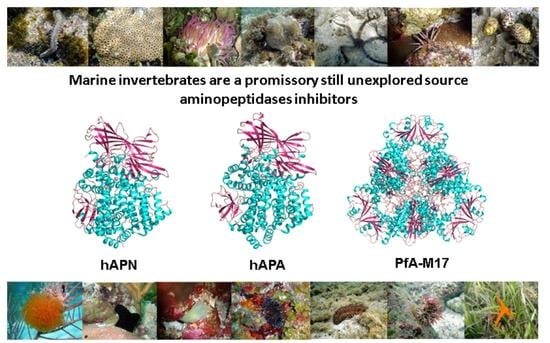
Marine Invertebrates: A Promissory Still Unexplored Source of Inhibitors of Biomedically Relevant Metallo Aminopeptidases Belonging to the M1 and M17 Families
Abstract
:1. Introduction
2. M1 and M17 Metalloexopeptidase Inhibitors Isolated from Marine Invertebrates
2.1. Metallopeptidases: General Characteristics and Classification
2.2. Clan MA: Subclan MA (E)
2.3. M1 Family of Metalloaminopeptidases
2.4. Inhibitors of M1 Family Isolated from Marine Invertebrates
2.4.1. A Specific Inhibitor of Thyrotropin-Releasing Hormone-Degrading Ectoenzyme/Pyroglutamyl Aminopeptidase II Isolated from a Marine Organism
2.4.2. Inhibitors of Aminopeptidase N Isolated from Marine Organisms
Psammaplin A
Identification of Inhibitory Activity of Mammalian APN in Marine Invertebrates from Cuban Coastline
Inhibitors of Aminopeptidase A Isolated from Marine Organisms
2.5. Clan MF: Family M17
2.6. Inhibitors of M17 Leucyl Aminopeptidases from Marine Organisms
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The Merops Database of Proteolytic Enzymes, Their Substrates and Inhibitors in 2017 and a Comparison with Peptidases in the Panther Database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Drag, M.; Salvesen, G.S. Emerging Principles in Protease-Based Drug Discovery. Nat. Rev. Drug Discov. 2010, 9, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Deu, E.; Verdoes, M.; Bogyo, M. New Approaches for Dissecting Protease Functions to Improve Probe Development and Drug Discovery. Nat. Struct. Mol. Biol. 2012, 19, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kryvalap, Y.; Czyzyk, J. The Role of Proteases and Serpin Protease Inhibitors in Β-Cell Biology and Diabetes. Biomolecules 2022, 12, 67. [Google Scholar] [CrossRef]
- Rai, M.; Curley, M.; Coleman, Z.; Demontis, F. Contribution of Proteases to the Hallmarks of Aging and to Age-Related Neurodegeneration. Aging Cell 2022, 21, e13603. [Google Scholar] [CrossRef]
- Verhulst, E.; Garnier, D.; De Meester, I.; Bauvois, B. Validating Cell Surface Proteases as Drug Targets for Cancer Therapy: What Do We Know, and Where Do We Go? Cancers 2022, 14, 624. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Drugs and Drug Candidates from Marine Sources: An Assessment of the Current State of Play. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef]
- Drinkwater, N.; Lee, J.; Yang, W.; Malcolm, T.R.; McGowan, S. M1 Aminopeptidases as Drug Targets: Broad Applications or Therapeutic Niche? FEBS J. 2017, 284, 1473–1488. [Google Scholar] [CrossRef]
- Drinkwater, N.; Malcolm, T.R.; McGowan, S. M17 Aminopeptidases Diversify Function by Moderating Their Macromolecular Assemblies and Active Site Environment. Biochimie 2019, 166, 38–51. [Google Scholar] [CrossRef]
- Hammers, D.; Carothers, K.; Lee, S. The Role of Bacterial Proteases in Microbe and Host-Microbe Interactions. Curr. Drug Targets 2022, 23, 222–239. [Google Scholar] [CrossRef]
- Carvalho, L.A.; Bernardes, G.J. The Impact of Activity-Based Protein Profiling in Malaria Drug Discovery. ChemMedChem 2022, 17, e202200174. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Hortelano, I.; Alcolea, V.; Font, M.; Pérez-Silanes, S. Examination of Multiple Trypanosoma cruzi Targets in a New Drug Discovery Approach for Chagas Disease. Bioorganic Med. Chem. 2022, 58, 116577. [Google Scholar] [CrossRef] [PubMed]
- Vermelho, A.B.; Cardoso, V.; Mansoldo, F.R.P.; Supuran, C.T.; Cedrola, S.M.L.; Rodrigues, I.A.; Rodrigues, G.C. Chagas Disease: Drug Development and Parasite Targets. In Antiprotozoal Drug Development and Delivery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 49–81. [Google Scholar]
- Mukherjee, R.; Dikic, I. Regulation of Host-Pathogen Interactions Via the Ubiquitin System. Annu. Rev. Microbiol. 2022, 76, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Salomon, E.; Schmitt, M.; Marapaka, A.K.; Stamogiannos, A.; Revelant, G.; Schmitt, C.; Alavi, S.; Florent, I.; Addlagatta, A.; Stratikos, E.; et al. Aminobenzosuberone Scaffold as a Modular Chemical Tool for the Inhibition of Therapeutically Relevant M1 Aminopeptidases. Molecules 2018, 23, 2607. [Google Scholar] [CrossRef] [PubMed]
- Albiston, A.L.; Ye, S.; Chai, S.Y. Membrane Bound Members of the M1 Family: More Than Aminopeptidases. Protein Pept. Lett. 2004, 11, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Carl-McGrath, S.; Lendeckel, U.; Ebert, M.; Röcken, C. Ectopeptidases in Tumour Biology: A Review. Histol. Histopathol. 2006, 12, 1339–1353. [Google Scholar]
- Mucha, A.; Drag, M.; Dalton, J.P.; Kafarski, P. Metallo-Aminopeptidase Inhibitors. Biochimie 2010, 92, 1509–1529. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Adhikari, N.; Jha, T. Design of Aminopeptidase N Inhibitors as Anti-Cancer Agents. J. Med. Chem. 2018, 61, 6468–6490. [Google Scholar] [CrossRef]
- Alvarez, C.; Pazos, F.; Soto, C.; Laborde, R.; Lanio, M.E. Pore-Forming Toxins from Sea Anemones: From Protein-Membrane Interaction to Its Implications for Developing Biomedical Applications. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2020; pp. 129–183. [Google Scholar]
- Alvarez, C.; Soto, C.; Cabezas, S.; Alvarado-Mesén, J.; Laborde, R.; Pazos, F.; Ros, U.; Hernández, A.M.; Lanio, M.E. Panorama of the Intracellular Molecular Concert Orchestrated by Actinoporins, Pore-Forming Toxins from Sea Anemones. Toxins 2021, 13, 567. [Google Scholar] [CrossRef]
- Martell, E.M.; González-Garcia, M.; Ständker, L.; Otero-González, A.J. Host Defense Peptides as Immunomodulators: The Other Side of the Coin. Peptides 2021, 146, 170644. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Otero-González, A.; Ghattas, M.; Ständker, L. Discovery, Optimization, and Clinical Application of Natural Antimicrobial Peptides. Biomedicines 2021, 9, 1381. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Ruocco, N.; Mutalipassi, M.; Costantini, M.; Zupo, V.; Coppola, D.; De Pascale, D.; Lauritano, C. Ten-Year Research Update Review: Antiviral Activities from Marine Organisms. Biomolecules 2020, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Fusetani, N. Enzyme Inhibitors from Marine Invertebrates. J. Nat. Prod. 2007, 70, 689–710. [Google Scholar] [CrossRef]
- Pujiastuti, D.Y.; Amin, M.N.G.; Alamsjah, M.A.; Hsu, J.-L. Marine Organisms as Potential Sources of Bioactive Peptides That Inhibit the Activity of Angiotensin I-Converting Enzyme: A Review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef]
- Tischler, D. A Perspective on Enzyme Inhibitors from Marine Organisms. Mar. Drugs 2020, 18, 431. [Google Scholar] [CrossRef] [PubMed]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frangež, R.; Svenson, J. Natural Cholinesterase Inhibitors from Marine Organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Alonso-del-Rivero, M.; Trejo, S.A.; Rodriguez de la Vega, M.; González, Y.; Bronsoms, S.; Canals, F.; Delfín, J.; Diaz, J.; Aviles, F.X.; Chávez, M.A. A Novel Metallocarboxypeptidase-Like Enzyme from the Marine Annelid Sabellastarte magnifica–a Step into the Invertebrate World of Proteases. FEBS J. 2009, 276, 4875–4890. [Google Scholar] [CrossRef]
