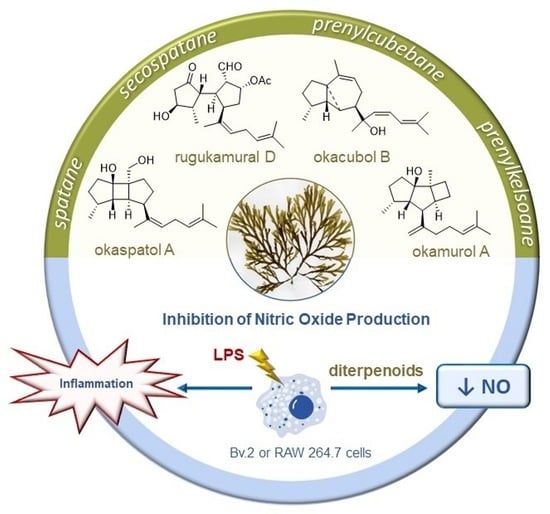
Rugulopteryx-Derived Spatane, Secospatane, Prenylcubebane and Prenylkelsoane Diterpenoids as Inhibitors of Nitric Oxide Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spatane Diterpenoids 1–7
2.2. Secospatane Diterpenoids 8–12
2.3. Prenylcubebane Diterpenoids 13–15
2.4. Prenylkelsoane Diterpenoid 16
2.5. Inhibitory Activity of NO Production
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Algae Collection
3.3. Extraction and Isolation
3.4. Characterization of Compounds
3.5. Cell Culture
3.6. Analysis of the Cellular Viability by Crystal Violet Staining
3.7. Analysis of Nitrites (NO2−)
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Clerck, O.; Leliaert, F.; Verbruggen, H.; Lane, C.E.; De Paula, J.C.; Payo, D.A.; Coppejans, E. A revised classification of the Dictyoteae (Dictyotales, Paheophyceae) based on rbcL and 26S ribosomal DNA sequence analysis. J. Phycol. 2006, 42, 1271–1288. [Google Scholar] [CrossRef]
- Hwang, I.K.; Lee, W.J.; Kim, H.S.; De Clerck, O. Taxonomic reappraisal of Dilophus okamurae (Dictyotales, phaeophyta) from the western Pacific ocean. Phycologia 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 11 February 2023).
- García-Gómez, J.C.; Sempere-Valverde, J.; Gonzalez, A.R.; Martínez-Chacón, M.; Olaya-Ponzone, L.; Sánchez-Moyano, E.; Ostalé-Valriberas, E.; Megina, C. From exotic to invasive in record time: The extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the Strait of Gibraltar. Sci. Total Environ. 2020, 704, 135408. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.N.; Wells, R.J. A series of new diterpenes from the brown alga Dilophus marginatus (Dictyotaceae). Aust. J. Chem. 1982, 35, 129–144. [Google Scholar] [CrossRef]
- Ochi, M.; Masui, N.; Kotsuki, H.; Miura, I.; Tokoroyama, T. The structures of fukurinolal and fukurinal, two new diterpenoids from the brown seaweed Dilophus okamurai Dawson. Chem. Lett. 1982, 11, 1927–1930. [Google Scholar] [CrossRef]
- Kurata, K.; Shiraishi, K.; Takato, T.; Taniguchi, K.; Suzuki, M. A new feeding-deterrent diterpenoid from the brown alga Dilophus okamurai Dawson. Chem. Lett. 1988, 17, 1629–1632. [Google Scholar] [CrossRef]
- Kurata, K.; Suzuki, M.; Shiraishi, K.; Taniguchi, K. Spatane-type diterpenes with biological activity from the brown alga Dilophus okamurai. Phytochemistry 1988, 27, 1321–1324. [Google Scholar] [CrossRef]
- Kurata, K.; Taniguchi, K.; Shiraishi, K.; Suzuki, M. Structures of secospatane-type diterpenes with feeding-deterrent activity from the brown alga Dilophus okamurai. Tetrahedron Lett. 1989, 30, 1567–1570. [Google Scholar] [CrossRef]
- Kurata, K.; Taniguchi, K.; Shiraishi, K.; Suzuki, M. Feeding-deterrent diterpenes from the brown alga Dilophus okamurai. Phytochemistry 1990, 29, 3453–3455. [Google Scholar] [CrossRef]
