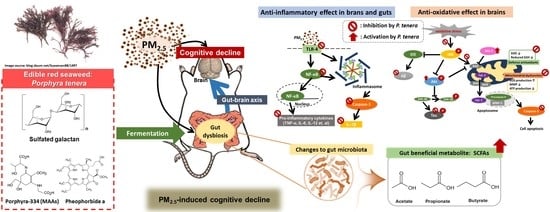
Porphyra tenera Protects against PM2.5-Induced Cognitive Dysfunction with the Regulation of Gut Function
Abstract
:1. Introduction
2. Results
2.1. Ameliorating Effect on Learning and Memory Impairment
2.2. Inhibitory Effect of Neuroinflammation
2.3. Antioxidant Effect of P. tenera
2.4. Expression of Cognition-Mediated Protein
2.5. Main Compound Analysis
2.6. Variation in Microbiome
2.7. Expression of Tight Junction Proteins
2.8. Inhibitory Effect of Inflammation in Gut and Blood Serum
2.9. Fecal Short-Chain Fatty Acids (SCFAs) Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of P. tenera Extract
4.3. Animal Experimental Design
4.4. Whole Body Exposure of PM2.5
4.5. Behavioral Tests
4.5.1. Y-Maze Test
4.5.2. Morris Water Maze (MWM) Test
4.6. Mitochondrial Tests
4.6.1. Isolation of Mitochondria
4.6.2. Mitochondrial ROS Contents
4.6.3. Mitochondrial Membrane Potential (MMP)
4.6.4. ATP Level
4.7. MDA Content
4.8. Western Blot Assay
4.9. Measurement of Cytokine Content
4.10. Bioactive Chemical Analysis of P. tenera
4.10.1. Molecular Weight Analysis
4.10.2. Sulfate Contents
4.10.3. Monosaccharide Composition
4.10.4. Major Bioactive Compound Analysis
4.11. Gut Microbiome Analysis
4.12. Measurement of SCFAs Contents
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Møller, P.; Loft, S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ. Health Perspect. 2010, 118, 1126–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulton, P.V.; Yang, W. Air pollution, oxidative stress, and Alzheimer’s disease. J. Environ. Public Health 2012, 2012, 472751. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Geng, X.; Stone, C.; Cosky, E.E.; Ji, Y.; Du, H.; Zhang, K.; Sun, Q.; Ding, Y. PM2.5 exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environ. Toxicol. 2019, 34, 530–538. [Google Scholar] [CrossRef]
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicol. Environ. Saf. 2019, 174, 344–352. [Google Scholar] [CrossRef]
- Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. PM2.5 exposure in association with AD-related neuropathology and cognitive outcomes. Environ. Pollut. 2022, 292, 118320. [Google Scholar] [CrossRef]
- Shou, Y.; Zhu, X.; Zhu, D.; Yin, H.; Shi, Y.; Chen, M.; Lu, L.; Qian, Q.; Zhao, D.; Hu, Y. Ambient PM2.5 chronic exposure leads to cognitive decline in mice: From pulmonary to neuronal inflammation. Toxicol. Lett. 2020, 331, 208–217. [Google Scholar] [CrossRef]
- Xie, J.-J.; Yuan, C.-G.; Xie, J.; Shen, Y.-W.; He, K.-Q.; Zhang, K.-G. Speciation and bioaccessibility of heavy metals in PM2.5 in Baoding city, China. Environ. Pollut. 2019, 252, 336–343. [Google Scholar] [CrossRef]
- Cian, R.E.; Drago, S.R.; De Medina, F.S.; Martínez-Augustin, O. Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Lee, S.-W.; Kwon, Y.-S.; Park, J.G.; Baek, K.-H. Chemical characterization and antioxidant potential of volatile oil from an edible seaweed Porphyra tenera (Kjellman, 1897). Chem. Cent. J. 2017, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Bito, T.; Teng, F.; Watanabe, F. Bioactive compounds of edible purple laver Porphyra sp. (Nori). J. Agric. Food Chem. 2017, 65, 10685–10692. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Ki, K.-N.; Chung, H.-Y. Proximate composition, amino acid, mineral, and heavy metal content of dried laver. Prev. Nutr. Food Sci. 2013, 18, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, Y.; Zhaohui, Z.; Wenshan, S.; Bafang, L.; Hu, H. Protective effect of MAAs extracted from Porphyra tenera against UV irradiation-induced photoaging in mouse skin. J. Photochem. Photobiol. B Biol. 2019, 192, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.-H.; Kim, Y.-Y.; Park, H.-Y. Air pollution and central nervous system disease: A review of the impact of fine particulate matter on neurological disorders. Front. Public Health 2020, 921, 575330. [Google Scholar] [CrossRef]
