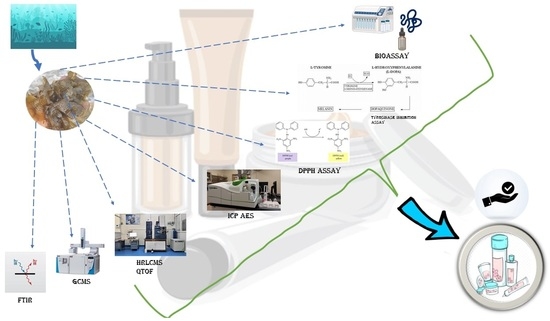
Comprehensive Phytochemical Analysis and Bioactivity Evaluation of Padina boergesenii: Unveiling Its Prospects as a Promising Cosmetic Component
Abstract
:1. Introduction
2. Results and Discussion
2.1. Collection of Brown Alga P. boergesenii
2.2. Functional Group Analysis of P. boergesenii Using FTIR Spectroscopy
2.3. Characterization of Ethanolic Extracts of P. boergesenii Using GCMS
2.4. Comprehensive Profiling of Methanol-Extracted Phycocompounds in P. boergesenii Using GCMS Analysis
2.5. Comprehensive Characterization of Phycocompounds in P. boergesenii Using HRLCMS QTOF
2.6. Quantification of Amino Acids in P. boergesenii Using HRLCMS
2.7. Elemental Analysis of P. boergesenii Using the ICP-AES Technique
2.8. Determination of Pigment Content in Padina boergesenii
2.8.1. Quantification of Chlorophylls, Carotenoids, Fucoxanthin, Phycoerythrin, and Phycocyanin
2.8.2. Estimation of Chlorophylls and Lycopene
2.8.3. Quantification of Chlorophylls and Total Carotenoids
2.9. Analysis of Total Polyphenol Content in P. boergesenii
2.10. Estimation of Total Protein Content in P. boergesenii
2.11. Antioxidant Analysis of P. boergesenii Extracts Using the DPPH Method
2.12. Assessment of Tyrosinase Inhibition Activity of P. boergesenii Extracts
3. Materials and Methods
3.1. Collection, Transportation, and Storage Protocol for the Brown Alga P. boergesenii
3.2. Functional Group Analysis of P. boergesenii Using FTIR Spectroscopy
3.3. Characterization of Phycocompounds in P. boergesenii by GCMS Analysis
3.4. Comprehensive Profiling of Methanol-Extracted Phycocompounds in P. boergesenii Using GCMS Analysis
3.5. Comprehensive Characterization of Phycocompounds in P. boergesenii Using HRLCMS QTOF
3.6. Quantification of Amino Acids in P. boergesenii Using HRLCMS
3.7. Elemental Analysis of P. boergesenii Using the ICP-AES Technique
3.7.1. Sample Preparation and Digestion
3.7.2. Element Analysis by ICP-AES
3.8. Quantification of Pigments in P. boergesenii
3.8.1. Quantification of Chlorophylls, Carotenoids, Fucoxanthin, Phycoerythrin, and Phycocyanin
- Set 1. Equations for estimation of chlorophyll and carotenoids in 100% methanol.
| 1. Chl a (μg/mL) = −2.0780 × (A632 − A750) − 6.5079 × (A652 − A750) + 16.2127 × (A665 − A750) − 2.1372 × (A696 − A750) |
| 2. Chl b (μg/mL) = −2.9450 × (A632 − A750) − 32.1228 × (A652 − A750) + 13.8255 × (A665 − A750) − 3.0097 × (A696 − A750) |
| 3. Chl c (μg/mL) = 34.0115 × (A632 − A750) − 12.7873 × (A652 − A750) + 1.4489 × (A665 − A750) − 2.5812 × (A696 − A750) |
| 4. Chl d (μg/mL) = −0.3411 × (A632 − A750) + 0.1129 × (A652 − A750) – 0.2538 × (A665 − A750) + 12.9508 × (A696 − A750) |
| 5. Total Chl (μg/mL) = Chl a + Chl b + Chl c + Chl d |
| 6. Carotenoids (μg/mL) = 4 × (A480 − A750) |
| 100% Ethanol |
- Set 2. Equations for estimation of chlorophyll and carotenoids in 100% ethanol.
| 1. Chl a (µg/mL) = 0.0604 × (A632 − A750) − 4.5224 × (A649 − A750) + 13.2969 × (A665 − A750) − 1.7453 × (A696 − A750) |
| 2. Chl b (µg/mL) = −4.1982 × (A632 − A750) + 25.7205 (A649 − A750) − 7.4096 × (A665 − A750) − 2.7418 × (A696 − A750) |
| 3. Chl c (µg/mL) = 28.4593 × (A632 − A750) − 9.9944 (A649 − A750) − 1.9344 × (A665 − A750) − 1.8093 × (A696 − A750) |
| 4. Chl d (µg/mL) = −0.2007 × (A632 − A750) + 0.0848 (A649 − A750) − 0.1909 × (A665 − A750) + 12.1302 × (A696 − A750) |
| 5. Total Chl (μg/mL) = Chl a + Chl b + Chl c + Chl d |
- Set 3. Equations for estimation of fucoxanthin, phycoerythrin, and phycocyanin.
| DMSO: Water (4:1, v/v) |
| Fucoxanthin (µg/mL) = 7.60 × (A480 − A750) − 5.55 × [(A631 − A750) + (A582 − A750) − 0.297 × (A665 − A750)] − 0.377 × (A665 − A750) |
| Phosphate Buffer (pH = 6.8) |
| 1. Phycoerythrin (µg/mL) = [(A565 − A750)/2.41 × 106] × 240,000 × 103 |
| 2.Phycocyanin (µg/mL) = [(A618 − A750)/1.90 × 106] × 264,000 × 103 |
3.8.2. Estimation of Chlorophylls and Lycopene
- Set 4. Equations for Chlorophyll a, Chlorophyll b, and Lycopene.