- Alonso, I.P.; Méndez, L.R.; Almeida, F.; Tresano, M.E.V.; Sánchez, Y.A.; Hernández-Zanuy, A.; Álvarez-Lajonchere, L.; Díaz, D.; Sánchez, B.; Florent, I. Marine Organisms: A Source of Biomedically Relevant Metallo M1, M2 and M17 Exopeptidase Inhibitors. Rev. Cuba. Cienc. Biológicas 2020, 8, 1–36. [Google Scholar]
- Chávez, M.; Delfín, J.; Díaz, J.; Pérez, U.; Martínez, J.; González, J.; Márquez, M.; Más, R. Caracterización de un Inhibidor de Proteasas Obtenido de la Anémona S. helianthus. Rev. CENIC 1988, 19, 82. [Google Scholar]
- Delfin, J.; Gonzalez, Y.; Diaz, J.; Chavez, M. Proteinase Inhibitor from Stichodactyla helianthus: Purification, Characterization and Immobilization. Arch. Med. Res. 1994, 25, 199–204. [Google Scholar]
- Delfin, J.; Martinez, I.; Antuch, W.; Morera, V.; Gonzalez, Y.; Rodriguez, R.; Marquez, M.; Saroyan, A.; Larionova, N.; Diaz, J. Purification, Characterization and Immobilization of Proteinase Inhibitors from Stichodactyla helianthus. Toxicon 1996, 34, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Lenarčič, B.; Ritonja, A.; Štrukelj, B.; Turk, B.; Turk, V. Equistatin, a New Inhibitor of Cysteine Proteinases from Actinia equina, Is Structurally Related to Thyroglobulin Type-1 Domain. J. Biol. Chem. 1997, 272, 13899–13903. [Google Scholar] [CrossRef] [PubMed]
- González, Y.; Araujo, M.; Oliva, M.; Sampaio, C.; Chávez, M. Purification and Preliminary Characterization of a Plasma Kallikrein Inhibitor Isolated from Sea Hares Aplysia dactylomela Rang, 1828. Toxicon 2004, 43, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Reytor, M.L.; González, Y.; Pascual, I.; Hernández, A.; Chávez M, Á.; Alonso del Rivero, M. Screening of Protease Inhibitory Activity in Extracts of Five Ascidian Species from Cuban Coasts. Biotecnol. Apl. 2011, 28, 77–82. [Google Scholar]
- Alonso-Del-Rivero, M.; Trejo, S.; Reytor, M.L.; Rodriguez-De-La-Vega, M.; Delfin, J.; Diaz, J.; Gonzalez, M.L.R.; Canals, F.; Chavez, M.A.; Aviles, F.X. Tri-Domain Bifunctional Inhibitor of Metallocarboxypeptidases a and Serine Proteases Isolated from Marine Annelid Sabellastarte magnifica. J. Biol. Chem. 2012, 287, 15427–15438. [Google Scholar] [CrossRef] [PubMed]
- Salas-Sarduy, E.; Cabrera-Muñoz, A.; Cauerhff, A.; González-González, Y.; Trejo, S.A.; Chidichimo, A.; de los Angeles Chávez-Planes, M.; José Cazzulo, J. Antiparasitic Effect of a Fraction Enriched in Tight-Binding Protease Inhibitors Isolated from the Caribbean Coral Plexaura homomalla. Exp. Parasitol. 2013, 135, 611–622. [Google Scholar] [CrossRef]
- González, L.; Sánchez, R.E.; Rojas, L.; Pascual, I.; García-Fernández, R.; Chávez, M.A.; Betzel, C. Screening of Protease Inhibitory Activity in Aqueous Extracts of Marine Invertebrates from Cuban Coast. Am. J. Anal. Chem. 2016, 7, 319–331. [Google Scholar] [CrossRef]
- Salas-Sarduy, E.; Guerra, Y.; Covaleda Cortés, G.; Avilés, F.X.; Chávez Planes, M.A. Identification of Tight-Binding Plasmepsin Ii and Falcipain 2 Inhibitors in Aqueous Extracts of Marine Invertebrates by the Combination of Enzymatic and Interaction-Based Assays. Mar. Drugs 2017, 15, 123. [Google Scholar] [CrossRef]
- Covaleda, G.; Trejo, S.A.; Salas-Sarduy, E.; del Rivero, M.A.; Chavez, M.A.; Aviles, F.X. Intensity Fading Maldi-Tof Mass Spectrometry and Functional Proteomics Assignments to Identify Protease Inhibitors in Marine Invertebrates. J. Proteom. 2017, 165, 75–92. [Google Scholar] [CrossRef]
- Hong, T.T.; Dat, T.T.H.; Cuong, P.V.; Cuc, N.T.K. Protease Inhibitors from Marine Sponge and Sponge-Associated Microorganisms. Vietnam. J. Sci. Technol. 2018, 56, 405–423. [Google Scholar] [CrossRef]
- Ikegami, S.; Kobayashi, H.; Myotoishi, Y.; Ohta, S.; Kato, K.H. Selective Inhibition of Exoplasmic Membrane Fusion in Echinoderm Gametes with Jaspisin, a Novel Antihatching Substance Isolated from a Marine Sponge. J. Biol. Chem. 1994, 269, 23262–23267. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.H.; Takemoto, K.; Kato, E.; Miyazaki, K.; Kobayashi, H.; Ikegami, S. Inhibition of Sea Urchin Fertilization by Jaspisin, a Specific Inhibitor of Matrix Metalloendoproteinase. Dev. Growth Differ. 1998, 40, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N.; Fujita, M.; Nakao, Y.; Matsunaga, S.; van Soest, R.W. Tokaramide a, a New Cathepsin B Inhibitor from the Marine Sponge Theonella Aff. mirabilis. Bioorganic Med. Chem. Lett. 1999, 9, 3397–3402. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; van Soest, R.W.; Itoh, Y.; Seiki, M.; Fusetani, N. Callysponginol Sulfate a, an Mt1-Mmp Inhibitor Isolated from the Marine Sponge Callyspongia tuncata. J. Nat. Prod. 2003, 66, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Seiki, M.; Itoh, Y.; Yamashita, J.; Van Soest, R.W.; Fusetani, N. Ageladine A: An Antiangiogenic Matrixmetalloproteinase Inhibitor from the Marine Sponge Agelas N Akamurai. J. Am. Chem. Soc. 2003, 125, 15700–15701. [Google Scholar] [CrossRef]
- Shim, J.S.; Lee, H.-S.; Shin, J.; Kwon, H.J. Psammaplin a, a Marine Natural Product, Inhibits Aminopeptidase N and Suppresses Angiogenesis in Vitro. Cancer Lett. 2004, 203, 163–169. [Google Scholar] [CrossRef]
- Pascual, I.; Gil-Parrado, S.; Cisneros, M.; Joseph-Bravo, P.; Dıaz, J.; Possani, L.D.; Charli, J.L.; Chávez, M. Purification of a Specific Inhibitor of Pyroglutamyl Aminopeptidase Ii from the Marine Annelide Hermodice carunculata: In Vivo Effects in Rodent Brain. Int. J. Biochem. Cell Biol. 2004, 36, 138–152. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Metalloproteinase Inhibitors: Status and Scope from Marine Organisms. Biochem. Res. Int. 2010, 2010, 845975. [Google Scholar] [CrossRef]
- del Rivero, M.A.; Reytor, M.L.; Trejo, S.A.; Chávez, M.A.; Avilés, F.X.; Reverter, D. A Noncanonical Mechanism of Carboxypeptidase Inhibition Revealed by the Crystal Structure of the Tri-Kunitz Smci in Complex with Human Cpa4. Structure 2013, 21, 1118–1126. [Google Scholar]
- Covaleda, G.; del Rivero, M.A.; Chávez, M.A.; Avilés, F.X.; Reverter, D. Crystal Structure of Novel Metallocarboxypeptidase Inhibitor from Marine Mollusk Nerita versicolor in Complex with Human Carboxypeptidase A4. J. Biol. Chem. 2012, 287, 9250–9258. [Google Scholar] [CrossRef]
- Cabrera-Muñoz, A.; Valiente, P.A.; Rojas, L.; Antigua, M.A.D.R.; Pires, J.R. Nmr Structure of Cmpi-Ii, a Non-Classical Kazal Protease Inhibitor: Understanding Its Conformational Dynamics and Subtilisin a Inhibition. J. Struct. Biol. 2019, 206, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Pascual Alonso, I.; Rivera Méndez, L.; Valdés-Tresanco, M.E.; Bounaadja, L.; Schmitt, M.; Arrebola Sánchez, Y.; Alvarez Lajonchere, L.; Charli, J.L.; Florent, I. Biochemical Evidences for M1-, M17- and M18-Like Aminopeptidases in Marine Invertebrates from Cuban Coastline. Z. Für Nat. C 2020, 75, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraj, H.M.; Sheeba, F.; Saba, A.; Khan, M. Marine Natural Products: A Lead for Anti-Cancer; NISCAIR-CSIR: New Delhi, India, 2012. [Google Scholar]
