Sesquiterpenes from Streptomyces qinglanensis and Their Cytotoxic Activity
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
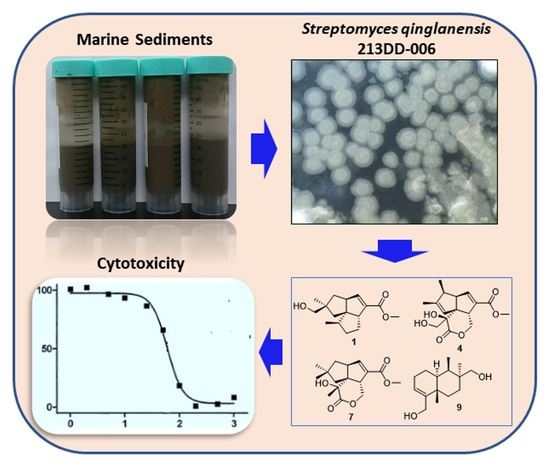
3.2. Isolation of the Microorganisms from Marine Sediment Samples
3.3. Isolation and Identification of the Strain 213DD-006
3.4. Fermentation of the Strain 213DD-006 and Extraction and Isolation of Metabolites
3.5. Computational Details
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donald, L.; Pipite, A.; Subramani, R.; Owen, J.; Keyzers, R.A.; Taufa, T. Streptomyces: Still the Biggest Producer of New Natural Secondary Metabolites, a Current Perspective. Microbiol. Res. 2022, 13, 418–465. [Google Scholar] [CrossRef]
- Ngel, M.; Paula, Y. Streptomyces as a Source of Antimicrobials: Novel Approaches to Activate Cryptic Secondary Metabolite Pathways. In Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods; Kırmusaoğlu, S., Ed.; IntechOpen: London, UK, 2019; pp. 119–130. [Google Scholar]
- Law, J.W.-F.; Law, L.N.-S.; Letchumanan, V.; Tan, L.T.-H.; Wong, S.H.; Chan, K.-G.; Ab Mutalib, N.-S.; Lee, L.-H. Anticancer Drug Discovery from Microbial Sources: The Unique Mangrove Streptomycetes. Molecules 2020, 25, 5365. [Google Scholar] [CrossRef] [PubMed]
- Salionov, D.S.; Poshekhontseva, V.Y.; Fokina, V.V.; Shutov, A.A.; Nikolaeva, V.M.; Vasiarov, G.G.; Titova, E.V.; Karasev, V.S.; Staroverov, S.M.; Donova, M.V. Biosynthesis of Tacrolimus by the Streptomyces tsukubensis VKM Ac-2618D Strain in the Presence of Polymeric Sorbents and Development of a Method for Its Isolation and Purification. Appl. Biochem. Microbiol. 2020, 56, 699–707. [Google Scholar] [CrossRef]
- Hotson, I.K. The avermectins: A new family of antiparasitic agents. J. S. Afr. Vet. Assoc. 1982, 53, 87–90. [Google Scholar]
- Niu, G.; Chater, K.F.; Tian, Y.; Zhang, J.; Tan, H. Specialised metabolites regulating antibiotic biosynthesis in Streptomyces spp. FEMS Microbiol. Rev. 2016, 40, 554–573. [Google Scholar] [CrossRef] [Green Version]
- Del Carratore, F.; Hanko, E.K.; Breitling, R.; Takano, E. Biotechnological application of Streptomyces for the production of clinical drugs and other bioactive molecules. Curr. Opin. Biotechnol. 2022, 77, 102762. [Google Scholar] [CrossRef]
- Lacey, H.J.; Rutledge, P.J. Recently Discovered Secondary Metabolites from Streptomyces Species. Molecules 2022, 27, 887. [Google Scholar] [CrossRef]
- Chen, Q.-F.; Liu, Z.-P.; Wang, F.-P. Natural sesquiterpenoids as cytotoxic anticancer agents. Mini-Rev. Med. Chem. 2011, 11, 1153–1164. [Google Scholar] [CrossRef]
- Le Bideau, F.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic Sesquiterpenes from Marine Origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef]