| Chlorophyll a (mg/100 mL) = 0.999 × A663 − 0.0989 × A645 |
| Chlorophyll b (mg/100 mL) = −0.328 × A663 + 1.77 × A645 |
| Lycopene (mg/100 mL) = −0.0485 × A663 + 0.204 × A645 + 0.372 × A505 − 0.0806 × A453 |
| A = Absorbance |
3.8.3. Estimation of Chlorophylls and Total Carotenoids
- Set 5. Equations for chlorophyll a, chlorophyll b, and total carotenoids.
| Chlorophyll a (µg/100 mL) = 12.25 × A663.6 − 2.25 × A646.6 |
| Chlorophyll b (µg/100 mL) = 20.31 × A646.6 + 4.91 × A663.6 |
| Total carotenoids (µg/100 mL) = [1000 × A470 − 2.27(Chl a) – 81.4 (Chl b)]/227 |
| A = Absorbance |
3.9. Analysis of Total Polyphenol Content in P. boergesenii
3.10. Estimation of Total Protein Content in P. boergesenii
3.11. Analysis of P. boergesenii Extracts Using the DPPH Method
3.12. Assessment of Tyrosinase Inhibition Activity of P. boergesenii Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient–Natural preservative: A case study. Comptes Rendus Chim. 2016, 19, 1077–1089. [Google Scholar] [CrossRef] [Green Version]
- Hauser, R.; Calafat, A.M. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlantézec, R.; Monfort, C.; Rouget, F.; Cordier, S. Maternal occupational exposure to solvents and congenital malformations: A prospective study in the general population. Occup. Environ. Med. 2009, 66, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.H. Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Desai, A.Y.; Mulye, V. Seaweed resources and utilization: An overview. Biotech. Express. 2015, 2, 22. [Google Scholar]
- Pereira, L.; Neto, J.M. (Eds.) Marine Algae: Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Marine Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.S.; Jena, M. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Venkatesan, K.; Sivadasan, D.; Alghazwani, Y.; Asiri, Y.I.; Prabahar, K.; Al-Qahtani, A.; Mohamed, J.M.M.; Khan, N.A.; Krishnaraju, K.; Paulsamy, P.; et al. Potential of seaweed biomass: Snake venom detoxifying action of brown seaweed Padina boergesenii against Naja naja venom. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Future J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- John, D.M. Marine Algae (Seaweeds) Associated with Coral Reefs in the Gulf. In Coral Reefs of the Gulf: Adaptation to Climatic Extremes; Springer: Berlin/Heidelberg, Germany, 2012; pp. 309–335. [Google Scholar]
- Guiry, M.D. AlgaeBase. World-Wide Electronic Publication. 2013. Available online: https://www.algaebase.org (accessed on 14 June 2023).
- Abuga, K.; Nyamweya, N. Alcohol-based hand sanitizers in COVID-19 prevention: A multidimensional perspective. Pharmacy 2021, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Lage, R.; Mendes, C.; Zugaib, B.M.; Abdalla, J.A.; Costa, A. Cosmeceutical Ingredients: Botanical and Nonbotanical Sources. Dly. Routine Cosmet. Dermatol. 2017, 1, 203–224. [Google Scholar]
- Zhao, X.; Zhou, M. Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects. Molecules 2022, 27, 5249. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.N.; Al Ramahi, R. Depigmentation and anti-aging treatment by natural molecules. Curr. Pharm. Des. 2019, 25, 2292–2312. [Google Scholar] [CrossRef]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S-and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Akhtar, Z.; Ali, S.I.; Abbas, N.; Ali, M.; Khan, M.Y.; Hasan, S.A.; Ahmed, S.; Manzoor, S.; Lutfi, Z. Evaluation of Antibacterial Potential of New Acid Dyes Based on Substituted Aryl Amines and Amino Hydroxy Sulfonic Acid. J. Chem. Soc. Pak. 2020, 42, 783–788. [Google Scholar]
- Demurtas, M.; Baldisserotto, A.; Lampronti, I.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Indole derivatives as multifunctional drugs: Synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indole hydrazones. Bioorganic Chem. 2019, 85, 568–576. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.T.; Barua, S.; Yoo, S.Y.; Hong, S.C.; Lee, K.B.; Lee, J. Seeking better topical delivery technologies of moisturizing agents for enhanced skin moisturization. Expert Opin. Drug Deliv. 2018, 15, 17–31. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Khan, Z.I.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Emerging role of microemulsions in cosmetics. Recent Pat. Drug Deliv. Formul. 2008, 2, 275–289. [Google Scholar] [CrossRef]
- Keng, P.S.; Basri, M.; Zakaria, M.R.S.; Rahman, M.A.; Ariff, A.B.; Rahman, R.A.; Salleh, A.B. Newly synthesized palm esters for cosmetics industry. Ind. Crops Prod. 2009, 29, 37–44. [Google Scholar] [CrossRef]
- Hayes, D.G. Fatty acids–based surfactants and their uses. In Fatty Acids; AOCS Press: Urbana, IL, USA, 2017; pp. 355–384. [Google Scholar]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef]