- Nakao, Y.; Oku, N.; Matsunaga, S.; Fusetani, N. Cyclotheonamides E2 and E3, New Potent Serine Protease Inhibitors from the Marine Sponge of the Genus Theonella. J. Nat. Prod. 1998, 61, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Hanessian, S.; Tremblay, M.; Petersen, J.F. The N-Acyloxyiminium Ion Aza-Prins Route to Octahydroindoles: Total Synthesis and Structural Confirmation of the Antithrombotic Marine Natural Product Oscillarin. J. Am. Chem. Soc. 2004, 126, 6064–6071. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; McCarthy, P.J.; Longley, R.E.; Pomponi, S.A.; Wright, A.E.; Lobkovsky, E.; Clardy, J. Discorhabdin P, a New Enzyme Inhibitor from a Deep-Water Caribbean Sponge of the Genus Batzella. J. Nat. Prod. 1999, 62, 173–175. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; McCarthy, P.J.; Longley, R.E.; Pomponi, S.A.; Wright, A.E. Secobatzellines a and B, Two New Enzyme Inhibitors from a Deep-Water Caribbean Sponge of the Genus Batzella. J. Nat. Prod. 1999, 62, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Schetz, J.A.; Kelly, M.; Peng, J.N.; Ang, K.K.; Flotow, H.; Leong, C.Y.; Ng, S.B.; Buss, A.D.; Wilkins, S.P. New Antiinfective and Human 5-Ht2 Receptor Binding Natural and Semisynthetic Compounds from the Jamaican Sponge Smenospongia aurea. J. Nat. Prod. 2002, 65, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Nishikawa, T.; Fusetani, N. Sodium 1-(12-Hydroxy) Octadecanyl Sulfate, an Mmp2 Inhibitor, Isolated from a Tunicate of the Family Polyclinidae. J. Nat. Prod. 2002, 65, 1936–1938. [Google Scholar] [CrossRef]
- Joe, M.-J.; Kim, S.-N.; Choi, H.-Y.; Shin, W.-S.; Park, G.-M.; Kang, D.-W.; Kim, Y.K. The Inhibitory Effects of Eckol and Dieckol from Ecklonia stolonifera on the Expression of Matrix Metalloproteinase-1 in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef]
- Harper, J.W.; Powers, J.C. Inhibitors of Metallo-Proteases. In Proteinase Inhibitors; Elsevier: Amsterdam, The Netherlands, 1986; pp. 219–298. [Google Scholar]
- Rawlings, N.D.; Barrett, A.J. Evolutionary Families of Peptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. [13] Evolutionary Families of Metallopeptidases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1995; pp. 183–228. [Google Scholar]
- Reeck, G.R.; de Haën, C.; Teller, D.C.; Doolittle, R.F.; Fitch, W.M.; Dickerson, R.E.; Chambon, P.; McLachlan, A.D.; Margoliash, E.; Jukes, T.H.; et al. Homology in Proteins and Nucleic Acids: A Terminology Muddle and a Way out of It. Cell 1987, 50, 667. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, J.Z.; Nordlund, P.; Thunnissen, M.M. Functional Properties and Molecular Architecture of Leukotriene A4 Hydrolase, a Pivotal Catalyst of Chemotactic Leukotriene Formation. Sci. World J. 2002, 2, 1734–1749. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.M.; Fukasawa, K.; Harada, M.; Hirose, J.; Izumi, T.; Shimizu, T. Aminopeptidase B Is Structurally Related to Leukotriene-A4 Hydrolase but Is Not a Bifunctional Enzyme with Epoxide Hydrolase Activity. Biochem. J. 1999, 339, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Klinke, T.; Rump, A.; Pönisch, R.; Schellenberger, W.; Müller, E.C.; Otto, A.; Klimm, W.; Kriegel, T.M. Identification and Characterization of Caape2–a Neutral Arginine/Alanine/Leucine-Specific Metallo-Aminopeptidase from Candida albicans. FEMS Yeast Res. 2008, 8, 858–869. [Google Scholar] [CrossRef]
- Wong, A.H.M.; Zhou, D.; Rini, J.M. The X-Ray Crystal Structure of Human Aminopeptidase N Reveals a Novel Dimer and the Basis for Peptide Processing. J. Biol. Chem. 2012, 287, 36804–36813. [Google Scholar] [CrossRef]
- Thunnissen, M.M.; Nordlund, P.; Haeggström, J.Z. Crystal Structure of Human Leukotriene A4 Hydrolase, a Bifunctional Enzyme in Inflammation. Nat. Struct. Biol. 2001, 8, 131–135. [Google Scholar] [CrossRef]
- Kyrieleis, O.J.; Goettig, P.; Kiefersauer, R.; Huber, R.; Brandstetter, H. Crystal Structures of the Tricorn Interacting Factor F3 from Thermoplasma Acidophilum, a Zinc Aminopeptidase in Three Different Conformations. J. Mol. Biol. 2005, 349, 787–800. [Google Scholar] [CrossRef]
- Ito, K.; Nakajima, Y.; Onohara, Y.; Takeo, M.; Nakashima, K.; Matsubara, F.; Ito, T.; Yoshimoto, T. Crystal Structure of Aminopeptidase N (Proteobacteria Alanyl Aminopeptidase) from Escherichia coli and Conformational Change of Methionine 260 Involved in Substrate Recognition. J. Biol. Chem. 2006, 281, 33664–33676. [Google Scholar] [CrossRef]
- McGowan, S.; Porter, C.J.; Lowther, J.; Stack, C.M.; Golding, S.J.; Skinner-Adams, T.S.; Trenholme, K.R.; Teuscher, F.; Donnelly, S.M.; Grembecka, J.; et al. Structural Basis for the Inhibition of the Essential Plasmodium Falciparum M1 Neutral Aminopeptidase. Proc. Natl. Acad. Sci. USA 2009, 106, 2537–2542. [Google Scholar] [CrossRef]
- Harbut, M.B.; Velmourougane, G.; Dalal, S.; Reiss, G.; Whisstock, J.C.; Onder, O.; Brisson, D.; McGowan, S.; Klemba, M.; Greenbaum, D.C. Bestatin-Based Chemical Biology Strategy Reveals Distinct Roles for Malaria M1-and M17-Family Aminopeptidases. Proc. Natl. Acad. Sci. USA 2011, 108, E526–E534. [Google Scholar] [CrossRef]
- Salomon, E.; Schmitt, M.; Mouray, E.; McEwen, A.G.; Bounaadja, L.; Torchy, M.; Poussin-Courmontagne, P.; Alavi, S.; Tarnus, C.; Cavarelli, J.; et al. Aminobenzosuberone Derivatives as Pfa-M1 Inhibitors: Molecular Recognition and Antiplasmodial Evaluation. Bioorganic Chem. 2020, 98, 103750. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, C.; Lin, Y.-L.; Li, F. Structural Insights into Central Hypertension Regulation by Human Aminopeptidase A. J. Biol. Chem. 2013, 288, 25638–25645. [Google Scholar] [CrossRef] [PubMed]
- Kochan, G.; Krojer, T.; Harvey, D.; Fischer, R.; Chen, L.; Vollmar, M.; von Delft, F.; Kavanagh, K.L.; Brown, M.A.; Bowness, P.; et al. Crystal Structures of the Endoplasmic Reticulum Aminopeptidase-1 (Erap1) Reveal the Molecular Basis for N-Terminal Peptide Trimming. Proc. Natl. Acad. Sci. USA 2011, 108, 7745–7750. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Zhong, Q.; Wu, Y.; Xia, W. Purification and Characterization of a Novel Angiotensin-I Converting Enzyme (Ace) Inhibitory Peptide Derived from Enzymatic Hydrolysate of Grass Carp Protein. Peptides 2012, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Gago, F. Computational Approaches to Enzyme Inhibition by Marine Natural Products in the Search for New Drugs. Mar. Drugs 2023, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.M.; Mohammad, K.A.; Sindi, I.A.; Mohamed, G.A.; Ibrahim, S.R.M. Unveiling the Efficacy of Sesquiterpenes from Marine Sponge Dactylospongia elegans in Inhibiting Dihydrofolate Reductase Using Docking and Molecular Dynamic Studies. Molecules 2023, 28, 1292. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Bravo, P.; Jaimes-Hoy, L.; Uribe, R.M.; Charli, J.L. 60 Years of Neuroendocrinology: TRH, the First Hypophysiotropic Releasing Hormone Isolated: Control of the Pituitary–Thyroid Axis. J. Endocrinol. 2015, 226, T85–T100. [Google Scholar] [CrossRef]
