Synthesis and Evaluation of Marine-Inspired Compounds Result in Hybrids with Antitrypanosomal and Antileishmanial Activities
Abstract
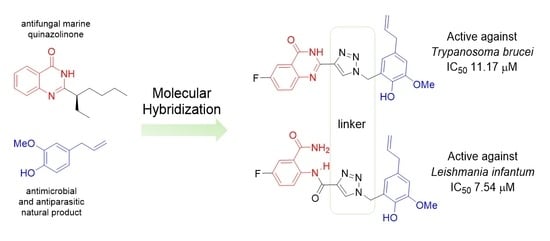
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Tests
2.2.1. Antiparasitic Activity
2.2.2. Antimicrobial Activity
3. Materials and Methods
3.1. Chemical Procedures
3.1.1. General Methods
3.1.2. Synthesis and Structure Elucidation
Synthesis of 4-allyl-2-methoxy-1-(prop-2-ynyloxy)benzene (2), 5-allyl-2-hydroxy-3-methoxybenzyl alcohol (3), and 2-[(azidoacetyl)amino]benzoic acid (12)
Synthesis of 4-allyl-2-azido-6-methoxyphenol (4)
Synthesis of Acetylenic Intermediates 6a and 7a
Synthesis of 2-(propioloylamino)benzamides 8a–8d
Synthesis of Triazoles 9a–9d, 13, and 14
Synthesis of Quinazolinones 10a–10c
3.2. Biological Tests
3.2.1. Antifungal and Antibacterial Assays
Compound Preparation
Fungal and Bacterial Strains
Antifungal Activity
Antibacterial Activity
3.2.2. Antiparasitic Assays
Parasite Cultures
Anti-T. brucei Activity
Anti-Leishmania Activity
3.2.3. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Theel, E.S.; Pritt, B.S. Parasites. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Duarte, D.; Carvalho, C.; Oliveira, R.; Santarém, N.; Palmeira, A.; Resende, D.I.S.P.; Silva, A.M.S.; Moreira, R.; Kijjoa, A.; et al. Indole-containing pyrazino[2,1-b]quinazoline-3,6-diones active against plasmodium and trypanosomatids. ACS Med. Chem. Lett. 2022, 13, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Dziduch, K.; Greniuk, D.; Wujec, M. The current directions of searching for antiparasitic drugs. Molecules 2022, 27, 1534. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Trypanosomiasis, Human African (Sleeping Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 3 October 2023).
- Schmidt, R.S.; Macêdo, J.P.; Steinmann, M.E.; Salgado, A.G.; Bütikofer, P.; Sigel, E.; Rentsch, D.; Mäser, P. Transporters of Trypanosoma brucei-phylogeny, physiology, pharmacology. FEBS J. 2018, 285, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E. Update on human African trypanosomiasis (sleeping sickness). J. Neurol. 2019, 266, 2334–2337. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 3 October 2023).
- Jones, A.J.; Grkovic, T.; Sykes, M.L.; Avery, V.M. Trypanocidal activity of marine natural products. Mar. Drugs 2013, 11, 4058–4082. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Zhang, L.; Demain, A. Natural Products: Drug Discovery and Therapeutic Medicine; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.-M.; Yang, L.-Y.; Huang, S.-S.; Chigor, V.N.; Eze, E.A.; Pan, L.-X.; Zhang, T.; Yang, D.-F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: A review of recent articles. Infect. Dis. Poverty 2021, 10, 9. [Google Scholar] [CrossRef]
- He, D.; Wang, M.; Zhao, S.; Shu, Y.; Zeng, H.; Xiao, C.; Lu, C.; Liu, Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Fu, H.; Zhong, H.; Hong, K.; Zhu, W. Antifungal quinazolinones from marine-derived Bacillus cereus and their preparation. Bioorg. Med. Chem. Lett. 2011, 21, 4005–4007. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; Gazolla, P.A.R.; da Silva, A.M.; Borsodi, M.P.G.; Bergmann, B.R.; Ferreira, R.S.; Vaz, B.G.; Vasconcelos, G.A.; Lima, W.P. Synthesis and leishmanicidal activity of eugenol derivatives bearing 1,2,3-triazole functionalities. Eur. J. Med. Chem. 2018, 146, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.B.; Caldas, I.S.; Paula, F.R.; Rodrigues, C.C.; Carvalho, D.T.; Dias, D.F. Synthesis, activity, and molecular modeling studies of 1,2,3-triazole derivatives from natural phenylpropanoids as new trypanocidal agents. Chem. Biol. Drug Des. 2020, 95, 124–129. [Google Scholar] [CrossRef]
- Kelleher, J.; McAuliffe, M.; Moynihan, H.; Mullins, N. Studies on the preparation and crystal polymorphism of 2-acetamidobenzamide and related compounds. Arkivoc 2007, 16, 209–226. [Google Scholar] [CrossRef]
- Irfan, M.; Aneja, B.; Yadava, U.; Khan, S.I.; Manzoor, N.; Daniliuc, C.G.; Abid, M. Synthesis, QSAR and anticandidal evaluation of 1,2,3-triazoles derived from naturally bioactive scaffolds. Eur. J. Med. Chem. 2015, 93, 246–254. [Google Scholar] [CrossRef]