- Yu, L.; Dai, H.-F.; Zhao, Y.-X.; Zuo, W.-J.; Dong, W.-H.; Mei, W.-L.; Zeng, H.-C. Two new sesquiterpene derivatives from soil actinomycete Streptomyces albospinus 15-4-2. Phytochem. Lett. 2013, 6, 110–112. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Chen, S.; Wu, W.; Sun, P. Isolation and Identification of Pentalenolactone Analogs from Streptomyces sp. NRRL S-4. Molecules 2021, 26, 7377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ding, N.; Jiang, Y.; Zhang, J.; Ma, J.; Chen, X.; Liu, J.; Han, L.; Huang, X. Albaflavenoid, a new tricyclic sesquiterpenoid from Streptomyces violascens. J. Antibiot. 2016, 69, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-B.; Ma, M.; Deng, Z.-X.; Hong, K. Genotype-driven isolation of enterocin with novel bioactivities from mangrove-derived Streptomyces qinglanensis 172205. Appl. Microbiol. Biotechnol. 2015, 99, 5825–5832. [Google Scholar] [CrossRef] [PubMed]
- Haruo, S.; Tōru, S.; Jun, U.; Setsuo, T.; Hiroshi, Y. Studies on the biosynthesis of pentalenolactone, part II isolation of pentalehic acid and pentalenolactone H. Tetrahedron Lett. 1978, 19, 4411–4412. [Google Scholar]
- Izawa, S.; Akutsu, H.; Atomi, T.; Kawabata, S.; Sasaki, K. AA-57, a new antibiotic related to pentalenolactone. J. Antibiot. 1978, 31, 729–731. [Google Scholar] [CrossRef] [Green Version]
- You, Z.; Omura, S.; Ikeda, H.; Cane, D.E. Pentalenolactone Biosynthesis. Molecular Cloning and Assignment of Biochemical Function to PtlH, A Non-Heme Iron Dioxygenase of Streptomyces avermitilis. J. Am. Chem. Soc. 2006, 128, 6566–6567. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wang, Y.; Zhang, M.; Ikeda, H.; Deng, Z.; Cane, D.E. Product-Mediated Regulation of Pentalenolactone Biosynthesis in Streptomyces Species by the MarR/SlyA Family Activators PenR and PntR. J. Bacteriol. 2013, 195, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Ihara, M.; Katogi, M.; Fukumoto, K.; Kametani, T. Total synthesis of (±)-pentalenene, (±)-pentalenic acid, and (±)deoxypentalenic acid through an intramolecular double Michael reaction. J. Chem. Soc. Perkin Trans. 1 1988, 11, 2963–2970. [Google Scholar] [CrossRef]
- Williard, P.G.; Sohng, J.K.; Cane, D.E. The X-ray crystal structure of pentalenolactone F methyl ester (epi-pentalenolactone F). J. Antibiot. 1988, 41, 130–133. [Google Scholar] [CrossRef] [Green Version]
- De Guzman, F.S.; Copp, B.R.; Mayne, C.L.; Concepcion, G.P.; Mangalindan, G.C.; Barrows, L.R.; Ireland, C.M. Bolinaquinone: A Novel Cytotoxic Sesquiterpene Hydroxyquinone from a Philippine Dysidea Sponge. J. Org. Chem. 1998, 63, 8042–8044. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Shen, X.; Guo, Y.-W. A novel sesquiterpene quinone from Hainan sponge Dysidea villosa. Bioorganic Med. Chem. Lett. 2009, 19, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Uyeda, M.; Mizukami, M.; Yokomizo, K.; Suzuki, K. Pentalenolactone I and Hygromycin A, Immunosuppressants produced by Streptomyces filipinensis and Streptomyces hygroscopicus. Biosci. Biotechnol. Biochem. 