- Miya, G.M.; Oriola, A.O.; Payne, B.; Cuyler, M.; Lall, N.; Oyedeji, A.O. Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties. Molecules 2023, 28, 3434. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; Ferreira, G.D.C.; Trevenzoli, I.H.; Oliveira, K.D.J.; de Melo Reis, R.A. Fatty acids, antioxidants and physical activity in brain aging. Nutrients 2017, 9, 1263. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural compounds and products from an anti-aging perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Chao, C.; Génot, C.; Rodriguez, C.; Magniez, H.; Lacourt, S.; Fievez, A.; Len, C.; Pezron, I.; Luart, D.; Van Hecke, E. Emollients for cosmetic formulations: Towards relationships between physico-chemical properties and sensory perceptions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 156–164. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Dixit, D.; Reddy, C.R.K. Non-targeted secondary metabolite profile study for deciphering the cosmeceutical potential of red marine macro alga Jania rubens—An LCMS-based approach. Cosmetics 2017, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Taofiq, O.; Barreiro, M.F.; Ferreira, I.C. The role of bioactive compounds and other metabolites from mushrooms against skin disorders-a systematic review assessing their cosmeceutical and nutricosmetic outcomes. Curr. Med. Chem. 2020, 27, 6926–6965. [Google Scholar] [CrossRef]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba Oil: An updated comprehensive review on chemistry, pharmaceutical uses, and toxicity. Polymers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, M.; Song, L. A review of fatty acids influencing skin condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T. Chemical and Physical Properties of Emollients. In Treatment of Dry Skin Syndrome: The Art and Science of Moisturizers; Springer: Berlin/Heidelberg, Germany, 2012; pp. 399–417. [Google Scholar]
- Husein el Hadmed, H.; Castillo, R.F. Cosmeceuticals: Peptides, proteins, and growth factors. J. Cosmet. Dermatol. 2016, 15, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Podder, I.; Gokhale, N.; Jagadeesan, S.; Garg, V.K. Use of vegetable oils in dermatology: An overview. Int. J. Dermatol. 2017, 56, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Kelm, G.R.; Wickett, R.R. The role of fatty acids in cosmetic technology. In Fatty Acids; AOCS Press: Urbana, IL, USA, 2017; pp. 385–404. [Google Scholar]
- Cochran, S.; Anthonavage, M. Fatty acids, fatty alcohols, synthetic esters and glycerin applications in the cosmetic industry. In Lipids and Skin Health; Springer International Publishing: Cham, Switzerland, 2014; pp. 311–319. [Google Scholar]
- Yu, R.J.; Van Scott, E.J. Alpha-hydroxyacids and carboxylic acids. J. Cosmet. Dermatol. 2004, 3, 76–87. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- Silva, R.O.; Sousa, F.B.M.; Damasceno, S.R.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.M.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Marzo, N.; Pérez-Sánchez, A.; Barrajón-Catalán, E.; Castillo, J.; Herranz-López, M.; Micol, V. Rosemary diterpenes and flavanone aglycones provide improved genoprotection against uv-induced DNA damage in a human skin cell model. Antioxidants 2020, 9, 255. [Google Scholar] [CrossRef] [Green Version]
- Ribeaucourt, D.; Bissaro, B.; Lambert, F.; Lafond, M.; Berrin, J.G. Biocatalytic oxidation of fatty alcohols into aldehydes for the flavors and fragrances industry. Biotechnol. Adv. 2022, 56, 107787. [Google Scholar] [CrossRef]
- Kang, S.Y.; Um, J.Y.; Chung, B.Y.; Lee, S.Y.; Park, J.S.; Kim, J.C.; Park, C.W.; Kim, H.O. Moisturizer in patients with inflammatory skin diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed. Dermatol. 2020, 4, 12. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Clark, A.K.; Sivamani, R.K.; Shi, V.Y. Natural oils for skin-barrier repair: Ancient compounds now backed by modern science. Am. J. Clin. Dermatol. 2018, 19, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Mikail, M.A. Anti-aging skincare: The natural and organic way. In Anti-Aging Pharmacology; Academic Press: Cambridge, MA, USA, 2023; pp. 269–284. [Google Scholar]
- Ashawat, M.; Banchhor, M.; Saraf, S.; Saraf, S. Herbal Cosmetics: Trends in Skin Care Formulation. Pharmacogn. Rev. 2009, 3, 82. [Google Scholar]
- Dahiya, S.; Dahiya, R. Potential of colloidal carriers for nanocosmeceutical applications. In Nanocosmeceuticals; Academic Press: Cambridge, MA, USA, 2022; pp. 169–208. [Google Scholar]
- Mohiuddin, A.K. Skin care creams: Formulation and use. Dermatol. Clin. Res. 2019, 5, 238–271. [Google Scholar]
- Yu, M.; Wan, S.; Song, H.; Zhang, Y.; Wang, C.; Wang, H.; Wang, H. Sensory-based identification of aroma-active compounds in hotpot seasoning before and after boiling. Molecules 2021, 26, 5727. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
- Abdelhamed, F.M.; Abdeltawab, N.F.; ElRakaiby, M.T.; Shamma, R.N.; Moneib, N.A. Antibacterial and anti-inflammatory activities of Thymus vulgaris essential oil nanoemulsion on acne vulgaris. Microorganisms 2022, 10, 1874. [Google Scholar] [CrossRef]
- Abozeid, D.; Fawzy, G.; Issa, M.; Abdeltawab, N.; Soliman, F. Medicinal Plants and their Constituents in the Treatment of Acne vulgaris. Biointerface Res. Appl. Chem. 2023, 13, 189. [Google Scholar]