- Ninomiya, M.; Hirohara, H.; Onishi, J.I.; Kusumi, T. Chemical study and absolute configuration of a new marine secospatane from the brown alga Dilophus okamurae. J. Org. Chem. 1999, 64, 5436–5440. [Google Scholar] [CrossRef]
- Yamase, H.; Umemoto, K.; Ooi, T.; Kusumi, T. Structures and absolute stereochemistry of five new secospatanes and a spatane isolated from the brown alga Dilophus okamurai Dawson. Chem. Pharm. Bull. 1999, 47, 813–818. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamada, H.; Kurata, K. Dictyterpenoids A and B, two novel diterpenoids with feeding-deterrent activity from the brown alga Dilophus okamurae. J. Nat. Prod. 2002, 65, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A novel chemical weapon involved in the invasive capacity of the alga Ruguloptexyx okamurae in the Strait of Gibraltar. Estuar. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar] [CrossRef]
- Cuevas, B.; Arroba, A.I.; De los Reyes, C.; Gómez-Jaramillo, L.; González-Montelongo, M.C.; Zubía, E. Diterpenoids from the brown alga Rugulopteryx okamurae and their anti-inflammatory activity. Mar. Drugs 2021, 19, 677. [Google Scholar] [CrossRef]
- Van Altena, I.A.; McGivern, J. New spatane-derived diterpenoids from the Australian brown alga Dictyota fenestrata. Aust. J. Chem. 1992, 45, 541–551. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Fenical, W.; Van Engen, D.; Clardy, J. Isolation and structure of spatol, a potent inhibitor of cell replication from the brown seaweed Spatoglossum schmittii. J. Am. Chem. Soc. 1980, 102, 7991–7993. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Fenical, W. Spatane diterpenoids from the tropical marine algae Spatoglossum schmittii and Spatoglossum howleii (Dictyotaceae). J. Org. Chem. 1983, 48, 3325–3329. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Fenical, W.; Sultanbawa, M.U.S. Spatane diterpenoids from the tropical marine alga Stoechospermum marginatum (Dictyotaceae). J. Org. Chem. 1981, 46, 2233–2241. [Google Scholar] [CrossRef]
- Rao, C.B.; Pullaiah, K.C.; Surapaneni, R.K.; Suryaprabha, R.; Raju, V.S.N. A new spatane diterpenoid from Stoechospermum marginatum. Ind. J. Chem. B 1987, 26B, 79–80. [Google Scholar]
- Wahidullah, S.; Kamat, S.Y.; Paknikar, S.K.; Bates, R.B. 5(R)-Acetoxyspata-13,17-diene, a novel diterpenoid from the brown alga Stoechospermum marginatum. Planta Med. 1988, 54, 270. [Google Scholar] [CrossRef]
- Venkateswarlu, Y.; Biabani, M.A.F. A spatane diterpene from the brown alga Stoechospermum marginatum. Phytochemistry 1995, 40, 331–333. [Google Scholar] [CrossRef]
- De Rosa, S.; Iodice, C.; Khalaghdoust, M.; Oryan, S.; Rustaiyan, A. Spatane diterpenoids from the brown alga Stoechospermum marginatum (Dictyotaceae). Phytochemistry 1999, 51, 1009–1012. [Google Scholar] [CrossRef]