- Ibañez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Mohibbullah, M.; Hannan, M.A.; Park, I.-S.; Moon, I.S.; Hong, Y.-K. The edible red seaweed Gracilariopsis chorda promotes axodendritic architectural complexity in hippocampal neurons. J. Med. Food 2016, 19, 638–644. [Google Scholar] [CrossRef]
- dos Santos, M.M.; Assreuy, A.M.S.; Oliveira, L.G.F.; de Alencar, D.B.; Sampaio, S.S.; Sampaio, A.H.; Chaves, E.M.C.; Sampaio, A.; Aragão, G.F. Ethanolic Extract of the Red Algae Meristiella echinocarpa (Areschoug) Confers Neuroprotection in Mice. J. Health Biol. Sci. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, H.-J.; Heo, H.J. Ecklonia cava Attenuates PM2.5-Induced Cognitive Decline through Mitochondrial Activation and Anti-Inflammatory Effect. Mar. Drugs 2021, 19, 131. [Google Scholar] [CrossRef]
- Bueno, B.G.; Caso, J.; Madrigal, J.L.M.; Leza, J.C. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci. Biobehav. Rev. 2016, 64, 134–147. [Google Scholar] [CrossRef]
- Yang, Y.; Yun, D.; Dong, B.; Geng, Y.; Wan, Y. VIP alleviates sepsis-induced cognitive dysfunction as the TLR-4/NF-κB signaling pathway is inhibited in the hippocampus of rats. J. Mol. Histol. 2022, 53, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.-E.; Shin, C.Y.; Han, S.-H.; Kwon, K.J. Astaxanthin suppresses PM2.5-induced neuroinflammation by regulating Akt phosphorylation in BV-2 microglial cells. Int. J. Mol. Sci. 2020, 21, 7227. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, M.; Ahn, C.-B.; Je, J.-Y. Enzymatic extracts from edible red algae, Porphyra tenera, and their antioxidant, anti-acetylcholinesterase, and anti-inflammatory activities. Food Sci. Biotechnol. 2010, 19, 1551–1557. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Han, H.J.; Shin, E.J.; Heo, H.J. Improving effect of Porphyra tenera extract on ultra-fine dust-mediated inflammation. J. Korean Soc. Food Sci. Nutr. 2020, 49, 295–303. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Z.; Geng, L.; Wang, J.; Zhang, Q. In vitro evaluation of the neuroprotective effect of oligo-porphyran from Porphyra yezoensis in PC12 cells. J. Appl. Phycol. 2019, 31, 2559–2571. [Google Scholar] [CrossRef]
- Sun, T.; Liang, H.; Xue, M.; Liu, Y.; Gong, A.; Jiang, Y.; Qin, Y.; Yang, J.; Meng, D. Protective effect and mechanism of fucoidan on intestinal mucosal barrier function in NOD mice. Food Agric. Immunol. 2020, 31, 939–953. [Google Scholar] [CrossRef]
- Djordjevic, A.; Spasic, S.; Jovanovic-Galovic, A.; Djordjevic, R.; Grubor-Lajsic, G. Oxidative stress in diabetic pregnancy: SOD, CAT and GSH-Px activity and lipid peroxidation products. J. Matern.-Fetal Neonatal Med. 2004, 16, 367–372. [Google Scholar] [CrossRef]
- Zhao, K.; Luo, G.; Giannelli, S.; Szeto, H.H. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem. Pharmacol. 2005, 70, 1796–1806. [Google Scholar] [CrossRef]
- Sastre, J.; Pallardó, F.V.; de la Asunción, J.G.; Viña, J. Mitochondria, oxidative stress and aging. Free Radic. Res. 2000, 32, 189–198. [Google Scholar] [CrossRef]
- Cenini, G.; Voos, W. Mitochondria as potential targets in Alzheimer disease therapy: An update. Front. Pharmacol. 2019, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D.; Tsai, C.L. Relationship between antioxidant activity and maturity of peanut hulls. J. Agric. Food Chem. 1993, 41, 67–70. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complementary Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, I.S.; Kim, M.; Son, K.-T.; Jeong, Y.; Jeon, Y.