- Mistry, N. Guidelines for formulating anti-pollution products. Cosmetics 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Kalasariya, H.S.; Patel, N.B.; Yadav, A.; Perveen, K.; Yadav, V.K.; Munshi, F.M.; Yadav, K.K.; Alam, S.; Jung, Y.K.; Jeon, B.H. Characterization of fatty acids, polysaccharides, amino acids, and minerals in marine macroalga Chaetomorpha crassa and evaluation of their potentials in skin cosmetics. Molecules 2021, 26, 7515. [Google Scholar] [CrossRef]
- Torres, A.; Rego, L.; Martins, M.S.; Ferreira, M.S.; Cruz, M.T.; Sousa, E.; Almeida, I.F. How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals 2023, 16, 573. [Google Scholar] [CrossRef]
- van Smeden, J.; Janssens, M.; Kaye, E.C.; Caspers, P.J.; Lavrijsen, A.P.; Vreeken, R.J.; Bouwstra, J.A. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol. 2014, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jaricot, M.; Malhiac, C.; Chao, C.; Merlaud, F.; Grisel, M.; Savary, G. Understanding of the residual odour of fatty esters used as emollient in cosmetic products. Int. J. Cosmet. Sci. 2022, 44, 685–702. [Google Scholar] [CrossRef]
- Benchagra, L.; Berrougui, H.; Islam, M.O.; Ramchoun, M.; Boulbaroud, S.; Hajjaji, A.; Fulop, T.; Ferretti, G.; Khalil, A. Antioxidant effect of moroccan pomegranate (Punica granatum L. sefri variety) extracts rich in punicalagin against the oxidative stress process. Foods 2021, 10, 2219. [Google Scholar]
- Choi, D.Y.; Lee, Y.J.; Hong, J.T.; Lee, H.J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Kaur, T.; Malhotra, S.K.; Gambhir, M.L. Moisturizers: The slippery road. Indian J. Dermatol. 2016, 61, 279. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin care and rejuvenation by cosmeceutical facial mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K. Topical peptide treatments with effective anti-aging results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending anti-aging peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y. Anti-aging and sunscreens: Paradigm shift in cosmetics. Adv. Pharm. Bull. 2019, 9, 348. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.R.; Mohite, S.K. Potential role of peptides for development of cosmeceutical skin products. Res. J. Top. Cosmet. Sci. 2020, 11, 77–82. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: A randomized, double-blind, placebo-controlled study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 987–1000. [Google Scholar] [CrossRef]
- Eldeen, I.M.S.; Elgorashi, E.E.; Van Staden, J. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. J. Ethnopharmacol. 2005, 102, 457–464. [Google Scholar] [CrossRef]
- Rembe, J.D.; Fromm-Dornieden, C.; Stuermer, E.K. Effects of vitamin B complex and vitamin C on human skin cells: Is the perceived effect measurable? Adv. Ski. Wound Care 2018, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.D.; Rajpurohit, D. Antioxidant and antimicrobial activity of nutmeg (Myristica fragrans). In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 831–839. [Google Scholar]
- Thapa, N.; Thapa, P.; Bhandari, J.; Niraula, P.; Shrestha, N.; Shrestha, B.G. Study of phytochemical, antioxidant and antimicrobial activity of Artocarpus heterophyllus. Nepal J. Biotechnol. 2016, 4, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Leandro, A.; Pereira, L.; Gonçalves, A. Diverse Applications of Marine Macroalgae. Mar. Drugs. 2019, 18, 17. [Google Scholar] [CrossRef] [Green Version]
- Corsetti, G.; D’Antona, G.; Dioguardi, F.S.; Rezzani, R. Topical application of dressing with amino acids improves cutaneous wound healing in aged rats. Acta Histochem. 2010, 112, 497–507. [Google Scholar] [CrossRef]
- Veis, A.; Anesey, J. Modes of intermolecular cross-linking in mature insoluble collagen. J. Biol. Chem. 1965, 240, 3899–3908. [Google Scholar] [CrossRef]
- Choi, H.-R.; Kang, Y.-A.; Ryoo, S.-J.; Shin, J.-W.; Na, J.-I.; Huh, C.-H.; Park, K.-C. Stem cell recovering effect of copper-free GHK in skin. J. Pept. Sci. 2012, 18, 685–690. [Google Scholar] [CrossRef]
- Murakami, H.; Shimbo, K.; Inoue, Y.; Takino, Y.; Kobayashi, H. Importance of amino acid composition to improve skin collagen protein synthesis rates in UV-irradiated mice. Amino Acids. 2011, 42, 2481–2489. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, M.; Yokose, U.; Hachiya, A.; Fujimura, T.; Tsukahara, K.; Kawada, H.; Kitahara, T.; Takema, Y.; Terui, T.; Nakagawa, H. Improvement of crow’s feet lines by topical application of 1-carbamimidoyl-L-proline (CLP). Eur. J. Dermatol. 2013, 23, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Morioka, Y.; Kitaura, Y.; Iwatsuki, K.; Shimomura, Y.; Oishi, Y. Branched-chain amino acids regulate type I tropocollagen and type III tropocollagen syntheses via modulation of mTOR in the skin. Biosci. Biotechnol. Biochem. 2018, 82, 611–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puviani, M.; Agostinis, F.; Milani, M. Barrier repair therapy for facial atopic eczema with a non-steroidal emollient cream containing rhamnosoft, ceramides and iso-leucine. A six-case report series. Minerva Pediatr. 2014, 66, 307–311. [Google Scholar]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serre, C.; Busuttil, V.; Botto, J.-M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardana, K.; Garg, V.K. An observational study of methionine-bound zinc with antioxidants for mild to moderate acne vulgaris. Dermatol. Ther. 2010, 23, 411–418. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids. 2010, 40, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.M.; Ahn, S.K.; Denda, M.; Brown, B.E.; Crumrine, D.; Kimutai, L.K.; Kömüves, L.; Lee, S.H.; Feingold, K.R. Modulations in Epidermal Calcium Regulate the Expression of Differentiation-Specific Markers. J. Investig. Dermatol. 2002, 119, 1128–1136. [Google Scholar] [CrossRef] [Green Version]