- Li, L.; Sheng, L.; Wang, C.-Y.; Zhou, Y.-B.; Huang, H.; Li, X.-B.; Li, J.; Mollo, E.; Gavagnin, M.; Guo, Y.-W. Diterpenes from the Hainan soft coral Lobophytum cristatum Tixier Durivault. J. Nat. Prod. 2011, 74, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-C.; Wu, Y.-J.; Su, J.-H.; Lin, W.-T.; Lin, Y.-S. A new spatane diterpenoid from the cultured soft coral Sinularia leptoclados. Mar. Drugs 2013, 11, 114–123. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Gruner, M.; Lübken, T.; Islamov, D.; Kataeva, O.; Andriamanantoanina, H.; Bauer, I.; Knölker, H.-J. Chemical constituents of the soft corals Sinularia vanderlandi and Sinularia gravis from the coast of Madagascar. Org. Biomol. Chem. 2016, 14, 989–1001. [Google Scholar] [CrossRef]
- Chen, D.; Li, D.; Shen, S.; Cheng, W. Terpenoids from a Chinese gorgonian Anthogorgia sp. and their antifouling activities. Chin. J. Chem. 2012, 30, 1459–1463. [Google Scholar] [CrossRef]
- Cao, F.; Shao, C.-L.; Liu, Y.-F.; Zhu, H.-J.; Wang, C.Y. Cytotoxic serrulatane-type diterpenoids from the gorgonian Euplexaura sp. and their absolute configurations by vibrational circular dichroism. Sci. Rep. 2017, 7, 12548. [Google Scholar] [CrossRef]
- Crews, P.; Rodríguez, J.; Jaspars, M. Organic Structural Analysis, 2nd ed.; Oxford University Press: New York, NY, USA, 2010; pp. 85–87. [Google Scholar]
- Le Bideau, F.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef]
- Rinkel, J.; Lauterbach, L.; Dickschat, J.S. Spata-13,17-diene synthase-an enzyme with sesqui-, di-, and seterterpene synthase activity from Streptomyces xinghaiensis. Angew. Chem. Int. Ed. 2017, 56, 16385–16389. [Google Scholar] [CrossRef]
- Chinnababu, B.; Reddy, S.P.; Rao, P.S.; Reddy, V.L.; Kumar, B.S.; Rao, J.V.; Prakasham, R.S.; Babu, K.S. Isolation, semi-synthesis and bio-evaluation of spatane derivatives from the brown algae Stoechospermum marginatum. Bioorg. Med. Chem. Lett. 2015, 25, 2479–2483. [Google Scholar] [CrossRef]
- Velatooru, L.R.; Baggu, C.B.; Janapala, V.R. Spatane diterpinoid from the brown algae, Stoechospermum marginatum induces apoptosis via ROS induced mitochondrial mediated caspase dependent pathway in murine B16F10 melanoma cells. Mol. Carcinogen. 2016, 55, 2222–2235. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Minhas, R.; Bansal, Y.; Bansal, G. Inducible nitric oxide synthase inhibitors: A comprehensive update. Med. Res. Rev. 2020, 40, 823–855. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituens fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Ye, B.-R.; Kim, E.-A.; Kim, J.; Kim, M.-S.; Lee, W.W.; Ahn, G.-N.; Kang, N.; Jung, W.-K.; Heo, S.-J. Bis(3-bromo-4,5-dihydroxybenzyl)ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177. [Google Scholar] [CrossRef]