2001, 65, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Liu, Y.; Chen, L.; Xu, M.; Naowarojna, N.; Lee, N.; Chen, L.; Zhu, D.; Hong, X.; Deng, Z.; et al. Biochemical Characterization of a Multifunctional Mononuclear Nonheme Iron Enzyme (PtlD) in Neopentalenoketolactone Biosynthesis. Org. Lett. 2019, 21, 7592–7596. [Google Scholar] [CrossRef]
- Choi, B.-K.; Trinh, P.T.H.; Lee, H.-S.; Choi, B.-W.; Kang, J.S.; Ngoc, N.T.D.; Van, T.T.T.; Shin, H.J. New Ophiobolin Derivatives from the Marine Fungus Aspergillus flocculosus and Their Cytotoxicities against Cancer Cells. Mar. Drugs 2019, 17, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Cho, H.; Lee, D.K.; Ha, J.; Choi, B.J.; Jeong, J.H.; Ryu, J.-H.; Kang, J.S.; Jeon, R. Discovery of 5-Phenoxy-2-aminopyridine Derivatives as Potent and Selective Irreversible Inhibitors of Bruton’s Tyrosine Kinase. Int. J. Mol. Sci. 2020, 21, 8006. [Google Scholar] [CrossRef]




| 1 | 4 | 7 | 9 | |
|---|---|---|---|---|
| Pos. | δH, Mult (J in Hz) | δH, Mult (J in Hz) | δH, Mult (J in Hz) | δH, Mult (J in Hz) |
| 1 | 1.68, dd (13.0, 8.9) 1.42 (13.2, 6.0) | 2.85, m | 1.75, dd (12.9, 10.2) 1.56, ovl | 1.82, m 1.25, dd (11.2, 6.6) |
| 2 | 2.15, m | |||
| 3 | 1.76, d (13.5), H-3a 1.58, d (13.6), H-3b | 5.41, brs | 2.16, d (13.4), H-3a 1.69, d (13.3), H-3b | 5.53, s |
| 5 | 3.02, d (9.1) | 3.12, m | 3.27, m | |
| 6 | 1.57, m | |||
| 7 | 6.64, dd (2.2, 1.5) | 6.78, t (1.9) | 6.81, t (2.1) | 1.49, m 1.07, ovl |
| 8 | 2.94, ddd (9.0, 5.8, 2.7) | 3.24, dt (8.5, 2.9) | 3.34, ovl | |
| 9 | 1.91, m | 1.53, m | ||
| 10 | 0.97, d (7.1) | 3.90, d (11.5) 3.44, d (11.5) | 1.56, s | 1.86, m |
| 11 | 1.64, m 1.36, m | 4.02, m | ||
| 12 | 2.00, m 1.40, ovl | 5.22, dd (11.2, 5.3) 4.39, dd (11.2, 0.6) | 4.70, dd (12.4, 5.0) 4.56, dd (12.4, 1.9) | 1.04, s |
| 13 | 1.08, s | |||
| 14 | 3.29, d (3.9) | 1.68, t (1.30) | 0.99, s | 0.92, d (7.6) |
| 15 | 1.04, s | 1.03, d (7.40) | 1.06, s | 3.46, d (10.7) 3.18, d (10.7) |
| 16 | 3.70, s | 3.75, s | 3.75, s |
| 1 | 4 | 7 | 9 | |
|---|---|---|---|---|
| Pos. | δC, Type | δC, Type | δC, Type | δC, Type |
| 1 | 41.8, CH2 | 45.9, CH | 45.6, CH2 | 25.5, CH2 |
| 2 | 47.1, C | 146.8, C | 41.4, C | 27.4, CH2 |
| 3 | 44.3, CH2 | 125.9, CH | 51.5, CH2 | 122.6, CH |
| 4 | 65.5, C | 64.4, C | 62.2, C | 148.0, C |
| 5 | 58.6, CH | 51.3, CH | 54.0, CH | 38.9, C |
| 6 | 138.2, C | 135.1, C | 134.5, C | 32.0, CH2 |
| 7 | 148.8, CH | 147.0, CH | 152.6, CH | 26.8, CH2 |
| 8 | 60.0, CH | 56.6, CH | 57.1, CH | 38.8, C |
| 9 | 45.6, CH | 78.2, C | 74.8, C | 41.7, CH |
| 10 | 17.3, CH3 | 63.4, CH2 | 22.5, CH3 | 41.8, CH |
| 11 | 34.3, CH2 | 174.2, C | 178.3, C | 62.7, CH2 |
| 12 | 30.1, CH2 | 69.9, CH2 | 69.4, CH2 | 21.4, CH3 |
