content by 40% | [158] |
| Nannochloropsis oceanica | Overexpression of RuBisCO activase | Growth productivity | Growth rate and photosynthesis increase by 32 and 28%, respectively, induced under low level of CO2 | [164] |
| Nannochloropsis oceanica | Overexpression of type 2 diacylglycerol acyltransferase (DGAT) | Lipid content | 69% increase in neutral lipid content | [154] |
| Phaeodactylum tricornutum | Overexpression of glycerol-3-phosphate acyltransferase 2 (GPAT2) | Lipid content | 2.9-fold increase in TAG content | [155] |
| Phaeodactylum tricornutum | Overexpression of malic enzyme | Lipid content | 2.5-fold increase in total lipid content | [159] |
| Phaeodactylum tricornutum | Overexpression of type 2 DGAT | Lipid content | 76% increase in EPA content | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trovão, M.; Schüler, L.M.; Machado, A.; Bombo, G.; Navalho, S.; Barros, A.; Pereira, H.; Silva, J.; Freitas, F.; Varela, J. Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production. Mar. Drugs 2022, 20, 440. https://doi.org/10.3390/md20070440
Trovão M, Schüler LM, Machado A, Bombo G, Navalho S, Barros A, Pereira H, Silva J, Freitas F, Varela J. Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production. Marine Drugs. 2022; 20(7):440. https://doi.org/10.3390/md20070440
Chicago/Turabian StyleTrovão, Mafalda, Lisa M. Schüler, Adriana Machado, Gabriel Bombo, Sofia Navalho, Ana Barros, Hugo Pereira, Joana Silva, Filomena Freitas, and João Varela. 2022. "Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production" Marine Drugs 20, no. 7: 440. https://doi.org/10.3390/md20070440
APA StyleTrovão, M., Schüler, L. M., Machado, A., Bombo, G., Navalho, S., Barros, A., Pereira, H., Silva, J., Freitas, F., & Varela, J. (2022). Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production. Marine Drugs, 20(7), 440. https://doi.org/10.3390/md20070440