- Ko, E.-Y.; Heo, S.-J.; Cho, S.-H.; Lee, W.; Kim, S.-Y.; Yang, H.-W.; Ahn, G.; Cha, S.-H.; Kwon, S.-H.; Jeong, M.S.; et al. 3-Bromo-5-(ethoxymethyl)-1,2-benzenediol inhibits LPS-induced proinflammatory responses by preventing ROS production and downregulating NF-kB in vitro in a zebrafish model. Int. Immunopharm. 2019, 67, 98–105. [Google Scholar] [CrossRef]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Ahn, G.-N.; Kang, S.-M.; Kang, D.-H.; Affan, A.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef]
- Tamman, M.A.; Daskalaki, M.G.; Tsoureas, N.; Kolliniati, O.; Mahdy, A.; Kampranis, S.C.; Tsatsanis, C.; Roussis, V.; Ioannou, E. Secondary metabolites with anti-inflammatory activity from Laurencia majuscula collected in the Red Sea. Mar. Drugs 2023, 21, 79. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Kang, M.-C.; Lee, W.-W.; Lee, H.-S.; Kamada, T.; Vairappan, C.S.; Jeon, Y.-J. 5β-hydroxypalisadin B isolated from the red alga Laurencia snackeyi attenuates inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Algae 2014, 29, 333–341. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and related diterpenes from the red alga Laurencia inhibit inflammatory bowel disease in mice by suppressing M1 and promoting M2-like macrophage responses. Mar. Drugs 2019, 17, 97. [Google Scholar] [CrossRef]
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Pang, S.M. Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglial cells. J. Appl. Phycol. 2018, 30, 3261–3270. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]









| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, m (J in Hz) | δC, Type | δH, m (J in Hz) | |
| 1 | 36.6, CH | 2.12, m | 36.8, CH | 2.06, m |
| 2 | 34.5, CH2 | 1.78, m 1.28, m | 34.7, CH2 | 1.75, m 1.29, m |
| 3 | 38.4, CH2 | 2.08, ddd (13.2,13.2,6.2) 1.51, ddd (13.2,13.2,6.9) | 38.7, CH2 | 2.08, ddd (13.1,13.1,6.4) 1.48, ddd (13.1,13.1,6.8) |
| 4 | 52.2, C | 52.9, C | ||
| 5 | 79.9, CH | 5.49, d (4.7) | 75.7, CH | 4.17, d (4.5) |
| 6 | 37.0, CH2 | 2.46, ddd (13.6,13.6,4.7) 1.78, m | 38.6, CH2 | 2.26, ddd (13.3,13.3,4.5) 1.64, m |
| 7 | 42.7, CH | 3.19, ddd (13.6,5.9,5.9) | 46.4, CH | 2.97, ddd (13.3,5.5,5.5) |
| 8 | 44.0, CH | 1.84, m | 41.4, CH | 1.72, m |
| 9 | 49.6, CH | 2.15, m | 48.2, CH | 1.87, br dd (6.4,4.5) |
| 10 | 82.4, C | 82.4, C | ||
| 11 | 14.5, CH3 | 0.82, d (6.6) | 15.1, CH3 | 0.80, d (6.8) |
| 12 | 13.6, CH3 | 0.94, s | 14.0, CH3 | 1.02, s |
| 13 | 135.0, C | 149.5, C | ||
| 14 | 24.0, CH3 | 1.76, d (1.1) | 108.9, CH2 | 4.82, s; 4.76, s |
| 15 | 127.3, CH | 5.21, br t (7.2) | 37.8, CH2 | 1.95, m |
| 16 | 27.8, CH2 | 2.70, m | 27.7, CH2 | 2.19, m 1.99, m |
| 17 | 124.5, CH | 5.07, br t (7.2) | 125.4, CH | 5.11, br t (7.0) |
| 18 | 131.8, C | 132.3, C | ||
| 19 | 25.9, CH3 | 1.67, d (1.1) | 25.9, CH3 | 1.66, d (1.0) |
| 20 | 17.8, CH3 | 1.63, br s | 17.7, CH3 | 1.60, br s |