- Kronenberg, H.M.; Shlomo Melmed, M.D.; Polonsky, K.S.; Wilson, J.D.; Foster, D.W.; Kronenberg, H.M. Williams Textbook of Endocrinology; Saunders: Philadelphia, PA, USA, 2002. [Google Scholar]
- Charli, J.L.; Rodríguez-Rodríguez, A.; Hernández-Ortega, K.; Cote-Vélez, A.; Uribe, R.M.; Jaimes-Hoy, L.; Joseph-Bravo, P. The Thyrotropin-Releasing Hormone-Degrading Ectoenzyme, a Therapeutic Target? Front. Pharm. 2020, 11, 640. [Google Scholar] [CrossRef]
- A Kelly, J. Thyrotropin-Releasing Hormone: Basis and Potential for Its Therapeutic Use. Essays Biochem. 1995, 30, 133. [Google Scholar]
- Charli, J.-L.; Mendez, M.; Vargas, M.-A.; Cisneros, M.; Assai, M.; Joseph-Bravo, P.; Wilk, S. Pyroglutamyl Peptidase Ii Inhibition Specifically Increases Recovery of Trh Released from Rat Brain Slices. Neuropeptides 1989, 14, 191–196. [Google Scholar] [CrossRef]
- Kelly, J.A.; Slator, G.R.; Tipton, K.F.; Williams, C.H.; Bauer, K. Kinetic Investigation of the Specificity of Porcine Brain Thyrotropin-Releasing Hormone-Degrading Ectoenzyme for Thyrotropin-Releasing Hormone-Like Peptides. J. Biol. Chem. 2000, 275, 16746–16751. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.A.; Scalabrino, G.A.; Slator, G.R.; Cullen, A.A.; Gilmer, J.F.; Lloyd, D.G.; Bennett, G.W.; Bauer, K.; Tipton, K.F.; Williams, C.H. Structure–Activity Studies with High-Affinity Inhibitors of Pyroglutamyl-Peptidase Ii. Biochem. J. 2005, 389, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Vargas, M.A.; Uribe, R.M.; Pascual, I.; Lazcano, I.; Yiotakis, A.; Matziari, M.; Joseph-Bravo, P.; Charli, J.-L. Anterior Pituitary Pyroglutamyl Peptidase Ii Activity Controls Trh-Induced Prolactin Release. Peptides 2008, 29, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Sinko, R.; Mohácsik, P.; Kővári, D.; Penksza, V.; Wittmann, G.; Mácsai, L.; Fonseca, T.L.; Bianco, A.C.; Fekete, C.; Gereben, B. Different Hypothalamic Mechanisms Control Decreased Circulating Thyroid Hormone Levels in Infection and Fasting-Induced Non-Thyroidal Illness Syndrome in Male Thyroid Hormone Action Indicator Mice. Thyroid 2023, 33, 109–118. [Google Scholar] [CrossRef]
- Barnieh, F.M.; Loadman, P.M.; Falconer, R.A. Is Tumour-Expressed Aminopeptidase N (Apn/Cd13) Structurally and Functionally Unique? Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188641. [Google Scholar] [CrossRef]
- Wickström, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (Cd13) as a Target for Cancer Chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef]
- Ni, J.; Wang, X.; Shang, Y.; Li, Y.; Chen, S. Cd13 Inhibition Augments Dr4-Induced Tumor Cell Death in a P-Erk1/2-Independent Manner. Cancer Biol. Med. 2021, 18, 569. [Google Scholar] [CrossRef]
- Schreiter, A.; Gore, C.; Labuz, D.; Fournie-Zaluski, M.C.; Roques, B.P.; Stein, C.; Machelska, H. Pain Inhibition by Blocking Leukocytic and Neuronal Opioid Peptidases in Peripheral Inflamed Tissue. FASEB J. 2012, 26, 5161–5171. [Google Scholar] [CrossRef]
- Bonnard, E.; Poras, H.; Nadal, X.; Maldonado, R.; Fournié-Zaluski, M.C.; Roques, B.P. Long-Lasting Oral Analgesic Effects of N-Protected Aminophosphinic Dual Enk Ephalinase Inhibitors (Denki S) in Peripherally Controlled Pain. Pharmacol. Res. Perspect. 2015, 3, e0116. [Google Scholar] [CrossRef]
- Arrebola, Y.; Rivera, L.; Pedroso, A.; McGuire, R.; Tresanco, M.E.V.; Bergado, G.; Charli, J.-L.; Sánchez, B.; Alonso, I.P. Bacitracin Is a Non-Competitive Inhibitor of Porcine M1 Family Neutral and Glutamyl Aminopeptidases. Nat. Prod. Res. 2021, 35, 2958–2962. [Google Scholar] [CrossRef]
- Melzig, M.F.; Bormann, H. Betulinic Acid Inhibits Aminopeptidase N Activity. Planta Med. 1998, 64, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Pascual Alonso, I.; Bounaadja, L.; Sánchez, L.; Rivera, L.; Tarnus, C.; Schmitt, M.; Garcia, G.; Diaz, L.; Hernandez-Zanuy, A.; Sánchez, B.; et al. Aqueous Extracts of Marine Invertebrates from Cuba Coastline Display Neutral Aminopeptidase Inhibitory Activities and Effects on Cancer Cells and Plasmodium Falciparum Parasites. Indian J. Nat. Prod. Resour. 2017, 8, 107–119. [Google Scholar]
- Pascual, I.; Valiente, P.A.; García, G.; Valdés-Tresanco, M.E.; Arrebola, Y.; Díaz, L.; Bounaadja, L.; Uribe, R.M.; Pacheco, M.C.; Florent, I.; et al. Discovery of Novel Non-Competitive Inhibitors of Mammalian Neutral M1 Aminopeptidase (Apn). Biochimie 2017, 142, 216–225. [Google Scholar] [CrossRef]
- Pascual-Alonso, I.; Alonso-Bosch, R.; Cabrera-Muñoz, A.; Perera, W.H.; Charli, J.L. Methanolic Extracts of Paratoid Gland Secretions from Cuban Peltophryne Toads Contain Inhibitory Activities against Peptidases with Biomedical Relevance. Biotecnol. Apl. 2019, 36, 2221–2227. [Google Scholar]
- Alonso, I.P.; Méndez, L.R.; García, F.A.; Valdés-Tresanco, M.E.; Bosch, R.A.; Perera, W.H.; Sánchez, Y.A.; Bergado, G.; Ramírez, B.S.; Charli, J.-L. Bufadienolides Preferentially Inhibit Aminopeptidase N among Mammalian Metallo-Aminopeptidases; Relationship with Effects on Human Melanoma Mewo Cells. Int. J. Biol. Macromol. 2023, 229, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Sorolla, M.A.; Krishnan, P.D.G.; Sorolla, A. From Seabed to Bedside: A Review on Promising Marine Anticancer Compounds. Biomolecules 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Farran, B.; Nagaraju, G.P. Understanding the Function of the Tumor Microenvironment, and Compounds from Marine Organisms for Breast Cancer Therapy. World J. Biol. Chem. 2021, 12, 15. [Google Scholar] [CrossRef]
- Aldrich, L.N.; Burdette, J.E.; de Blanco, E.C.; Coss, C.C.; Eustaquio, A.S.; Fuchs, J.R.; Kinghorn, A.D.; MacFarlane, A.; Mize, B.K.; Oberlies, N.H.; et al. Discovery of Anticancer Agents of Diverse Natural Origin. J. Nat. Prod. 2022, 85, 702–719. [Google Scholar] [CrossRef]
- Nuzzo, G.; Senese, G.; Gallo, C.; Albiani, F.; Romano, L.; D’ippolito, G.; Manzo, E.; Fontana, A. Antitumor Potential of Immunomodulatory Natural Products. Mar. Drugs 2022, 20, 386. [Google Scholar] [CrossRef]
- Saeed, A.F.; Su, J.; Ouyang, S. Marine-Derived Drugs: Recent Advances in Cancer Therapy and Immune Signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef]
- Jung, J.H.; Sim, C.J.; Lee, C.-O. Cytotoxic Compounds from a Two-Sponge Association. J. Nat. Prod. 1995, 58, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, H.-S.; Seo, Y.; Rho, J.-R.; Cho, K.W.; Paul, V.J. New Bromotyrosine Metabolites from the Sponge Aplysinella rhax. Tetrahedron 2000, 56, 9071–9077. [Google Scholar] [CrossRef]
