Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing
Abstract
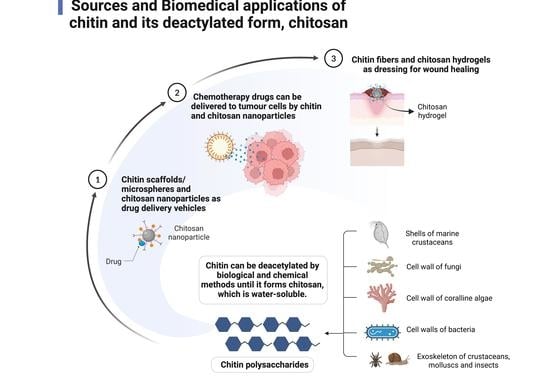
:1. Introduction
2. Chitosan as a Possible Drug Delivery Agent
3. Application of Chitin and Chitosan in Cancer Therapy
4. Potential for Chitin and Chitosan in Wound Healing
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Parhi, R. Chitin and Chitosan in Drug Delivery. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–239. ISBN 978-3-030-16581-9. [Google Scholar]
- Ahmad, A.; Mubarak, N.M.; Naseem, K.; Tabassum, H.; Rizwan, M.; Najda, A.; Kashif, M.; Bin-Jumah, M.; Hussain, A.; Shaheen, A.; et al. Recent Advancement and Development of Chitin and Chitosan-Based Nanocomposite for Drug Delivery: Critical Approach to Clinical Research. Arab. J. Chem. 2020, 13, 8935–8964. [Google Scholar] [CrossRef]
- Dave, U.; Somanader, E.; Baharlouei, P.; Pham, L.; Rahman, M.A. Applications of Chitin in Medical, Environmental, and Agricultural Industries. J. Mar. Sci. Eng. 2021, 9, 1173. [Google Scholar] [CrossRef]
- Rahman, M.A.; Halfar, J. First Evidence of Chitin in Calcified Coralline Algae: New Insights into the Calcification Process of Clathromorphum Compactum. Sci. Rep. 2015, 4, 6162. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Halfar, J.; Adey, W.H.; Nash, M.; Paulo, C.; Dittrich, M. The Role of Chitin-Rich Skeletal Organic Matrix on the Crystallization of Calcium Carbonate in the Crustose Coralline Alga Leptophytum Foecundum. Sci. Rep. 2019, 9, 11869. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Shoeb Khan, M.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and Its Derivatives: Structural Properties and Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Singh, S.K. Solubility of Lignin and Chitin in Ionic Liquids and Their Biomedical Applications. Int. J. Biol. Macromol. 2019, 132, 265–277. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic Liquids in the Processing and Chemical Modification of Chitin and Chitosan for Biomedical Applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Broek, L.A.; Boeriu, C.G.; Stevens, C.V. Chitin and Chitosan: Properties and Applications; Wiley series in renewable resources; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-45046-7. [Google Scholar]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Oh, J.-W. Reviewing Chitin/Chitosan Nanofibers and Associated Nanocomposites and Their Attained Medical Milestones. Polymers 2021, 13, 2330. [Google Scholar] [CrossRef]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Rasti, H.; Parivar, K.; Baharara, J.; Iranshahi, M.; Namvar, F. Chitin from the Mollusc Chiton: Extraction, Characterization and Chitosan Preparation. Iran. J. Pharm. Res. IJPR 2017, 16, 366–379. [Google Scholar] [PubMed]
- Anraku, M.; Ifuku, S.; Iohara, D.; Hirayama, F.; Otagiri, M.; Gebicki, J.M. Future Aspects of Biomedical Applications of Chitin and Chitosan in Diseases Associated with Oxidative Stress. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 589–608. ISBN 978-0-12-817966-6. [Google Scholar]
- Tissera, W.M.J.C.; Rathnayake, S.I.; Abeyrathne, E.D.N.S.; Nam, K.-C. An Improved Extraction and Purification Method for Obtaining High-Quality Chitin and Chitosan from Blue Swimmer (Portunus Pelagicus) Crab Shell Waste. Food Sci. Biotechnol. 2021, 30, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Özel, N.; Elibol, M. A Review on the Potential Uses of Deep Eutectic Solvents in Chitin and Chitosan Related Processes. Carbohydr. Polym. 2021, 262, 117942. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachapur, V.L.; Guemiza, K.; Rouissi, T.; Sarma, S.J.; Brar, S.K. Novel Biological and Chemical Methods of Chitin Extraction from Crustacean Waste Using Saline Water: Novel Biological and Chemical Methods of Chitin Extraction. J. Chem. Technol. Biotechnol. 2016, 91, 2331–2339. [Google Scholar] [CrossRef] [Green Version]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in Chitin Analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and Characterization of Chitin and Chitosan from Fishery Waste by Chemical Method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2012, 51, 12. [Google Scholar]