- Matz, H.; Orion, E.; Wolf, R. Balneotherapy in dermatology. Dermatol. Ther. 2003, 16, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Denda, M.; Katagiri, C.; Hirao, T.; Maruyama, N.; Takahashi, M. Some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch. Dermatol. Res. 1999, 291, 560–563. [Google Scholar] [CrossRef]
- Schempp, C.M.; Dittmar, H.C.; Hummler, D.; Simon-Haarhaus, B.; Schöpf, E.; Simon, J.C.; Schulte-Mönting, J. Magnesium ions inhibit the antigen-presenting function of human epidermal Langerhans cells in vivo and in vitro. Involvement of ATPase, HLA-DR, B7 molecules, and cytokines. J. Investig. Dermatol. 2000, 115, 680–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration, HHS. Skin protectant drug products for over-the-counter human use; final monograph. Final rule. Fed. Regist. 2003, 68, 33362–33381. [Google Scholar]
- Food and Drug Administration. Sunscreen Drug Products for Over-the-Counter Human Use. Amendment to the Tentative Final Monograph; Enforcement Policy. Fed. Regist. 2019, 63, 6204–6275. [Google Scholar]
- Higdon, J.; Drake, V.J. An Evidenced-Based Approach to Vitamins and Minerals, 2nd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2012; pp. 157–168. [Google Scholar]
- Antoniou, C.; Stefanaki, C. Cosmetic camouflage. J. Cosmet. Dermatol. 2006, 5, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Ed. 2008, 19, 969–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler Jr, J.F.; Woolery-Lloyd, H.; Waldorf, H.; Saini, R. Innovations in natural ingredients and their use in skin care. J. Drugs Dermatol. JDD 2010, 9 (Suppl. 6), S72–S81. [Google Scholar]
- Leenutaphong, V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol. Photoimmunol. Photomed. 1995, 11, 198–203. [Google Scholar] [CrossRef]
- O’Connor, I.; O’Brien, N. Modulation of UVA light-induced oxidative stress by β-carotene, lutein and astaxanthin in cultured fibroblasts. J. Dermatol. Sci. 1998, 16, 226–230. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Yang, C.M.; Chang, K.W.; Yin, M.H.; Huang, H.M. Methods for the determination of the chlorophylls and their derivatives. Taiwania 1998, 43, 116–122. [Google Scholar]
- Souza, B.W.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.N.; Heo, S.J.; Kang, S.M.; Ahn, G.; Jeon, Y.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. Vitr. 2010, 24, 1648–1654. [Google Scholar] [CrossRef]
- Farabegoli, F.; Santaclara, F.J.; Costas, D.; Alonso, M.; Abril, A.G.; Espiñeira, M.; Ortea, I.; Costas, C. Exploring the Anti-Inflammatory Effect of Inulin by Integrating Transcriptomic and Proteomic Analyses in a Murine Macrophage Cell Model. Nutrients 2023, 15, 859. [Google Scholar] [CrossRef]
- Dixit, D.C.; Reddy, C.R.K.; Balar, N.; Suthar, P.; Gajaria, T.; Gadhavi, D.K. Assessment of the nutritive, biochemical, antioxidant and antibacterial potential of eight tropical macro algae along Kachchh coast, India as human food supplements. J. Aquat. Food Prod. Technol. 2018, 27, 61–79. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- El-Shafei, R.; Hegazy, H.; Acharya, B. A review of antiviral and antioxidant activity of bioactive metabolite of macroalgae within an optimized extraction method. Energies 2021, 14, 3092. [Google Scholar] [CrossRef]
- Monteiro, M.; Santos, R.A.; Iglesias, P.; Couto, A.; Serra, C.R.; Gouvinhas, I.; Barros, A.; Oliva-Teles, A.; Enes, P.; Díaz-Rosales, P. Effect of extraction method and solvent system on the phenolic content and antioxidant activity of selected macro-and microalgae extracts. J. Appl. Phycol. 2020, 32, 349–362. [Google Scholar] [CrossRef]
- Im, D.Y. Antioxidative activity and tyrosinase inhibitory activity of the extract and fractions from Arctium lappa roots and analysis of phenolic compounds. Korean J. Pharmacogn. 2014, 45, 141–146. [Google Scholar]
- Jo, J.H.; Kim, D.; Lee, S.; Lee, T.K. Total phenolic contents and biological activities of Korean seaweed extracts. Food Sci. Biotechnol. 2005, 14, 798–802. [Google Scholar]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, S.; Yousefzadi, M.; Nezhad, S.B.M.; Pozharitskaya, O.N.; Shikov, A.N. Utilization of the ethyl acetate fraction of Padina boergesenii as a natural UV filter in sunscreen cream formulation. Life 2023, 13, 239. [Google Scholar] [CrossRef]