-J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Venkatraman, K.L.; Mehta, A. Health benefits and pharmacological effects of Porphyra species. Plant Foods Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Zhang, H.; Liu, J.; Ouyang, J.-M. Repair activity and crystal adhesion inhibition of polysaccharides with different molecular weights from red algae Porphyra yezoensis against oxalate-induced oxidative damage in renal epithelial cells. Food Funct. 2019, 10, 3851–3867. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, L.; Zhang, J.; Wang, J.; Zhang, Q.; Duan, D.; Zhang, Q. Oligo-porphyran ameliorates neurobehavioral deficits in parkinsonian mice by regulating the PI3K/Akt/Bcl-2 pathway. Mar. Drugs 2018, 16, 82. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free. Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef] [Green Version]
- Rehman, I.U.; Ahmad, R.; Khan, I.; Lee, H.J.; Park, J.; Ullah, R.; Choi, M.J.; Kang, H.Y.; Kim, M.O. Nicotinamide Ameliorates Amyloid Beta-Induced Oxidative Stress-Mediated Neuroinflammation and Neurodegeneration in Adult Mouse Brain. Biomedicines 2021, 9, 408. [Google Scholar] [CrossRef]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease. Front. Pharmacol. 2016, 6, 321. [Google Scholar] [CrossRef] [Green Version]
- Stanley, M.; Macauley, S.L.; Holtzman, D.M. Changes in insulin and insulin signaling in Alzheimer’s disease: Cause or consequence? J. Exp. Med. 2016, 213, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Syad, A.N.; Devi, K.P. Assessment of anti-amyloidogenic activity of marine red alga G. acerosa against Alzheimer’s beta-amyloid peptide 25–35. Neurol. Res. 2015, 37, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, H.; Fu, L.; Ci, F.; Mao, X. Porphyran and oligo-porphyran originating from red algae Porphyra: Preparation, biological activities, and potential applications. Food Chem. 2021, 349, 129209. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.H.; Ko, G.; Jo, H.; Oh, T.; Ahn, B.; Unno, T. Anti-Inflammatory properties and gut microbiota modulation of Porphyra tenera extracts in dextran sodium sulfate-induced colitis in mice. Antioxidants 2020, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-M.; Xu, S.-S.; Li, L.; Pan, T.-M.; Shi, C.-L.; Liu, H.; Cao, M.-J.; Su, W.-J.; Liu, G.-M. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hwang, J.-Y.; Park, H.-B.; Yadav, D.; Oda, T.; Jin, J.-O. Porphyran isolated from Pyropia yezoensis inhibits lipopolysaccharide-induced activation of dendritic cells in mice. Carbohydr. Polym. 2020, 229, 115457. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [Green Version]
- Righi, V.; Parenti, F.; Schenetti, L.; Mucci, A. Mycosporine-like amino acids and other phytochemicals directly detected by high-resolution NMR on Klamath (Aphanizomenon flos-aquae) blue-green algae. J. Agric. Food Chem. 2016, 64, 6708–6715. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Bird, A.; Kopec, R.E. The Metabolism and Potential Bioactivity of Chlorophyll and Metallo-chlorophyll Derivatives in the Gastrointestinal Tract. Mol. Nutr. Food Res. 2021, 65, 2000761. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Bork, P.M.; Schmitz, M.L.; Rimpler, H.; Frei, B.; Sticher, O. Pheophorbide A from Solanum diflorum interferes Please add the name of the publisher and the location of it.with NF-κB activation. Planta Med. 2001, 67, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.; An, Y.; Zhou, J.; Yang, J.; Zhang, Y.; Yang, J.; Wang, L.; Li, X.; Lu, D.; Zhong, J. Subchronic exposure to concentrated ambient PM2.5 perturbs gut and lung microbiota as well as metabolic profiles in mice. Environ. Pollut. 2021, 272, 115987. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef]
- Gareau, M.G. Microbiota-gut-brain axis and cognitive function. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health And Disease. Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 817, pp. 357–371. [Google Scholar]