- Singh, V.; Prathap, S. Reaction of isoeugenol with formaldehyde in basic medium: Formation of trans-4-(4-hydroxy-3-hydroxymethyl-5-methoxy) phenyl-5-methyl-1, 3-dioxane and its transformation into the tricyclo [5.2. 2.02, 6] undecane System. J. Chem. Res. Synop. 1997, 11, 422–423. [Google Scholar] [CrossRef]
- Magnani, J.L.P.; John, M.; Sarkar, A.K.; Vohra, Y.U.; Baek, M.-G. Preparation of Galactopyranosyl-cyclohexyl Derivatives as E-Selectin Antagonists. US Patent No. 17/522, 378, 19 November 2021. [Google Scholar]
- Aarjane, M.; Slassi, S.; Tazi, B.; Maouloua, M.; Amine, A. Novel series of acridone-1,2,3-triazole derivatives: Microwave-assisted synthesis, DFT study and antibacterial activities. J. Chem. Sci. 2019, 131, 85. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Ismail, M.F.; Amr, A.E.-G.E.; Naglah, A.M. Synthesis, antiproliferative, and antioxidant evaluation of 2-pentylquinazolin-4(3H)-one(thione) derivatives with DFT Study. Molecules 2019, 24, 3787. [Google Scholar] [CrossRef]
- Lee, E.S.; Son, J.K.; Na, Y.H.; Jahng, Y. Synthesis and biological properties of selected 2-aryl-4(3H)-quinazolinones. Heterocycl. Commun. 2004, 10, 325–330. [Google Scholar] [CrossRef]
- Ornelas, C.; Ruiz Aranzaes, J.; Cloutet, E.; Alves, S.; Astruc, D. Click assembly of 1,2,3-triazole-linked dendrimers, including ferrocenyl dendrimers, which sense both oxo anions and metal cations. Angew. Chem. Int. Ed. Engl. 2007, 46, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Fekner, T.; Müller-Bunz, H.; Guiry, P.J. Synthesis, resolution, and application of cyclobutyl- and adamantyl-quinazolinap ñigands. Eur. J. Org. Chem. 2008, 30, 5055–5066. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 25th Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Sereno, D.; Cavaleyra, M.; Zemzoumi, K.; Maquaire, S.; Ouaissi, A.; Lemesre, J.L. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob. Agents Chemother. 1998, 42, 3097–3102. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Anti-Parasitic Activity IC50 (µM) 95% CI | Cytotoxicity CC50 (µM) 95% CI | SI | ||
|---|---|---|---|---|---|
| L. infantum Promastigotes | T. brucei | THP-1 | L. infantum | T. brucei | |
| 9a | >40 | >40 | >100 | - | -- |
| - | - | - | |||
| 9b | >40 | 16.39 | >100 | - | >6 |
| - | (14.04–19.06) | - | |||
| 9c | 7.54 (5.85–9.58) | 21.03 | 45.82 | 6 | 2 |
| (15.93–28.62) | (38.04–55.30) | ||||
| 9d | >40 | 15.92 | > 100 | - | >6 |
| - | (13.42–18.89) | - | |||
| 10a | >40 | >40 | >100 | - | -- |
| - | - | - | |||
| 10b | >40 | 19.9 | >100 | - | >5 |
| - | (17.73–22.29) | - | |||
| 10c | >40 | 11.17 | >100 | - | >9 |
| - | (7.97–15.28) | - | |||
| 13 | >40 | >40 | >100 | - | -- |
| - | - | - | |||
| 14 | >40 | 31.68 | >100 | - | >3 |
| - | (27.98–36.34) | - | |||
| Eugenol | >40 | >40 | >100 | - | - |
| - | - | - | |||
| Pentamidine | NT | 0.0056 | 37.71 (31.75–43.57) | 6734 | |
| (0.0052–0.006) | |||||
| Miltefosine | 10.98 (10.17–12.15) | NT | 29.38 (23.97–36.02) | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.T.; Teixeira, M.; Luelmo, S.; Santarém, N.; Pinto, E.; Cordeiro-da-Silva, A.; Sousa, E. Synthesis and Evaluation of Marine-Inspired Compounds Result in Hybrids with Antitrypanosomal and Antileishmanial Activities. Mar. Drugs 2023, 21, 551. https://doi.org/10.3390/md21110551
Carvalho DT, Teixeira M, Luelmo S, Santarém N, Pinto E, Cordeiro-da-Silva A, Sousa E. Synthesis and Evaluation of Marine-Inspired Compounds Result in Hybrids with Antitrypanosomal and Antileishmanial Activities. Marine Drugs. 2023; 21(11):551. https://doi.org/10.3390/md21110551
Chicago/Turabian StyleCarvalho, Diogo Teixeira, Melissa Teixeira, Sara Luelmo, Nuno Santarém, Eugénia Pinto, Anabela Cordeiro-da-Silva, and Emília Sousa. 2023. "Synthesis and Evaluation of Marine-Inspired Compounds Result in Hybrids with Antitrypanosomal and Antileishmanial Activities" Marine Drugs 21, no. 11: 551. https://doi.org/10.3390/md21110551
APA StyleCarvalho, D. T., Teixeira, M., Luelmo, S., Santarém, N., Pinto, E., Cordeiro-da-Silva, A., & Sousa, E. (2023). Synthesis and Evaluation of Marine-Inspired Compounds Result in Hybrids with Antitrypanosomal and Antileishmanial Activities. Marine Drugs, 21(11), 551. https://doi.org/10.3390/md21110551