- Moubayed, N.M.; Al Houri, H.J.; Al Khulaifi, M.M.; Al Farraj, D.A. Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J. Biol. Sci. 2017, 24, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Suman, T.Y.; Rajasree, S.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 1: GC–MS qualitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, J.; Palani, S. Phytochemical screening, GC-MS analysis and antioxidant activity of marine algae Chlorococcum humicola. World J. Pharm. Pharm. Sci. 2016, 5, 1154–1167. [Google Scholar]
- Tatipamula, V.B.; Killari, K.N.; Prasad, K.; Rao, G.S.N.K.; Talluri, M.R.; Vantaku, S.; Bilakanti, D.; Srilakshmi, N. Cytotoxicity studies of the chemical constituents from marine algae Chara baltica. Indian J. Pharm. Sci. 2019, 81, 815–823. [Google Scholar] [CrossRef]
- Mustapa, A.N.; Martin, Á.; Mato, R.B.; Cocero, M.J. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind. Crops Prod. 2015, 74, 83–94. [Google Scholar] [CrossRef]
- Ragunathan, V.; Pandurangan, J.; Ramakrishnan, T. Gas chromatography-mass spectrometry analysis of methanol extracts from marine red seaweed Gracilaria corticata. Pharmacogn. J. 2019, 11, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Cyriac, B.; Eswaran, K. GC-MS determination of bioactive components of Gracilaria dura (C. Agardh) J. Agardh. Sci. Res. Rep. 2015, 5, 100–105. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Carlsson, N.G.; Undeland, I. Quantification of total fatty acids in microalgae: Comparison of extraction and transesterification methods. Anal. Bioanal. Chem. 2014, 406, 7313–7322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, Y.; Jiao, D.; Lu, C.; Lou, Y.; Li, N.; Wang, G.; Wang, H. Synthesis and release of fatty acids under the interaction of Ulva pertusa and Heterosigma akashiwo by stable isotope analysis. Ecotoxicol. Environ. Saf. 2021, 210, 111852. [Google Scholar] [CrossRef] [PubMed]
- Salunke, M.; Wakure, B.; Wakte, P. HR-LCMS assisted phytochemical screening and an assessment of anticancer activity of Sargassum Squarrossum and Dictyota Dichotoma using in vitro and molecular docking approaches. J. Mol. Struct. 2022, 1270, 133833. [Google Scholar] [CrossRef]
- Salunke, M.A.; Wakure, B.S.; Wakte, P.S. High-resolution liquid chromatography and mass spectrometry (HR-LCMS) assisted phytochemical profiling and an assessment of anticancer activities of Gracilaria foliifera and Turbinaria conoides using in vitro and molecular docking analysis. J. Biomol. Struct. Dyn. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N.; Braun, C.; Kvaskoff, D. Extraction and analysis of mycosporine-like amino acids in marine algae. Nat. Prod. Mar. Algae Methods Protoc. 2015, 1308, 119–129. [Google Scholar]
- Javith, M.A.; Balange, A.K.; Xavier, M.; Hassan, M.A.; Sanath Kumar, H.; Nayak, B.B.; Krishna, G. Comparative studies on the chemical composition of inland saline reared Litopenaeus vannamei. J. Culin. Sci. Technol. 2022, 20, 336–349. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Montoya, N.G. A high-resolution reverse-phase liquid chromatography method for the analysis of mycosporine-like amino acids (MAAs) in marine organisms. Mar. Biol. 2005, 146, 237–252. [Google Scholar] [CrossRef]
- Falandysz, J.; Szymczyk, K.; Ichihashi, H.; Bielawski, L.; Gucia, M.; Frankowska, A.; Yamasaki, S.I. ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit. Contam. 2001, 18, 503–513. [Google Scholar] [CrossRef]
- Murugaiyan, K.; Narasimman, S. Elemental composition of Sargassum longifolium and Turbinaria conoides from Pamban Coast, Tamilnadu. Int. J. Res. Biol. Sci. 2012, 2, 137–140. [Google Scholar]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Wijaya, C.; Elya, B.; Yanuar, A. Study of tyrosinase inhibitory activity and phytochemical screening of Cassia Fistula L. Leaves. Int. J. Appl. Pharm. 2018, 10, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Maheswari, M.U.; Reena, A.; Sivaraj, C. GC-MS analysis, antioxidant and antibacterial activity of the brown algae, Padina tetrastromatica. Int. J. Pharm. Sci. Res. 2017, 8, 4014–4020. [Google Scholar]
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H. Antioxidant and anti-tyrosinase activities from Piper officinarum C. DC (Piperaceae). J. Appl. Pharm. Sci. 2014, 4, 87–91. [Google Scholar]







| Frequency (cm−1) | Intensity | Band Assignments | Functional Group |
|---|---|---|---|
| 604.94 | S | C-Cl stretch | Halo compound |
| S | C-Br stretch | Halo compound | |
| 659.08 | S | C-Br stretch | Halo compound |
| S | C-Cl stretch | Halo compound | |
| 751.88 | S | C-Cl stretch | Halo compound |
| S | C-H bend | 1,2-Disubstituted | |
| S | C-H bend | Monosubstituted | |
| 1038.02 | S | S=O stretch | Sulfoxide |
| S | C-F stretch | Fluoro compound | |
| M | C-N stretch | Amine | |
| 1099.89 | S | C-F starch | Fluoro compound |
| S | C-O starch | Aliphatic ether | |
| S | C-O starch | Secondary alcohol | |
| M | C-N stretch | Amine | |
| 1126.96 | S | C-F stretch | Fluoro compound |
| S | C-O stretch | Tertiary alcohol | |
| S | C-O stretch | Aliphatic ether | |
| M | C-N starch | Amine | |
| 1196.56 | S | C-F stretch | Fluoro compound |