- Pina, I.C.; Gautschi, J.T.; Wang, G.Y.S.; Sanders, M.L.; Schmitz, F.J.; France, D.; Cornell-Kennon, S.; Sambucetti, L.C.; Remiszewski, S.W.; Perez, L.B.; et al. Psammaplins from the Sponge Pseudoceratina P Urpurea: Inhibition of Both Histone Deacetylase and DNA Methyltransferase. J. Org. Chem. 2003, 68, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Liu, Y.; Hong, J.; Lee, C.O.; Cho, H.; Kim, D.K.; Im, K.S.; Jung, J.H. New Bromotyrosine Derivatives from an Association of Two Sponges, Jaspis W Ondoensis and Poecillastra W Ondoensis. J. Nat. Prod. 2003, 66, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, D.; Sun, D.; Yang, S.; Hu, G.; Wu, Z.; Zhao, L. Synthesis of the Marine Bromotyrosine Psammaplin F and Crystal Structure of a Psammaplin a Analogue. Molecules 2010, 15, 8784–8795. [Google Scholar] [CrossRef]
- Mujumdar, P.; Teruya, K.; Tonissen, K.F.; Vullo, D.; Supuran, C.T.; Peat, T.S.; Poulsen, S.A. An Unusual Natural Product Primary Sulfonamide: Synthesis, Carbonic Anhydrase Inhibition, and Protein X-Ray Structures of Psammaplin C. J. Med. Chem. 2016, 59, 5462–5470. [Google Scholar] [CrossRef]
- Kim, D.; Lee, I.S.; Jung, J.H.; Yang, S.I. Psammaplin a, a Natural Bromotyrosine Derivative from a Sponge, Possesses the Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus and the DNA Gyrase-Inhibitory Activity. Arch. Pharmacal Res. 1999, 22, 25–29. [Google Scholar] [CrossRef]
- TTabudravu, J.; Eijsink, V.; Gooday, G.; Jaspars, M.; Komander, D.; Legg, M.; Synstad, B.; van Aalten, D. Psammaplin a, a Chitinase Inhibitor Isolated from the Fijian Marine Sponge Aplysinella rhax. Bioorganic Med. Chem. 2002, 10, 1123–1128. [Google Scholar] [CrossRef]
- Revelant, G.; Al-Lakkis-Wehbe, M.; Schmitt, M.; Alavi, S.; Schmitt, C.; Roux, L.; Al-Masri, M.; Schifano-Faux, N.; Maiereanu, C.; Tarnus, C.; et al. Exploring S1 Plasticity and Probing S1′ Subsite of Mammalian Aminopeptidase N/Cd13 with Highly Potent and Selective Aminobenzosuberone Inhibitors. Bioorganic Med. Chem. 2015, 23, 3192–3207. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, H.S.; Kang, Y.J.; Yoon, S.; Lee, J.; Choi, W.S.; Jung, J.H.; Kim, H.S. Psammaplin a Induces Sirtuin 1-Dependent Autophagic Cell Death in Doxorubicin-Resistant Mcf-7/Adr Human Breast Cancer Cells and Xenografts. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 401–410. [Google Scholar] [CrossRef]
- Ratovitski, E.A. Tumor Protein (Tp)-P53 Members as Regulators of Autophagy in Tumor Cells Upon Marine Drug Exposure. Mar. Drugs 2016, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-D.; Li, J.; Du, L.; Mahdi, F.; Le, T.P.; Chen, W.-L.; Swanson, S.M.; Watabe, K.; Nagle, D.G. Biochemical and Anti-Triple Negative Metastatic Breast Tumor Cell Properties of Psammaplins. Mar. Drugs 2018, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Jung, J.H.; Na, Y.J.; Kim, H.S. A Natural Histone Deacetylase Inhibitor, Psammaplin a, Induces Cell Cycle Arrest and Apoptosis in Human Endometrial Cancer Cells. Gynecol. Oncol. 2008, 108, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.H.; Chie, E.K.; Da Young, P.; Kim, I.A.; Kim, I.H. Dnmt (DNA Methyltransferase) Inhibitors Radiosensitize Human Cancer Cells by Suppressing DNA Repair Activity. Radiat. Oncol. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Kim, D.H.; Shin, J.; Kwon, H.J. Psammaplin a Is a Natural Prodrug That Inhibits Class I Histone Deacetylase. Exp. Mol. Med. 2007, 39, 47–55. [Google Scholar] [CrossRef]
- Jing, Q.; Hu, X.; Ma, Y.; Mu, J.; Liu, W.; Xu, F.; Li, Z.; Bai, J.; Hua, H.; Li, D. Marine-Derived Natural Lead Compound Disulfide-Linked Dimer Psammaplin A: Biological Activity and Structural Modification. Mar. Drugs 2019, 17, 384. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Hughes, R.; Pfefferkorn, J.A.; Barluenga, S.; Roecker, A.J. Combinatorial Synthesis through Disulfide Exchange: Discovery of Potent Psammaplin a Type Antibacterial Agents Active against Methicillin-Resistant Staphylococcus aureus (MRSA). Chem. –A Eur. J. 2001, 7, 4280–4295. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Hughes, R.; Pfefferkorn, J.A.; Barluenga, S. Optimization and Mechanistic Studies of Psammaplin a Type Antibacterial Agents Active against Methicillin-Resistant Staphylococcus aureus (MRSA). Chem. –A Eur. J. 2001, 7, 4296–4310. [Google Scholar] [CrossRef]
- Alonso, I.P.; Sanchez, Y.M.A.; Ruiz, G.A.; González, M.L.R.; Hernández-Zanuy, A. Identificación De Actividad Inhibidora De Aminopeptidasa N Aislada De Riñón Porcino, En Invertebrados Marinos De La Plataforma Insular De La Habana/Identification of Porcine Kidney Aminopeptidase N Inhibitory Activity, in Marine Invertebrates from Havana Coastline. Rev. Cuba. Cienc. Biológicas 2016, 5, 15. [Google Scholar]
- Alonso, I.P.; Pedroso, A.; Sánchez, Y.M.A.; Valdés-Tresanco, M.E.; Méndez, L.R.; Fortun, S. La Aminopeptidasa a De Mamíferos: Características Bioquímicas, Funciones Fisiológicas Y Su Implicación En Procesos Fisiopatológicos En Humanos/Aminopeptidase a from Mammals: Biochemical Characteristics, Physiological Roles and Implication in Physiopathological Processes in Humans. Rev. Cuba. Cienc. Biológicas 2018, 6, 20. [Google Scholar]
- Göhring, B.; Holzhausen, H.; Meye, A.; Heynemann, H.; Rebmann, U.; Langner, J.; Riemann, D. Endopeptidase 24.11/Cd10 Is Down-Regulated in Renal Cell Cancer. Int. J. Mol. Med. 1998, 2, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Tonna, S.; Dandapani, S.V.; Uscinski, A.; Appel, G.B.; Schlöndorff, J.S.; Zhang, K.; Denker, B.M.; Pollak, M.R. Functional Genetic Variation in Aminopeptidase a (Enpep): Lack of Clear Association with Focal and Segmental Glomerulosclerosis (Fsgs). Gene 2008, 410, 44–52. [Google Scholar] [CrossRef]
- del Carmen Puertas, M.; Martínez-Martos, J.M.; Cobo, M.; Lorite, P.; Sandalio, R.M.; Palomeque, T.; Torres, M.I.; Carrera-González, M.P.; Mayas, M.D.; Ramírez-Expósito, M.J. Plasma Renin–Angiotensin System-Regulating Aminopeptidase Activities Are Modified in Early Stage Alzheimer’s Disease and Show Gender Differences but Are Not Related to Apolipoprotein E Genotype. Exp. Gerontol. 2013, 48, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Blanco, L.; Sanz, B.; Perez, I.; Sánchez, C.E.; Cándenas, M.L.; Pinto, F.M.; Gil, J.; Casis, L.; López, J.I.; Larrinaga, G. Altered Glutamyl-Aminopeptidase Activity and Expression in Renal Neoplasms. BMC Cancer 2014, 14, 386. [Google Scholar] [CrossRef]
- Bodineau, L.; Frugiere, A.; Marc, Y.; Inguimbert, N.; Fassot, C.; Balavoine, F.; Roques, B.; Llorens-Cortes, C. Orally Active Aminopeptidase a Inhibitors Reduce Blood Pressure: A New Strategy for Treating Hypertension. Hypertension 2008, 51, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Fowler, J.H.; Walling, L.L. Leucine Aminopeptidases: Diversity in Structure and Function. Biol. Chem. 2006, 387, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Scranton, M.A.; Yee, A.; Park, S.-Y.; Walling, L.L. Plant Leucine Aminopeptidases Moonlight as Molecular Chaperones to Alleviate Stress-Induced Damage. J. Biol. Chem. 2012, 287, 18408–18417. [Google Scholar] [CrossRef]