- Kaya, M.; Baran, T.; Karaarslan, M. A New Method for Fast Chitin Extraction from Shells of Crab, Crayfish and Shrimp. Nat. Prod. Res. 2015, 29, 1477–1480. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Schimpf, C.; Rafaja, D.; Brendler, E.; Viehweger, C.; Żółtowska-Aksamitowska, S.; Petrenko, I.; et al. Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena Versicolor Spider Molt Cuticle. Molecules 2019, 24, 3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally Drug-Loaded Chitin: Isolation and Applications. Mar. Drugs 2019, 17, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Lv, S.; Zhong, Y.; Jiang, X. Injectable Hydroxypropyl Chitin Hydrogels Embedded with Carboxymethyl Chitin Microspheres Prepared via a Solvent-Free Process for Drug Delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 1564–1583. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and Its Use in Imaging and Drug Delivery (Review). Biomed. Rep. 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-Based Nanoparticles: An Overview of Biomedical Applications and Its Preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [Green Version]
- Shinde, U.A.; Joshi, P.N.; Jain, D.D.; Singh, K. Preparation and Evaluation of N-Trimethyl Chitosan Nanoparticles of Flurbiprofen for Ocular Delivery. Curr. Eye Res. 2019, 44, 575–582. [Google Scholar] [CrossRef]
- Zhao, R.; Li, J.; Wang, J.; Yin, Z.; Zhu, Y.; Liu, W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 2017, 18, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.; Weyers, M.; Steenekamp, J.H.; Steyn, J.D.; Gouws, C.; Hamman, J.H. Drug Bioavailability Enhancing Agents of Natural Origin (Bioenhancers) That Modulate Drug Membrane Permeation and Pre-Systemic Metabolism. Pharmaceutics 2019, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric Coating of Oral Solid Dosage Forms as a Tool to Improve Drug Bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C.; Holmström, M.O. Perspectives on Interferon-Alpha in the Treatment of Polycythemia Vera and Related Myeloproliferative Neoplasms: Minimal Residual Disease and Cure? Semin. Immunopathol. 2019, 41, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, A.; Tsomos, E.; Hammerstad, S.S.; Tomer, Y. Interferon Alpha: The Key Trigger of Type 1 Diabetes. J. Autoimmun. 2018, 94, 7–15. [Google Scholar] [CrossRef]
- Talpaz, M.; Mercer, J.; Hehlmann, R. The Interferon-Alpha Revival in CML. In Chronic Myeloid Leukemia; Hehlmann, R., Ed.; Hematologic Malignancies; Springer International Publishing: Cham, Switzerland, 2021; pp. 179–226. ISBN 978-3-030-71913-5. [Google Scholar]
- Pires de Mello, C.P.; Tao, X.; Kim, T.H.; Bulitta, J.B.; Rodriquez, J.L.; Pomeroy, J.J.; Brown, A.N. Zika Virus Replication Is Substantially Inhibited by Novel Favipiravir and Interferon Alpha Combination Regimens. Antimicrob. Agents Chemother. 2018, 62, e01983-17. [Google Scholar] [CrossRef] [Green Version]
- Fleischmann, W.R.; Koren, S.; Fleischmann, C.M. Orally Administered Interferons Exert Their White Blood Cell Suppressive Effects via a Novel Mechanism. Proc. Soc. Exp. Biol. Med. 1992, 201, 200–207. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Edison, T.N.J.I.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan Nanopolymers: An Overview of Drug Delivery against Cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef]