| S | C-O stretch | Tertiary alcohol | |
| S | C-O stretch | Ester | |
| M | C-N stretch | Amine | |
| 1320.30 | S | C-F stretch | Fluoro compound |
| S | C-N stretch | Aromatic amine | |
| S | S=O stretch | Sulfone | |
| M | O-H bend | Phenol | |
| 1343.50 | S | C-F Stretch | Fluoro compound |
| S | S=O Stretch | Sulfonate | |
| S | S=O Stretch | Sulfonamide | |
| S | S=O Stretch | Sulfonic acid | |
| S | S=O Stretch | Sulfone | |
| M | O-H bend | Phenol | |
| 1521.37 | S | N-O stretch | Nitro compound |
| 1648.98 | M | C=N Stretch | Imine/Oxime |
| M | C=C stretch | Alkene (disubstituted) | |
| M | C=C stretch | Alkene | |
| M | C=C stretch | Conjugated alkene | |
| M | N-H bend | Amine | |
| M | C=C stretch | Cyclic alkene | |
| S | C=C Stretch | Alkene (monosubstituted) | |
| 1656.71 | M | C=C stretch | Alkene (disubstituted) |
| M | C=N Stretch | Imine/Oxime | |
| M | C=C stretch | Alkene (vinyldehyd) | |
| W | C-H bend | Aromatic compound | |
| 1873.25 | W | C-H bend | Aromatic compound |
| 2859.29 | SB | O-H stretch | Carboxylic acid |
| WB | O-H stretch | Alcohol | |
| SB | N-H Stretch | Amine salt | |
| M | C-H stretch | Alkane | |
| 2925.03 | SB | O-H stretch | Carboxylic acid |
| WB | O-H stretch | Alcohol | |
| SB | N-H Stretch | Amine salt | |
| M | C-H stretch | Alkane | |
| 2959.83 | SB | O-H stretch | Carboxylic acid |
| WB | O-H stretch | Alcohol | |
| SB | N-H Stretch | Amine salt | |
| M | C-H stretch | Alkane | |
| 3404.51 | SB | O-H stretch | Alcohol |
| 3686.79 | MS | O-H Stretch | Alcohol |
| No. | Name of Compound | PubChem ID | Mol. Formula | Mol. Weight (g/mol) | Retention Time (min) | Kovats Index (iu) | % Peak Area |
|---|---|---|---|---|---|---|---|
| 1. | Decane, 6-ethyl-2-methyl- | 43923 | C13H28 | 184.36 | 12.42 | 1185 | 4.32 |
| 2. | Cyclopentanol, 2-methyl-, trans- | 6432271 | C6H12O | 100.16 | 16.42 | 849 | 2.31 |
| 3. | Pentadecanoic acid, tripropylsilyl ester | 632007 | C24H50O2Si | 398.7 | 20.07 | 2484 | 1.68 |
| 4. | 3,7,11,15-Tetramethylhexadec-2-en-1-ol | 145386 | C20H40O | 296.5 | 20.77 | 2045 | 28.64 |
| 5. | Cyclodecanol | 15166 | C10H20O | 156.26 | 21.19 | 1387 | 2.77 |
| 6. | 9-(2-Oxiranyl)-1-nonanol | 565484 | C11H22O2 | 186.29 | 21.51 | 1448 | 5.33 |
| 7. | Phthalic acid,6-ethyl-3-octyl butyl ester | 6423866 | C22H34O4 | 362.5 | 23.23 | 2505 | 16.65 |
| 8. | Hexadecanoic acid, ethyl ester | 12366 | C18H36O2 | 284.5 | 23.77 | 1978 | 6.61 |
| 9. | 17-Octadecen-1-ol acetate | 249903005 | C20H38O2 | 310.5 | 25.95 | 2167 | 5.50 |
| 10. | Cyclooctaneacetic acid, 2-oxo- | 536995 | C10H16O3 | 184.23 | 26.79 | 1647 | 2.35 |
| 11. | Bispalmitic acid 3-methyl-1,2-butanediyl | 549812 | C37H72O4 | 581 | 28.01 | 3905 | 0.87 |
| 12. | 2-Monolinolenin, 2TMS derivative | 5362857 | C27H52O4Si2 | 496.9 | 31.55 | 2804 | 3.25 |
| 13. | Octadecane, 3-ethyl-5-(2-ethylbutyl)- | 292285 | C26H54 | 366.7 | 34.20 | 2413 | 6.97 |
| 14. | Benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate | 5368209 | C25H36O2 | 368.6 | 34.60 | 2774 | 8.54 |
| 15. | Oxalic acid, allyl nonyl ester | 6420231 | C14H24O4 | 256.34 | 8.20 | 1738 | 4.19 |
| No. | Name of Compound | PubChem ID | Mol. Formula | Mol. Weight (g/mol) | % Peak Area | Kovats Index (iu) | Retention Time (min) |
|---|---|---|---|---|---|---|---|
| 1 | 1,2,4-Trioxolane, 3,5-dipropyl- | 536099 | C8H16O3 | 160.21 | 1.05 | 1086 | 14.45 |
| 2 | Phytol | 5366244 | C20H40O | 296.5 | 6.01 | 2045 | 15.47 |
| 3 | 1,4-Eicosadiene | 5365774 | C20H38 | 278.5 | 1.63 | 2007 | 16.14 |
| 4 | Hexadecanoic acid, methyl ester | 8181 | C17H34O2 | 270.5 | 2.31 | 1878 | 16.82 |
| 5 | n-Hexadecanoic acid | 985 | C16H32O2 | 256.42 | 17.77 | 1968 | 17.49 |
| 6 | 9-Dodecenoic acid, methyl ester, [E]- | 5362755 | C13H24O2 | 212.33 | 1.44 | 1489 | 19.66 |
| 7 | Hexahydrofarnesol | 138824 | C15H32O | 228.41 | 2.28 | 1563 | 19.90 |
| 8 | Oleic acid | 445639 | C18H34O2 | 282.5 | 27.85 | - | 20.39 |
| 38.33 | - | 11.44 | |||||
| 9 | Palmitic acid vinyl ester | 69658 | C18H34O2 | 282.5 | 2.18 | 1968 | 22.11 |
| 10 | 1,1’-Bicyclopentyl, 2-hexadecyl- | 291314 | C26H50 | 362.7 | 1 | 2653 | 22.83 |
| 11 | 9,17-Octadecadienal, [Z]- | 5365667 | C18H32O | 264.4 | 5.92 | 1997 | 24.91 |
| 3.13 | 1997 | 29.90 | |||||
| 12 | 17-Octadecynoic acid | 1449 | C18H32O2 | 280.4 | 3.34 | 2165 | 25.64 |
| 13 | 29-Methylisofucosterol | 6443745 | C30H50O | 426.7 | 3.71 | 2880 | 27.76 |
| 14 | Patchouli alcohol | 10955174 | C15H26O | 222.37 | 3.14 | 1420 | 31.19 |
| 15 | 9-Octadecenal | 5283381 | C18H34O | 266.5 | 2.36 | 2007 | 34.79 |
| Phycocompound | Class of Compound | Formula | Retention Time (min) | Mass (Da) | Abundance | Hits (DB) |
|---|---|---|---|---|---|---|
| 8-Hydroxy-2chlorodibenzofuran | Organochlorine compound | C12H7ClO2 | 0.828 | 218.0135 | 828077 | 2 |
| Sulfabenzamide | Sulfur compounds (sulfonamide) | C13H12N2O3S | 0.829 | 276.0549 | - | 4 |
| 3-((2-Methyl-3-furyl)sulfanyl)-2-butanone | Aryl sulfide (organosulfur compound) | C9H12O2S | 0.897 | 184.0573 | 631461 | 7 |
| 8-Amino caprylic acid | Omega-amino fatty acid (carboxylic acids) | C8H17NO2 | 1.175 | 159.1249 | 1110887 | 6 |