- Xu, M.; Mo, X.; Huang, H.; Chen, X.; Liu, H.; Peng, Z.; Chen, L.; Rong, S.; Yang, W.; Xu, S. Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ1–42-induced AD-like mice. Int. J. Biol. Macromol. 2020, 161, 258–270. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Y.; Wang, Y.; Wang, P.; Song, H.; Wang, F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 2019, 14, e0218384. [Google Scholar] [CrossRef]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2013, 61, 71–90. [Google Scholar] [CrossRef]
- Yang, H.-S.; Haj, F.G.; Lee, M.; Kang, I.; Zhang, G.; Lee, Y. Laminaria japonica extract enhances intestinal barrier function by altering inflammatory response and tight junction-related protein in lipopolysaccharide-stimulated Caco-2 cells. Nutrients 2019, 11, 1001. [Google Scholar] [CrossRef] [Green Version]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.-Y.; Huang, D.-Y.; Zhang, H.-J.; Wang, S.; Chen, X.-F. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int. Immunopharmacol. 2017, 50, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, S.; Jung, K.; Pham, T.N.A.; Yang, S.; Ahn, B. Potential Prebiotic and Anti-Obesity Effects of Codium fragile Extract. Appl. Sci. 2022, 12, 959. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, R.G. Morris water maze. Scholarpedia 2008, 3, 6315. [Google Scholar] [CrossRef]
- Wang, D.-M.; Li, S.-Q.; Wu, W.-L.; Zhu, X.-Y.; Wang, Y.; Yuan, H.-Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef]
- Lutfia, F.N.L.; Isnansetyo, A.; Susidarti, R.A.; Nursid, M. Chemical composition diversity of fucoidans isolated from three tropical brown seaweeds (Phaeophyceae) species. Biodivers. J. Biol. Divers. 2020, 21, 3170–3177. [Google Scholar] [CrossRef]









| Total Polysaccharide (%) | Mw (kDa) | Sulfate (%) | Relative Area (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Fucose | Rhamnose | Galactose | Glucose | Xylose | Others | |||
| 46.23 ± 0.39 | 220.49 | 43.25 | 6.52 | 7.83 | 44.24 | - | 23.18 | 18.23 |
| Retention Time (min) | ESI+ (m/z) | Collision Energy (eV) | Fragment Ion | Identified Compounds |
|---|---|---|---|---|
| 0.83 | 347.1450 | 25 | 303, 244, 227, 200, 186 | porphyra-334 isomer |
| 1.50 | 347.1450 | 25 | 303, 244, 227, 200, 186 | porphyra-334 isomer |
| 4.03 | 285.1438 | 25 | 241, 197 | palythene |
| 4.54 | 329.1333 | 20 | 279, 253, 233, 205, 187, 150 | palythenic acid |
| 9.87 | 593.2760 | 40 | 533, 460, 447 | pheophorbide a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, M.J.; Lee, H.L.; Moon, J.H.; Jeong, H.R.; Kim, H.-J.; Chung, M.-Y.; Heo, H.J. Porphyra tenera Protects against PM2.5-Induced Cognitive Dysfunction with the Regulation of Gut Function. Mar. Drugs 2022, 20, 439. https://doi.org/10.3390/md20070439
Park SK, Kang JY, Kim JM, Kim MJ, Lee HL, Moon JH, Jeong HR, Kim H-J, Chung M-Y, Heo HJ. Porphyra tenera Protects against PM2.5-Induced Cognitive Dysfunction with the Regulation of Gut Function. Marine Drugs. 2022; 20(7):439. https://doi.org/10.3390/md20070439
Chicago/Turabian StylePark, Seon Kyeong, Jin Yong Kang, Jong Min Kim, Min Ji Kim, Hyo Lim Lee, Jong Hyun Moon, Hye Rin Jeong, Hyun-Jin Kim, Min-Yu Chung, and Ho Jin Heo. 2022. "Porphyra tenera Protects against PM2.5-Induced Cognitive Dysfunction with the Regulation of Gut Function" Marine Drugs 20, no. 7: 439. https://doi.org/10.3390/md20070439
APA StylePark, S. K., Kang, J. Y., Kim, J. M., Kim, M. J., Lee, H. L., Moon, J. H., Jeong, H. R., Kim, H. -J., Chung, M. -Y., & Heo, H. J. (2022). Porphyra tenera Protects against PM2.5-Induced Cognitive Dysfunction with the Regulation of Gut Function. Marine Drugs, 20(7), 439. https://doi.org/10.3390/md20070439