- Wang, G.; Li, R.; Parseh, B.; Du, G. Prospects and Challenges of Anticancer Agents’ Delivery via Chitosan-Based Drug Carriers to Combat Breast Cancer: A Review. Carbohydr. Polym. 2021, 268, 118192. [Google Scholar] [CrossRef]
- Dev, A.; Mohan, J.C.; Sreeja, V.; Tamura, H.; Patzke, G.R.; Hussain, F.; Weyeneth, S.; Nair, S.V.; Jayakumar, R. Novel Carboxymethyl Chitin Nanoparticles for Cancer Drug Delivery Applications. Carbohydr. Polym. 2010, 79, 1073–1079. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical Applications of Chitin and Chitosan Based Nanomaterials—A Short Review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel Carboxymethyl Derivatives of Chitin and Chitosan Materials and Their Biomedical Applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Saewan, N. Utilization of Carboxymethyl Chitosan in Cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.; Li, L.; Su, X.; Wang, K.; Lu, Z.; Yuan, C.; Feng, J.; Yan, S.; Kong, B.; Song, K. Development and Evaluation of Novel Tumor-Targeting Paclitaxel-Loaded Nano-Carriers for Ovarian Cancer Treatment: In Vitro and in Vivo. J. Exp. Clin. Cancer Res. 2018, 37, 29. [Google Scholar] [CrossRef] [Green Version]
- Barani, M.; Bilal, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanotechnology in Ovarian Cancer: Diagnosis and Treatment. Life Sci. 2021, 266, 118914. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, M.; Wang, L.; Zielinski, R.J.; Qian, W.; Wang, Y.A.; Mohs, A.M.; Kairdolf, B.A.; Ji, X.; Capala, J.; Lipowska, M.; et al. Targeted Drug Delivery and Image-Guided Therapy of Heterogeneous Ovarian Cancer Using HER2-Targeted Theranostic Nanoparticles. Theranostics 2019, 9, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-Cured Glycol Chitosan Hydrogel for Ovarian Cancer Drug Delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Lu, X.; Feng, Y.-J. Glycogen Synthase Kinase-3β Positively Regulates the Proliferation of Human Ovarian Cancer Cells. Cell Res. 2006, 16, 671–677. [Google Scholar] [CrossRef]
- Du, X.; Yin, S.; Xu, L.; Ma, J.; Yu, H.; Wang, G.; Li, J. Polylysine and Cysteine Functionalized Chitosan Nanoparticle as an Efficient Platform for Oral Delivery of Paclitaxel. Carbohydr. Polym. 2020, 229, 115484. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Yousefi, B.; Asemi, Z.; Nikfar, B.; Mansournia, M.A.; Hallajzadeh, J. Chitosan: A Compound for Drug Delivery System in Gastric Cancer-a Review. Carbohydr. Polym. 2020, 242, 116403. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Upadhyay, S.; Singh, M.; Sharma, I.; Sharma, P.; Kamboj, P.; Saini, A.; Voraha, R.; Sharma, A.; Upadhyay, T.; et al. Chitin, Chitinases and Chitin Derivatives in Biopharmaceutical, Agricultural and Environmental Perspective. Biointerface Res. Appl. Chem. 2020, 11, 9985–10005. [Google Scholar] [CrossRef]
- Asiri, S.M.; Khan, F.A.; Bozkurt, A. Synthesis of Chitosan Nanoparticles, Chitosan-Bulk, Chitosan Nanoparticles Conjugated with Glutaraldehyde with Strong Anti-Cancer Proliferative Capabilities. Artif. Cells Nanomedicine Biotechnol. 2018, 46, S1152–S1161. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.; Priya, K.; Ilavenil, S.; Janani, B.; Vedarethinam, V.; Ramesh, T.; Arasu, M.V.; Al-Dhabi, N.A.; Kim, Y.-O.; Kim, H.-J. Shrimp Shells Extracted Chitin in Silver Nanoparticle Synthesis: Expanding Its Prophecy towards Anticancer Activity in Human Hepatocellular Carcinoma HepG2 Cells. Int. J. Biol. Macromol. 2020, 165, 1402–1409. [Google Scholar] [CrossRef]
- Santhana Panneer, D.; Tirunavukkarasu, S.; Sadaiyandi, V.; Rajendiran, N.; Mohammad, F.; Oh, W.-C.; Sagdevan, S. Antiproliferative Potentials of Chitin and Chitosan Encapsulated Gold Nanoparticles Derived from Unhatched Artemia Cysts. Chem. Phys. Lett. 2022, 790, 139345. [Google Scholar] [CrossRef]