| L-NIO | Amino acids, peptides, and proteins | C7H15N3O2 | 1.272 | 173.1157 | 449684 | 7 |
| Lys Gly | Dipeptide | C8H17N3O3 | 1.318 | 203.1262 | 189239 | 10 |
| Pirbuterol | Amino alcohols (ethanolamines) | C12H20N2O3 | 1.319 | 240.146 | - | 10 |
| N-Guanyl histamine | Amines | C6H11N5 | 1.329 | 153.1014 | 143807 | 6 |
| Pro Pro His | Pro-Pro-His is an oligopeptide. | C16H23N5O4 | 1.894 | 349.1722 | - | 10 |
| MeIQx | Quinoxalines (aromatic amine) | C11H11N5 | 2.375 | 213.1016 | 208953 | 10 |
| Salsolidine | Isoquinolines (organic heterocyclic compound) | C12H17NO2 | 2.937 | 207.1253 | - | 10 |
| Isopentenyladenine-9-glucoside | Organic heterocyclic compound (aminopurine) | C17H25N5O4 | 3.179 | 363.1882 | - | 10 |
| 2-Amino-1,7,9trimethylimidazo[4,5g]quinoxaline | Heterocyclic compounds, 2-ring (quinoxalines) | C12H13N5 | 3.408 | 227.1171 | 136941 | 7 |
| Niazirinin | Carbohydrates and carbohydrate derivatives | C16H19NO6 | 3.831 | 321.1201 | 116378 | 10 |
| Cryptopleurine | Alkaloids | C24H27NO3 | 3.88 | 377.2028 | 92057 | 10 |
| Beta-butoxyethyl nicotinate | An aromatic carboxylic acid and a member of pyridines | C12H17NO3 | 3.964 | 223.12 | 104913 | 7 |
| LG 100268 | Vitamin B complex (nicotinic acids) | C24H29NO2 | 4.033 | 363.2243 | 177426 | 7 |
| 2-(3’-Methylthio)propyl malic acid | Alcohol | C8H14O5S | 4.169 | 222.0578 | 92654 | 10 |
| Gln Phe Lys | Peptide (organic amino compound) | C20H31N5O5 | 4.387 | 421.2295 | 146961 | 10 |
| N-linoleoyl taurine | Fatty amide (fatty acid derivative) | C20H37NO4S | 4.6 | 387.2453 | 149019 | 10 |
| Tyr Ile Pro | Peptide | C20H29N3O5 | 4.632 | 391.2192 | 268855 | 10 |
| S-Decyl GSH | Peptide | C20H37N3O6S | 5.096 | 447.245 | 79144 | 5 |
| ORG 20599 | Steroids (pregnanediones) | C25H40ClNO3 | 5.171 | 437.2696 | 6034 | 10 |
| Nafronyl | Naphthalenes (benzenoid aromatic compound) | C24H33NO3 | 5.27 | 383.2521 | 82233 | 7 |
| Lys Met Lys | Oligopeptide | C17H35N5O4S | 5.533 | 405.2348 | 156109 | 7 |
| Benzenemethanol, 2-(2hydroxypropoxy)-3-methyl- | Aromatic ether | C11H16O3 | 5.68 | 196.109 | 719286 | 10 |
| Acetyl lycopsamine | Pyrrolizines (organic heterocyclic compound) | C17H27NO6 | 5.791 | 341.1862 | 215252 | 10 |
| Benzenemethanol, 2-(2hydroxypropoxy)-3-methyl- | Aromatic ether | C11H16O3 | 6.022 | 196.1092 | 130269 | 10 |
| 2,6-Dimethoxy-4-(1propenyl)phenol | Phenols | C11H14O3 | 6.247 | 194.0938 | 149475 | 10 |
| Azuleno(5,6-c)furan-1(3H)-one, 4,4a,5,6,7,7a,8,9-octahydro-3,4,8-trihydroxy-6,6,8-trimethyl- | Terpenoids (sesquiterpenoids) | C15H22O5 | 6.251 | 282.1453 | 113097 | 10 |
| Hericerin | Amides (lactams) | C27H33NO3 | 6.316 | 419.2504 | 325331 | 5 |
| Di-n-pentyl phthalate | Phthalate ester (phthalic acids) | C18H26O4 | 6.811 | 306.1819 | 155711 | 7 |
| N-Formyl-norleucyl-leucylphenylalanyl-methylester | Peptide | C23H35N3O5 | 7.036 | 433.2662 | 476213 | 2 |
| 2,4,6-Trimethyl-4-phenyl-1,3 dioxane | Dioxanes | C13H18O2 | 7.158 | 206.13 | 240568 | 10 |
| Sr.no | Amino Acids | H2 (nmol/mL) |
|---|---|---|
| 1 | Aspartic acid (Asp) | 2034.99 |
| 2 | Glutamic acid (Glu) | 1332.61 |
| 3 | Asparagine (Asn) | ND |
| 4 | Serine (Ser) | 768.22 |
| 5 | Glutamine (Gln) | ND |
| 6 | Histidine (His) | ND |
| 7 | Glycine (Gly) | 1215.22 |
| 8 | Threonine (Thr) | 527.17 |
| 9 | Arginine (Arg) | 498.77 |
| 10 | Alanine (Ala) | 1268.27 |
| 11 | Tyrosine (Tyr) | 233.26 |
| 12 | Cystine (Cys) | ND |
| 13 | Valine (Val) | 389.20 |
| 14 | Methionine (Met) | 293.46 |
| 15 | Norvaline (Nva) | ND |
| 16 | Tryptophan (Trp) | ND |
| 17 | Phenylalanine (Phe) | 392.66 |
| 18 | Isoleucine (Ile) | 244.84 |
| 19 | Leucine (Leu) | 808.49 |
| 20 | Lysine (Lys) | 354.69 |
| 21 | Hydroxyproline (Hyp) | 806.49 |
| Element | (Amount in %) |
|---|---|
| B | 0.013712 |
| Ca | 4.099749 |
| Cu | 0.000386 |
| Fe | 0.244448 |
| K | 2.709106 |
| Mg | 2.055089 |
| Zn | 0.004538 |
| Na | 0.073146 |
| Si | 20.17574 |
| Se | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalasariya, H.S.; Pereira, L.; Patel, N.B. Comprehensive Phytochemical Analysis and Bioactivity Evaluation of Padina boergesenii: Unveiling Its Prospects as a Promising Cosmetic Component. Mar. Drugs 2023, 21, 385. https://doi.org/10.3390/md21070385
Kalasariya HS, Pereira L, Patel NB. Comprehensive Phytochemical Analysis and Bioactivity Evaluation of Padina boergesenii: Unveiling Its Prospects as a Promising Cosmetic Component. Marine Drugs. 2023; 21(7):385. https://doi.org/10.3390/md21070385
Chicago/Turabian StyleKalasariya, Haresh S., Leonel Pereira, and Nikunj B. Patel. 2023. "Comprehensive Phytochemical Analysis and Bioactivity Evaluation of Padina boergesenii: Unveiling Its Prospects as a Promising Cosmetic Component" Marine Drugs 21, no. 7: 385. https://doi.org/10.3390/md21070385
APA StyleKalasariya, H. S., Pereira, L., & Patel, N. B. (2023). Comprehensive Phytochemical Analysis and Bioactivity Evaluation of Padina boergesenii: Unveiling Its Prospects as a Promising Cosmetic Component. Marine Drugs, 21(7), 385. https://doi.org/10.3390/md21070385