| CH3COO- | 172.6, C | |||
| CH3COO- | 21.2, CH3 | 2.03, s | ||
| Position | 3 | 4 | ||
|---|---|---|---|---|
| δC, Type | δH, m (J in Hz) | δC, Type | δH, m (J in Hz) | |
| 1 | 36.8, CH | 2.10, m | 37.0, CH | 2.06, m |
| 2 | 34.7, CH2 | 1.77, m 1.28, m | 34.5, CH2 | 1.75, m 1.30, m |
| 3 | 38.1, CH2 | 2.08, m 1.56, m | 38.0, CH2 | 2.03, m 1.46, m |
| 4 | 54.1, C | 48.2, C | ||
| 5 | 30.7, CH2 | 2.20, dd (12.8, 6.9) 1.59, m | 35.7, CH2 | 2.11, dd (12.7,6.7) 1.25, m |
| 6 | 30.1, CH2 | 2.10, m 1.72, m | 29.4, CH2 | 1.91, m 1.52, m |
| 7 | 45.5, CH | 2.82, ddd (12.7,5.8,5.8) | 52.2, CH | 2.44, m |
| 8 | 39.8, CH | 1.83, br dd (5.5, 5.2) | 41.6, CH | 1.71, br dd (5.5,4.8) |
| 9 | 49.9, CH | 2.08, m | 48.7, CH | 1.80, br dd (5.5, 5.1) |
| 10 | 81.8, C | 82.2, C | ||
| 11 | 14.4, CH3 | 0.81, d (6.4) | 15.1, CH3 | 0.79, d (6.7) |
| 12 | 65.0, CH2 | 3.63, d (10.8) 3.43, d (10.8) | 20.7, CH3 | 1.01, s |
| 13 | 135.9, C | 134.6, C | ||
| 14 | 24.2, CH3 | 1.76, d (1.2) | 17.9, CH3 | 1.59, br s |
| 15 | 126.7, CH | 5.14, br t (7.0) | 123.6, CH | 5.17, br t (7.1) |
| 16 | 27.8, CH2 | 2.71, m 2.65, m | 27.9, CH2 | 2.72, m 2.68, m |
| 17 | 124.7, CH | 5.07, br t (6.7) | 124.8, CH | 5.09, br t (7.2) |
| 18 | 131.7, C | 131.8, C | ||
| 19 | 25.9, CH3 | 1.66, d (1.1) | 25.9, CH3 | 1.66, br s |
| 20 | 17.8, CH3 | 1.61, br s | 17.8, CH3 | 1.62, br s |
| Position | δC, Type | δH, m (J in Hz) | Position | δC, Type | δH, m (J in Hz) |
|---|---|---|---|---|---|
| 1 | 43.5, CH | 2.50, m | 11 | 14.5, CH3 | 0.92, d (7.4) |
| 2 | 73.5, CH | 4.10, br d (5.6) | 12 | 201.7, CH | 9.59, d (2.3) |
| 3 | 45.1, CH2 | 2.41, dd (19.1,5.6) 2.07, m | 13 | 135.7, C | |
| 4 | 59.4, CH | 3.49, ddd (9.7,7.2,2.3) | 14 | 22.5, CH3 | 1.69, br s |
| 5 | 78.2, CH | 5.63, ddd (7.2,6.6,4.3)m | 15 | 129.8, CH | 5.26, br t (7.1) |
| 6 | 38.1, CH2 | 2.12, m 1.89, ddd (14.2,8.1,4.3) | 16 | 28.1, CH2 | 2.84, ddd (15.5,7.1,7.1) 2.72, ddd (15.7,7.1,7.1) |
| 7 | 41.7, CH | 3.57, m | 17 | 123.9, CH | 5.09, br t (7.1) |
| 8 | 39.3, CH | 2.93, ddd (9.8,9.7,9.3) | 18 | 132.6, C | |
| 9 | 51.4, CH | 2.64, dd (9.8,7.3) | 19 | 25.8, CH3 | 1.69, br s |
| 10 | 220.4, C | 20 | 17.9, CH3 | 1.65, br s | |
| CH3COO | 172.1, C | ||||
| CH3COO | 20.9, CH3 | 1.96, s |
| Position | 13 | 14 | ||
|---|---|---|---|---|
| δC, Type | δH, m (J in Hz) | δC, Type | δH, m (J in Hz) | |
| 1 | 35.8, CH | 2.30, m | 35.8, CH | 2.27, m |
| 2 | 30.8, CH2 | 1.68, m 0.88,m | 30.8, CH2 | 1.66,m 0.91, m |
| 3 | 30.7, CH2 | 2.08, ddd (12.3.11.6,8.3) 1.74, dd (12.3,7.8) | 30.7, CH2 | 2.07, ddd (12.3,11.5,8.3) 1.73, m |
| 4 | 137.9, C | 137.4, C | ||
| 5 | 118.1, CH | 5.25, br d (7.0) | 118.2, CH | 5.20, br d (7.0) |
| 6 | 27.5, CH2 | 1.84, m 1.64, m | 24.2, CH2 | 2.02, m 1.51, m |
| 7 | 34.1, CH | 3.02, m | 44.1, CH | 1.84, ddd (9.3,8.7,3.5) |