| 13 | 167.5, C | 166.1, C | 165.8, C | 22.7, CH3 |
| 14 | 70.9, CH2 | 14.5, CH3 | 29.5, CH3 | 11.9, CH3 |
| 15 | 24.6, CH3 | 15.6, CH3 | 31.8, CH3 | 71.7, CH2 |
| 16 | 51.8, CH3 | 52.2, CH3 | 52.1, CH3 |
| Compounds | ACHN | MDA-MB-231 | HCT-15 | PC-3 | NUGC-3 | NCI-H23 |
|---|---|---|---|---|---|---|
| 1 | >30 | >30 | >30 | >30 | >30 | >30 |
| 2 | >30 | >30 | >30 | >30 | >30 | >30 |
| 3 | >30 | >30 | >30 | >30 | >30 | >30 |
| 4 | 2.44 ± 0.27 | 3.22 ± 0.15 | 3.12 ± 0.02 | 2.88 ± 0.06 | 2.23 ± 0.14 | 2.60 ± 0.02 |
| 5 | 2.15 ± 0.17 | 2.88 ± 0.39 | 2.46 ± 0.35 | 3.46 ± 0.03 | 2.37 ± 0.38 | 2.73 ± 0.35 |
| 6 | 2.31 ± 0.06 | 2.63 ± 0.15 | 2.35 ± 0.11 | 2.92 ± 0.20 | 2.41 ± 0.09 | 2.75 ± 0.11 |
| 7 | >30 | >30 | >30 | >30 | >30 | >30 |
| 8 | 1.97 ± 0.13 | 2.71 ± 0.10 | 3.23 ± 0.12 | 3.24 ± 0.58 | 1.97 ± 0.44 | 2.40 ± 0.12 |
| 9 | >30 | >30 | >30 | >30 | >30 | >30 |
| Adr. | 0.095 ± 0.005 | 0.073 ± 0.009 | 0.080 ± 0.007 | 0.084 ± 0.010 | 0.092 ± 0.023 | 0.070 ± 0.007 |
| Compounds | HL-60 | Raji | K562 | RPMI-8402 | NALM6 | U266 | WSU-DLCL2 |
|---|---|---|---|---|---|---|---|
| 1 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| 2 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| 3 | 16.39 ± 2.38 | >30 | >30 | >30 | >30 | >30 | >30 |
| 4 | >30 | >30 | 28.23 ± 0.85 | 26.39 ± 0.63 | 17.75 ± 1.73 | >30 | >30 |
| 5 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| 6 | 22.58 ± 0.74 | 21.30 ± 3.88 | 8.37 ± 0.26 | 8.85 ± 0.43 | 10.65 ± 0.74 | >30 | 14.03 ± 1.17 |
| 7 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| 8 | >30 | >30 | 17.94 ± 1.05 | 25.37 ± 2.90 | 24.77 ± 1.22 | >30 | 28.26 ± 3.45 |
| 9 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| Adr. | 0.017 ± 0.001 | 0.008 ± 0.001 | 0.090 ± 0.002 | 0.017 ± 0.000 | 0.003 ± 0.000 | 0.035 ± 0.001 | 0.004 ± 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh, C.V.; Kang, J.S.; Yang, J.-W.; Kwon, J.-H.; Heo, C.-S.; Lee, H.-S.; Park, C.H.; Shin, H.J. Sesquiterpenes from Streptomyces qinglanensis and Their Cytotoxic Activity. Mar. Drugs 2023, 21, 361. https://doi.org/10.3390/md21060361
Anh CV, Kang JS, Yang J-W, Kwon J-H, Heo C-S, Lee H-S, Park CH, Shin HJ. Sesquiterpenes from Streptomyces qinglanensis and Their Cytotoxic Activity. Marine Drugs. 2023; 21(6):361. https://doi.org/10.3390/md21060361
Chicago/Turabian StyleAnh, Cao Van, Jong Soon Kang, Jeong-Wook Yang, Joo-Hee Kwon, Chang-Su Heo, Hwa-Sun Lee, Chan Hong Park, and Hee Jae Shin. 2023. "Sesquiterpenes from Streptomyces qinglanensis and Their Cytotoxic Activity" Marine Drugs 21, no. 6: 361. https://doi.org/10.3390/md21060361
APA StyleAnh, C. V., Kang, J. S., Yang, J.-W., Kwon, J.-H., Heo, C.-S., Lee, H.-S., Park, C. H., & Shin, H. J. (2023). Sesquiterpenes from Streptomyces qinglanensis and Their Cytotoxic Activity. Marine Drugs, 21(6), 361. https://doi.org/10.3390/md21060361