- Chao, W.S.; Gu, Y.-Q.; Pautot, V.; Bray, E.A.; Walling, L.L. Leucine Aminopeptidase Rnas, Proteins, and Activities Increase in Response to Water Deficit, Salinity, and the Wound Signals Systemin, Methyl Jasmonate, and Abscisic Acid. Plant Physiol. 1999, 120, 979–992. [Google Scholar] [CrossRef]
- Alén, C.; Sherratt, D.J.; Colloms, S. Direct Interaction of Aminopeptidase a with Recombination Site DNA in Xer Site-Specific Recombination. EMBO J. 1997, 16, 5188–5197. [Google Scholar] [CrossRef]
- Behari, J.; Stagon, L.; Calderwood, S.B. Pepa, a Gene Mediating Ph Regulation of Virulence Genes in Vibrio cholerae. J. Bacteriol. 2001, 183, 178–188. [Google Scholar] [CrossRef]
- Aly, A.S.; Vaughan, A.M.; Kappe, S.H. Malaria Parasite Development in the Mosquito and Infection of the Mammalian Host. Annu. Rev. Microbiol. 2009, 63, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E. Complex Nature of Malaria Parasite Hemoglobin Degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 5283–5284. [Google Scholar] [CrossRef] [PubMed]
- Skinner-Adams, T.S.; Stack, C.M.; Trenholme, K.R.; Brown, C.L.; Grembecka, J.; Lowther, J.; Mucha, A.; Drag, M.; Kafarski, P.; McGowan, S.; et al. Plasmodium falciparum Neutral Aminopeptidases: New Targets for Anti-Malarials. Trends Biochem. Sci. 2010, 35, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Calic, P.P.; Vinh, N.B.; Webb, C.T.; Malcolm, T.R.; Ngo, A.; Lowes, K.; Drinkwater, N.; McGowan, S.; Scammells, P.J. Structure-Based Development of Potent Plasmodium Falciparum M1 and M17 Aminopeptidase Selective and Dual Inhibitors Via S1’-Region Optimisation. Eur. J. Med. Chem. 2023, 248, 115051. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, N.; Vinh, N.B.; Mistry, S.N.; Bamert, R.S.; Ruggeri, C.; Holleran, J.P.; Loganathan, S.; Paiardini, A.; Charman, S.A.; Powell, A.K.; et al. Potent Dual Inhibitors of Plasmodium falciparum M1 and M17 Aminopeptidases through Optimization of S1 Pocket Interactions. Eur. J. Med. Chem. 2016, 110, 43–64. [Google Scholar] [CrossRef]
- Bauvois, B.; Dauzonne, D. Aminopeptidase-N/Cd13 (Ec 3.4. 11.2) Inhibitors: Chemistry, Biological Evaluations, and Therapeutic Prospects. Med. Res. Rev. 2006, 26, 88–130. [Google Scholar] [CrossRef]
- Kancharla, P.; Li, Y.; Yeluguri, M.; Dodean, R.A.; Reynolds, K.A.; Kelly, J.X. Total Synthesis and Antimalarial Activity of 2-(P-Hydroxybenzyl)-Prodigiosins, Isoheptylprodigiosin, and Geometric Isomers of Tambjamine Myp1 Isolated from Marine Bacteria. J. Med. Chem. 2021, 64, 8739–8754. [Google Scholar] [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.-M.; Yang, L.-Y.; Huang, S.-S.; Chigor, V.N.; Eze, E.A.; Pan, L.-X.; Zhang, T.; Yang, D.-F. Potentials of Marine Natural Products against Malaria, Leishmaniasis, and Trypanosomiasis Parasites: A Review of Recent Articles. Infect. Dis. Poverty 2021, 10, 1–19. [Google Scholar] [CrossRef]
- Zayed, A.; Negm, W.; Kabbash, A.; Ezzat, S.M. Marine-Derived Metabolites as Antimalarial Candidates Targeting Various Life Stages. J. Adv. Med. Pharm. Res. 2022, 3, 12–18. [Google Scholar] [CrossRef]
- Singh, H.; Parida, A.; Debbarma, K.; Ray, D.P.; Banerjee, P. Common Marine Organisms: A Novel Source of Medicinal Compounds. Int. J. Bioresour. Sci. 2020, 7, 39–49. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, Z.; Wang, J.; Tian, X.; Wang, C.; Cui, J.; Huo, X.; Feng, L.; Yu, Z.; Ma, X. Visual Analysis and Inhibitor Screening of Leucine Aminopeptidase, a Key Virulence Factor for Pathogenic Bacteria-Associated Infection. ACS Sens. 2021, 6, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, J.; Yang, H.; Peng, L.; Wang, K.; Wang, Y.; Zhao, X.; Liu, H.; Dou, C.; Shi, L.; et al. Leucine Aminopeptidase 3 Promotes Migration and Invasion of Breast Cancer Cells through Upregulation of Fascin and Matrix Metalloproteinases-2/9 Expression. J. Cell. Biochem. 2019, 120, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dai, G.; Wang, S.; Zhao, Y.; Wang, X.; Zhao, X.; Zhang, H.; Wei, L.; Zhang, L.; Guo, S.; et al. Inhibition of the Proliferation, Migration, and Invasion of Human Breast Cancer Cells by Leucine Aminopeptidase 3 Inhibitors Derived from Natural Marine Products. Anti-Cancer Drugs 2020, 31, 60–66. [Google Scholar] [CrossRef] [PubMed]





















| Enzyme | IUBMB * Nomenclature | Merops ID | Sources |
|---|---|---|---|
| Aminopeptidase N (APN) | EC 3.4.11.2 | M01.001 | Homo sapiens, Sus scrofa |
| Lysyl aminopeptidase | - | M01.002 | Escherichia coli |
| Aminopeptidase A (APA) | EC 3.4.11.7 | M01.003 | Homo sapiens |
| Leukotriene A4 hydrolase (LTA4H) | EC 3.3.2.6 | M01.004 | Homo sapiens |
| Alanyl aminopeptidase (bacterial-type) | EC 3.4.11.2 | M01.005 | Escherichia coli, Arabidopsis thaliana |
| Ape2 aminopeptidase | - | M01.006 | Saccharomyces cerevisiae |
| Aap1’ aminopeptidase | - | M01.007 | Saccharomyces cerevisiae |
| Thyrotropin-releasing hormone-degrading ectoenzyme or Pyroglutamyl-peptidase II (TRH-DE, PPII) | EC 3.4.19.6 | M01.008 | Homo sapiens, Mus musculus, Ratus novergicus |
| Aminopeptidase N (actinomycete-type) | - | M01.009 | Streptomyces lividans |
| Cytosol alanyl aminopeptidase | - | M01.010 | Homo sapiens, Arabidopsis thaliana, Caenorhabditis elegans |
| Insulin-regulated membrane aminopeptidase or cystinyl Aminopeptidase (IRAP) | EC 3.4.11.3 | M01.011 | Homo sapiens |
| Aminopeptidase G | - | M01.012 | Streptomyces coelicolor |
| Aminopeptidase N (insect) | - | M01.013 | Manduca sexta |
| Aminopeptidase B (APB) | EC 3.4.11.6 | M01.014 | Homo sapiens |
| Aminopeptidase H11 (nematode) | - | M01.015 | |
| Aminopeptidase Ey | EC 3.4.11.20 | M01.016 | Gallus gallus domesticus |
| TMA108 protein | - | M01.017 | Saccharomyces cerevisiae |
| Endoplasmic reticulum aminopeptidase 1 ERAP-1 | - | M01.018 | Homo sapiens |
| Tricorn interacting factor F2 | - | M01.020 | Thermoplasma acidophilum |
| Tricorn interacting factor F3 | - | M01.021 | Thermoplasma acidophilum |
| Arginyl aminopeptidase-like 1 | - | M01.022 | Homo sapiens |
| Endoplasmic reticulum aminopeptidase 2 ERAP-2 | - | M01.024 | Homo sapiens |
| Aminopeptidase-1 (Caenorhabditis-type) | - | M01.025 | Caenorhabditis elegans |
| Aminopeptidase Q | - | M01.026 | Homo sapiens |
| Aminopeptidase O (AP-O) | EC 3.4.11.- | M01.028 | Homo sapiens |
| M1 aminopeptidase (Plasmodium spp.) | EC 3.4.11.2 | M01.029 | Plasmodium falciparum |
| Aminopeptidase N2 (insect) | - | M01.030 | Manduca sexta |
| Cold-active aminopeptidase (Colwellia psychrerythraea)-Type peptidase | - | M01.031 | Colwellia psychrerythraea |