| 8 | 24.7, CH | 0.98, m | 21.49, CH | 1.24, m |
| 9 | 34.9, CH | 1.64, m | 35.3, CH | 1.49, dd (4.6,4.6) |
| 10 | 32.1, C | 32.1, C | ||
| 11 | 18.2, CH3 | 1.00, d (6.6) | 18.6, CH3 | 1.03, d (6.5) |
| 12 | 21.7, CH3 | 1.83, br s | 21.52, CH3 | 1.80, br s |
| 13 | 143.3, C | 78.4, C | ||
| 14 | 20.5, CH3 | 1.80, br d (1.0) | 28.1, CH3 | 1.42, s |
| 15 | 126.2, CH | 5.83, br d (10.9) | 134.0, CH | 5.38, d (12.1) |
| 16 | 123.0, CH | 6.52, dd (15.2,10.9) | 126.2, CH | 6.18, dd (12.1,11.7) |
| 17 | 140.4, CH | 5.67, d (15.2) | 123.4, CH | 6.72, br d (11.7) |
| 18 | 71.4, C | 136.2, C | ||
| 19 | 30.09 c, CH3 | 1.27, s | 26.6, CH3 | 1.78, br s |
| 20 | 30.07 c, CH3 | 1.27, s | 17.5, CH3 | 1.72, br s |
| Position | δC, Type | δH, m (J in Hz) | Position | δC, Type | δH, m (J in Hz) |
|---|---|---|---|---|---|
| 1 | 35.7, CH | 2.50, m | 11 | 17.8, CH3 | 0.92, d (7.0) |
| 2 | 32.2, CH2 | 2.02, m 1.34, m | 12 | 21.2, CH3 | 1.19, s |
| 3 | 34.4, CH2 | 1.75, m 1.44, m | 13 | 150.0, C | |
| 4 | 49.1, C | 14 | 110.5, CH2 | 4.94, br s 4.85, c | |
| 5 | 28.1, CH2 | 2.05, m 1.40, m | 15 | 37.7, CH2 | 1.89, m 1.84, m |
| 6 | 16.0, CH2 | 1.89, m 1.52, m | 16 | 27.4, CH2 | 2.09, m 2.05, m |
| 7 | 45.1, CH | 2.29, ddd (8.8,8.3,2.3) | 17 | 125.6, CH | 5.08, br t (7.0) |
| 8 | 45.5, CH | 2.38, dd (11.1,8.3) | 18 | 132.2, C | |
| 9 | 55.3, CH | 2.59, dd (11.1,6.4) | 19 | 25.9, CH3 | 1.65, br s |
| 10 | 94.6, C | 20 | 18.1, CH3 | 1.59, br s |
| Compound | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 6 | 8 | 10 | 11 | 12 | 14 | 16 | |
| Non-cytotoxic dose for Bv.2 (μM) | 10 | 25 | 10 | 10 | 0.1 | 0.5 | 0.5 | 0.1 | 1 | 10 |
| Non-cytotoxic dose for RAW 264.7 (μM) | 1 | 25 | 10 | 10 | 0.1 | 0.5 | 0.5 | 1 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, B.; Arroba, A.I.; de los Reyes, C.; Zubía, E. Rugulopteryx-Derived Spatane, Secospatane, Prenylcubebane and Prenylkelsoane Diterpenoids as Inhibitors of Nitric Oxide Production. Mar. Drugs 2023, 21, 252. https://doi.org/10.3390/md21040252
Cuevas B, Arroba AI, de los Reyes C, Zubía E. Rugulopteryx-Derived Spatane, Secospatane, Prenylcubebane and Prenylkelsoane Diterpenoids as Inhibitors of Nitric Oxide Production. Marine Drugs. 2023; 21(4):252. https://doi.org/10.3390/md21040252
Chicago/Turabian StyleCuevas, Belén, Ana I. Arroba, Carolina de los Reyes, and Eva Zubía. 2023. "Rugulopteryx-Derived Spatane, Secospatane, Prenylcubebane and Prenylkelsoane Diterpenoids as Inhibitors of Nitric Oxide Production" Marine Drugs 21, no. 4: 252. https://doi.org/10.3390/md21040252
APA StyleCuevas, B., Arroba, A. I., de los Reyes, C., & Zubía, E. (2023). Rugulopteryx-Derived Spatane, Secospatane, Prenylcubebane and Prenylkelsoane Diterpenoids as Inhibitors of Nitric Oxide Production. Marine Drugs, 21(4), 252. https://doi.org/10.3390/md21040252