- Mumyatova, V.A.; Balakina, A.A.; Sen’, V.D.; Pliss, E.M.; Terent’ev, A.A. Effect of Chitosan-(Poly)Nitroxides on Normal and Tumor Cells under Conditions of Induced Oxidative Stress. Bull. Exp. Biol. Med. 2019, 166, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Kurishima, K.; Miyazaki, K.; Watanabe, H.; Shiozawa, T.; Ishikawa, H.; Satoh, H.; Hizawa, N. Lung Cancer Patients with Synchronous Colon Cancer. Mol. Clin. Oncol. 2018, 8, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Kasinathan, A.; Ganesan, R.; Balasubramanian, A.; Bhaskaran, J.; Suresh, S.; Srinivasan, R.; Aravind, K.B.; Sivalingam, N. Curcumin Induces Apoptosis and Cell Cycle Arrest via the Activation of Reactive Oxygen Species–Independent Mitochondrial Apoptotic Pathway in Smad4 and P53 Mutated Colon Adenocarcinoma HT29 Cells. Nutr. Res. 2018, 51, 67–81. [Google Scholar] [CrossRef]
- Cheng, M.; Gao, X.; Wang, Y.; Chen, H.; He, B.; Xu, H.; Li, Y.; Han, J.; Zhang, Z. Synthesis of Glycyrrhetinic Acid-Modified Chitosan 5-Fluorouracil Nanoparticles and Its Inhibition of Liver Cancer Characteristics in Vitro and in Vivo. Mar. Drugs 2013, 11, 3517–3536. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, M.; Zou, R.; Mao, S.; Cong, P.; Hou, M.; Jin, H.; Zhao, Y.; Bao, Y. Adipose-Derived Mesenchymal Stem Cell-Loaded β-Chitin Nanofiber Hydrogel Promote Wound Healing in Rats. J. Mater. Sci. Mater. Med. 2022, 33, 12. [Google Scholar] [CrossRef]
- Ma, M.; Zhong, Y.; Jiang, X. Thermosensitive and PH-Responsive Tannin-Containing Hydroxypropyl Chitin Hydrogel with Long-Lasting Antibacterial Activity for Wound Healing. Carbohydr. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef] [PubMed]
- Balitaan, J.N.I.; Hsiao, C.-D.; Yeh, J.-M.; Santiago, K.S. Innovation Inspired by Nature: Biocompatible Self-Healing Injectable Hydrogels Based on Modified-β-Chitin for Wound Healing. Int. J. Biol. Macromol. 2020, 162, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and Chitosan in Selected Biomedical Applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Jafari, H.; Bernaerts, K.V.; Dodi, G.; Shavandi, A. Chitooligosaccharides for Wound Healing Biomaterials Engineering. Mater. Sci. Eng. C 2020, 117, 111266. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, Z.; Duan, B.; Zhang, L.; Yang, Z.; Wang, Y.; Ye, Q. Novel Fibers Fabricated Directly from Chitin Solution and Their Application as Wound Dressing. J. Mater. Chem. B 2014, 2, 3427. [Google Scholar] [CrossRef]
- Santos, T.C.; Höring, B.; Reise, K.; Marques, A.P.; Silva, S.S.; Oliveira, J.M.; Mano, J.F.; Castro, A.G.; Reis, R.L.; van Griensven, M. In Vivo Performance of Chitosan/Soy-Based Membranes as Wound-Dressing Devices for Acute Skin Wounds. Tissue Eng. Part A 2013, 19, 860–869. [Google Scholar] [CrossRef] [Green Version]
- Hemmingsen, L.M.; Škalko-Basnet, N.; Jøraholmen, M.W. The Expanded Role of Chitosan in Localized Antimicrobial Therapy. Mar. Drugs 2021, 19, 697. [Google Scholar] [CrossRef]
- Hemmingsen, L.M.; Julin, K.; Ahsan, L.; Basnet, P.; Johannessen, M.; Škalko-Basnet, N. Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Mar. Drugs 2021, 19, 269. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. https://doi.org/10.3390/md20070460
Baharlouei P, Rahman A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Marine Drugs. 2022; 20(7):460. https://doi.org/10.3390/md20070460
Chicago/Turabian StyleBaharlouei, Parnian, and Azizur Rahman. 2022. "Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing" Marine Drugs 20, no. 7: 460. https://doi.org/10.3390/md20070460
APA StyleBaharlouei, P., & Rahman, A. (2022). Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Marine Drugs, 20(7), 460. https://doi.org/10.3390/md20070460