| Lysyl aminopeptidase 1 (Streptomyces sp.) | - | M01.032 | Streptomyces albulus |
| Lysyl endopeptidase (Streptomyces albulus) | - | M01.033 | Streptomyces albulus |
| Leukotriene A4 hydrolase (Saccharomyces cerevisiae) | EC 3.3.2.6 | M01.034 | Saccharomyces cerevisiae |
| LePepA g.p. (Legionella pneumophila) | - | M01.035 | Legionella pneumophila |
| Enzyme | Source | Crystallographic Codes of the Structures at Protein Data Bank (PDB) |
|---|---|---|
| aminopeptidase N | Plasmodium falciparum | 3EBG, 3EBH, 3EBI, 3Q43, 3Q44, 3T8V, 4J3B, 4K5L, 4K5M, 4K5N, 4K5O,4K5P, 4R5T, 4R5V, 4R5X, 4ZQT, 4ZW3, 4ZW5, 4ZW6, 4ZW7, 4ZW8, 4ZX3, 4ZX4, 4ZX5, 4ZX6, 5XM7, 5Y19, 5Y1H, 5Y1K, 5Y1Q, 5Y1R, 5Y1S, 5Y1T, 5Y1V, 5Y1W, 5Y1X, 6EA1, 6EA2, 6EAA, 6EAB, 6EE3, 6EE4, 6EE6, 6EED, 6SBQ, 6SBR |
| aminopeptidase N | Escherichia coli | 2DQ6, 2DQM, 2HPO, 2HPT, 2ZXG, 3B2P, 3B2X, 3B34, 3B37, 3B3B, 3KED, 3PUU, 3QJX,4Q4E, 4Q4I, 4XMT, 4XMU, 4XMV, 4XMW, 4XMX, 4XM2, 4XN1, 4XN2, 4XN4, 4XN5, 4XN7, 4XN8, 4XN9, 4XNA, 4XNB, 4XND, 4X03, 4X04, 4X05, 5MFR, 5MFS, 5MFT, 5Y01, 5YQ1, 5YQ2, 5YQB, 6G8B |
| aminopeptidase N | Homo sapiens | 4FYQ, 4FYR, 4FYS, 4FYT, 5LHD, 6AKT |
| aminopeptidase N | Sus scrofa | 4FSC, 4FKE, 4FKH, 4FKK, 4HOM, 4NAQ, 4NZ8, 4OU3 |
| ERAP 1 | Homo sapiens | 2XDT, 2YD0, 3MDJ, 3QNF, 3RJO, 6Q4R |
| ERAP 2 | Homo sapiens | 3SE6, 4E36, 4JBS, 5AB0, 5AB2, 5CU5, 5J6S, 5KIV |
| aminopeptidase A | Homo sapiens | 4KX7, 4KX8, 4KX9, 4KXA, 4KXB, 4KXC, 4KXD |
| leukotriene A4 hydrolase | Homo sapiens | 1G6W, 1H19, 1HS6, 1SQM, 2R59, 2VJ8, 3B7R, 3B7S, 3B7T, 3B7U, 3CHO, 3CHP, 3CHQ, 3CHR, 3CHS, 3FH5, 3FH7, 3FH8, 3FHE, 3FTS, 3FTU, 3FTV, 3FTW, 3FTX, 3FTY, 3FTZ, 3FU0, 3FU3, 3FU5, 3FU6, 3FUD, 3FUE, 3FUF, 3FUH, 3FUI, 3FUJ, 3FUK, 3FUL, 3FUM, 3FUN, 3U9W, 4DPR, 4L2L, 4MKT, 4Ms6, 4RSY, 4RVB, 5AEN, 5BPP, 5FWQ, 5N3W, 5NI2, 5NI4, 5NI6, 5NIA, 5NID, 5NIE, 6ENB, 6ENC, 6END |
| leukotriene A4 hydrolase (Saccharomyces cerevisiae) | Saccharomyces cerevisiae (ATCC 204508/S288c) | 2XPY, 2XPZ, 2XQ0 |
| cold-active aminopeptidase (Colwellia psychrerythraea)-type peptidase | Colwellia psychrerythraea (34H/ATCC BAA-681) | 3CIA |
| tricorn interacting factor F3 | Thermoplasma acidophilum (ATCC 25905/DSM 1728/JCM 9062/NBRC 15155/AMRC-C165) | 1Z1W,1Z5H, 3Q7J |
| LePepA g.p. (Legionella pneumophila) | Legionella pneumophila | 5ZI5, 5ZI7, 5ZIE |
| TATA-binding protein-associated factor | Homo sapiens | 5FUR, 6MZC, 6MZL, 6MZM |
| IRAP (cystinil aminopeptidase) | Homo sapiens | 4P8Q, 4PJ6, 4Z7I, 5C97, 5MJ6 |
| Species | Phylum | [Protein] Crude Extract (mg/mL) | Inhibit Activity of DPP-IV (U/mg) | Inhibit Activity of TRH-DE (U/mg) |
|---|---|---|---|---|
| Caulerpa racemosa | Chlorophyta | 5.86 | - | - |
| Dictyosphaeria cavernosa | Chlorophycota | 8.73 | - | - |
| Halimeda opuntia | Chlorophycota | 14.72 | - | - |
| Halimeda incrassata | Chlorophycota | 10.23 | - | - |
| Bidens pilosa | Magnoliophyta | 22.78 | - | - |
| Ascidia sidneyense | Chordata | 57.27 | - | - |
| Molgula occidentalis | Chordata | 31.41 | - | - |
| Pyura vittata | Chordata | 58.53 | - | - |
| Phallusia nigra | Chordata | 25.90 | 93.00 | - |
| Microcosmus gamus | Chordata | 24.45 | - | - |
| Tectitethya cripta | Porifera | 0.90 | - | - |
| Mycale microsigmatosa | Porifera | 43.50 | 56.59 | - |
| Lima scabra | Mollusca | 51.55 | - | - |
| Aplisia dactilomela | Mollusca | 24.00 | - | - |
| Zoanthus pullchelus | Cnidaria | 15.57 | - | - |
| Plexaura homomalla | Cnidaria | 15.25 | - | - |
| Condylactis gigantea | Cnidaria | 36.60 | 79.46 | - |
| Stichodactyla helianthus | Cnidaria | 79.50 | 17.48 | - |
| Cassiopea xamachana | Cnidaria | 3.14 | - | - |
| Physalia physalis | Cnidaria | 13.84 | - | - |
| Palythoa caribaeorum | Cnidaria | 16.80 | 133.00 | - |
| Bartholomea annulata | Cnidaria | 56.50 | - | |
| Hermodice carunculata | Annelida | 62.41 | - | 24.00 |
| Sabellastarte magnifica | Annelida | 67.24 | - | - |
| Holothuria floridiana | Echinodermata | 18.84 | - | - |
| Holothuria mexicana | Echinodermata | 29.63 | - | - |
| Species | Phylum | pAPN Inhibitory Activity (U/mL) | pAPN Specific Inhibitory Activity (U/mg) | Pre-Incubation Time (min) | IC50 (mg/mL) |
|---|---|---|---|---|---|
| Phallusia nigra | Chordata | 2.3060 | 0.8363 | - | - |
| Lisoclinum verrilli | Chordata | 7.8770 | 0.7361 | 1 | 0.11 ± 0.06 |
| Ascidia sidneyensis | Chordata | 3.1760 | 0.6481 | - | - |
| Microcosmus guanus | Chordata | 7.1368 | 0.5366 | - | - |
| Esteinacidia turbinata | Chordata | 3.6340 | 0.5344 | - | - |
| Diplosoma listerianum | Chordata | 8.1300 | 0.4394 | 30 | 0.11 ± 0.26 |
| Poticlenum constellatum | Chordata | 3.2230 | 0.2984 | - | - |
| Eucidaris tribuloides | Echinodermata | 6.2526 | 0.3206 | 5 | 1.35 ± 0.19 |
| Ophiocoma echinata | Echinodermata | 8.2337 | 0.0874 | 60 | 2.39 ± 1.09 |
| Lebrunia danae | Echinodermata | - | - | - | - |
| Bryozoo sp1 | Bryozoa | 5.7240 | 0.0560 | - | - |
| Bryozoo sp2 | Bryozoa | 6.7600 | 1.1266 | 30 | 0.29 ± 0.05 |
| Hermodice carunculata | Annelida | - | - | - | - |
| Species | Phylum | NA-like Activity (×104 U/mg) | Crude Extracts hAPN sIA (U/mg) | 2.5% TCA Treated Extracts hAPN sIA (U/mg) |
|---|---|---|---|---|
| Cenchritis muricatus | Mollusca | ND | 0.46 | 5.20 |
| Nerita peloronta | Mollusca | 6.95 ± 2.84 | ND | 5.06 |
| Nerita versicolor | Mollusca | 12.56 ± 1.70 | ND | 2.21 |
| Lissodendoryx (Lissodendoryx) isodictyalis | Porifera | 1.51 ± 0.49 | ND | 171.92 |
| Tripneustes ventricosus | Echinodermata | 6.39 ± 0.07 | ND | 56.86 |
| Echinaster (Othilia) echinophorus | Echinodermata | 58.87 ± 12.62 | ND | 10.82 |
| Isostichopus badionotus | Echinodermata | ND | 1.56 | 33.81 |
| Stichodactyla helianthus | Cnidaria | 9.63 ± 2.68 | ND | 32.81 |
| Bunodosoma granuliferum | Cnidaria | 39.44 ± 5.45 | ND | 4.31 |
| Physalia physalis | Cnidaria | 2.04 ± 0.52 | ND | 13.13 |
| Species | Phylum | IC50 vs. pAPA (µg/mL) | IC50 vs. pAPN (µg/ML) | IC50 vs. hAPN (µg/mL) | IC50 vs. 3LL Viability (µg/mL) | IC50 vs. PC3 Viability (µg/mL) |
|---|---|---|---|---|---|---|
| Cenchritis muricatus | Mollusca | ND | ND | 450.20 ± 77.40 | 214.00 ± 46.50 | 352.90 ± 65.00 |
| Nerita peloronta | Mollusca | 487 ± 11.03 | 287.03 ± 12.00 | 237.80 ± 20.90 | 273.30 ± 78.80 | 299.10 ± 31.70 |
| Nerita versicolor | Mollusca | 92.23 ± 12.34 | 132.11 ± 22.05 | 370.00 ± 50.00 | 358.80 ± 70.20 | 289.70 ± 39.70 |
| Lissodendoryx (Lissodendoryx) isodictyalis | Porifera | 659.87 ± 10.65 | 613.24 ± 10.76 | 11.70 ± 2.70 | <5.00 | <5.00 |
| Tripneustes ventricosus | Echinodermata | 11.03 ± 0.12 | 13.35 ± 3.91 | 25.00 ± 3.10 | 39.90 ± 2.00 | 77.00 ± 3.90 |
| Echinaster (Othilia) echinophorus | Echinodermata | 182.01 ± 67.12 | 112.55 ± 23.21 | 198.20 ± 27.20 | 265.70 ± 29.60 | 405.60 ± 50.40 |
| Isostichopus badionotus | Echinodermata | 1005.12 ± 293.32 | 11.08 ± 0.27 | 69.70 ± 10.00 | 57.10 ± 2.70 | 83.10 ± 3.00 |
| Stichodactyla helianthus | Cnidaria | 256.3 ± 10.00 | 136.56 ± 22.87 | 103.60 ± 20.60 | 110.80 ± 13.20 | 58.10 ± 7.50 |
| Bunodosoma granuliferum | Cnidaria | ND | 98.02 ± 18.05 | 567.60 ± 88.00 | 786.80 ± 37.10 | 711.30 ± 29.30 |
| Physalia physalis | Cnidaria | ND | 198.92 ± 10.76 | 123.10 ± 21.30 | 257.30 ± 6.70 | 234.50 ± 5.00 |
| Bestatin (positive control) | - | 25.50 ± 2.35 | 6.54 ± 0.82 | 6.70 ± 1.90 | 0.54 ± 0.01 | 3.15 ± 0.72 |
| Amastatin (positive control) | - | 75.45 ± 4.55 | 58.32 ± 3.34 | 63.45 ± 7.61 | ND | ND |
| Enzyme | IUBMB * Nomenclature | Merops ID | Sources |
|---|---|---|---|
| Leucyl aminopeptidase 3 | 3.4.11.1 | M17.001 | Homo sapiens, Haemaphysalis longicornis, Mus musculus |
| Leucyl aminopeptidase (plant-type) | 3.4.11.1 | M17.002 | Solanum lycopersicum |
| PepA aminopeptidase | 3.4.11.10 | M17.003 | Escherichia coli, Pseudomonas aeruginosa |
| PepB aminopeptidase | 3.4.11.23 | M17.004 | Escherichia coli, Salmonella typhimurium |
| Mername-AA040 peptidase | - | M17.005 | Homo sapiens |
| Leucyl aminopeptidase-1 (Caenorhabditis-type) | - | M17.006 | Caenorhabditis elegans |
| M17 aminopeptidase (Plasmodium spp.) | 3.4.11.1 | M17.008 | Plasmodium spp. |
| Aminopeptidase yspII (Schizosaccharomyces sp.) | - | M17.009 | Schizosaccharomyces sp. |
| Leucyl aminopeptidase (Bacillus-type) | - | M17.010 | Geobacillus kaustophilus |
| Leucyl aminopeptidase (Fasciola-type) | - | M17.011 | Fasciola hepática |
| PwLAP aminopeptidase | - | M17.012 | Paragonimus westermani |
| Cysteinylglycinase (Treponema denticola)-like peptidase | - | M17.013 | Treponema denticola |
| LAPTc aminopeptidase | - | M17.014 | Trypanosoma cruzi, Leishmania major |
| Aminopeptidase pepZ (Staphylococcus sp.) | - | M17.015 | Staphylococcus aureus |
| Aminopeptidase A/I (Helicobacter-type) | - | M17.016 | Helicobacter pylori |
| similar to cytosol aminopeptidase (Rattus norvegicus) | - | M17.950 | Rattus norvegicus |
| At4g30920 (Arabidopsis thaliana) | - | M17.A01 | Arabidopsis thaliana |
| At4g30910 (Arabidopsis thaliana) | - | M17.A02 | Arabidopsis thaliana |
| At2g24200 (Arabidopsis thaliana) | - | M17.A03 | Arabidopsis thaliana |
| CG7340 g.p. (Drosophila melanogaster) | - | M17.A04 | Drosophila melanogaster |
| ZK353.6 (Caenorhabditis elegans) | - | M17.A05 | Caenorhabditis elegans |
| Enzyme | Source | Crystallographic Codes of the Structures at Protein Data Bank (PDB) |
|---|---|---|
| Leucyl aminopeptidase 3 | Bos taurus | 1BLL, 1BPM, 1BPN, 1LAM, 1LAN, 1LAP, 1LCP, 2EWB, 2J9A |
| Leucyl aminopeptidase (plant-type) | Solanum lycopersicum | 4KSI, 5D8N |
| PepA aminopeptidase | Escherichia coli | 1GYT |
| Francisella tularensis | 3PEI | |
| Pseudomonas putida | 3H8E, 3H8F, 3H8G | |
| Xanthomonas oryzae | 3JRU | |
| PepB aminopeptidase | Escherichia coli | 6OV8 |
| Yersinia pestis | 6CXD | |
| M17 aminopeptidase (Plasmodium spp.) | Plasmodium falciparum | 3KQX, 3KQZ, 3KR4, 3KR5, 3T8W, 4K3N, 4R6T, 4R7M, 4X2T |
| LAPTc aminopeptidase | Leishmania major | 5NTH |
| Trypanosoma brucei | 5NSK, 5NSM, 5NSQ, 5NTD | |
| Aminopeptidase A/I (Helicobacter-type) | Helicobacter pylori | 4ZI6, 4ZLA |
| ZK353.6 (Caenorhabditis elegans) | Caenorhabditis elegans | 2HB6, 2HC9 |
| Species | Phyla | Crude Extract rPfA-M17 sIA (U/mg) | Crude Extract hLAP sIA (U/mg) | 2.5% TCA Treated Extracts rPfA-M17 sIA (U/mg) | 2.5% TCA Treated Extracts hLAP sIA (U/mg) |
|---|---|---|---|---|---|
| Cenchritis muricatus | Mollusca | 0.40 | 0.16 | 7.56 | 4.30 |
| Nerita peloronta | Mollusca | ND | ND | 10.61 | 6.48 |
| Nerita versicolor | Mollusca | ND | ND | 7.17 | 5.58 |
| Lissodendoryx (Lissodendoryx) isodictyalis | Porifera | ND | 0.27 | 256.13 | 312.01 |
| Tripneustes ventricosus | Equinodermata | ND | ND | 41.85 | 43.31 |
| Echinaster (Othilia) echinophorus | Equinodermata | 0.11 | ND | 9.10 | 7.85 |
| Isostichopus badionotus | Equinodermata | 0.18 | 1.24 | 55.83 | 27.86 |
| Stichodactyla helianthus | Cnidaria | 0.55 | 0.68 | 38.15 | 18.67 |
| Bunodosoma granuliferum | Cnidaria | 0.19 | ND | 5.45 | 4.08 |
| Physalia physalis | Cnidaria | 0.40 | ND | 19.21 | 11.00 |
| Species | IC50 Value vs. rPfA-M17 (µg/mL) | IC50 vs. hLAP (µg/mL) | IC50hLAP/ IC50rPfA-M17 | IC50 Pf FcB1 (µg/mL) |
|---|---|---|---|---|
| Cenchritis muricatus | 113.40 ± 3.00 | 341.00 ± 110.00 | 3.00 | >400 |
| Nerita peloronta | 22.20 ± 2.70 | 329.50 ± 100.00 | 14.80 | 291.80 ± 38.50 |
| Nerita versicolor | 207.00 ± 30.60 | 12 429.60 ± 633.00 | 60.00 | 325.50 ± 0.80 |
| Lissodendoryx (Lissodendoryx) isodictyalis | 27.30 ± 9.40 | 66.30 ± 27.60 | 2.42 | 2.60 ± 0.60 |
| Tripneustes ventricosus | 84.80 ± 7.30 | 607.70 ± 300.50 | 7.16 | 0.24 ± 0.01 |
| Echinaster (Othilia) echinophorus | 127.50 ± 82.10 | 308.70 ± 100.00 | 2.42 | 201.60 ± 162.10 |
| Isostichopus badionotus | 86.70 ± 32.60 | 272.70 ± 63.50 | 3.13 | 183.70 ± 155.70 |
| Stichodactyla helianthus | 15.30 ± 6.20 | 234.90 ± 34.60 | 15.35 | >400 |
| Bunodosoma granuliferum | 509.20 ± 100.90 | 1171.00 ± 92.10 | 2.29 | >400 |
| Physalia physalis | 293.70 ± 100.00 | 550.00 ± 85.00 | 1.87 | 206.00 ± 84.00 |
| Bestatin (positive control) | 0.15 ± 0.02 | 11.83 ± 2.61 | 78.86 | 1.14 ± 0.27 |
| Amastatin (positive control) | 60.70 ± 19.84 | 158.05 ± 28.44 | 2.60 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual Alonso, I.; Almeida García, F.; Valdés Tresanco, M.E.; Arrebola Sánchez, Y.; Ojeda del Sol, D.; Sánchez Ramírez, B.; Florent, I.; Schmitt, M.; Avilés, F.X. Marine Invertebrates: A Promissory Still Unexplored Source of Inhibitors of Biomedically Relevant Metallo Aminopeptidases Belonging to the M1 and M17 Families. Mar. Drugs 2023, 21, 279. https://doi.org/10.3390/md21050279
Pascual Alonso I, Almeida García F, Valdés Tresanco ME, Arrebola Sánchez Y, Ojeda del Sol D, Sánchez Ramírez B, Florent I, Schmitt M, Avilés FX. Marine Invertebrates: A Promissory Still Unexplored Source of Inhibitors of Biomedically Relevant Metallo Aminopeptidases Belonging to the M1 and M17 Families. Marine Drugs. 2023; 21(5):279. https://doi.org/10.3390/md21050279
Chicago/Turabian StylePascual Alonso, Isel, Fabiola Almeida García, Mario Ernesto Valdés Tresanco, Yarini Arrebola Sánchez, Daniel Ojeda del Sol, Belinda Sánchez Ramírez, Isabelle Florent, Marjorie Schmitt, and Francesc Xavier Avilés. 2023. "Marine Invertebrates: A Promissory Still Unexplored Source of Inhibitors of Biomedically Relevant Metallo Aminopeptidases Belonging to the M1 and M17 Families" Marine Drugs 21, no. 5: 279. https://doi.org/10.3390/md21050279
APA StylePascual Alonso, I., Almeida García, F., Valdés Tresanco, M. E., Arrebola Sánchez, Y., Ojeda del Sol, D., Sánchez Ramírez, B., Florent, I., Schmitt, M., & Avilés, F. X. (2023). Marine Invertebrates: A Promissory Still Unexplored Source of Inhibitors of Biomedically Relevant Metallo Aminopeptidases Belonging to the M1 and M17 Families. Marine Drugs, 21(5), 279. https://doi.org/10.3390/md21050279