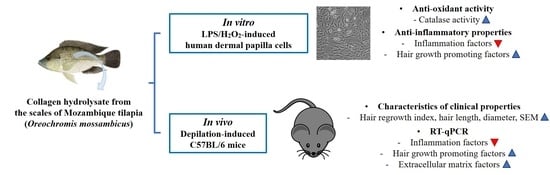
Collagen Hydrolysate from the Scales of Mozambique Tilapia (Oreochromis mossambicus) Improve Hair and Skin Health by Alleviating Oxidative Stress and Inflammation and Promoting Hair Growth and Extracellular Matrix Factors
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Effects of CH on H2O2-Induced hDPCs
2.2. Effects of CH on Cytokine mRNA Expression of LPS-Induced hDPCs
2.3. Visual Observation of Hair Growth after CH Oral Administration
2.4. Effects of CH on Hair Regrowth in Mice
2.5. Effects of CH on the Improvement of Clinical Characteristics of Hair
2.6. Effect of CH on Hair Shine Enhancement Measured by SEM
2.7. Effects of CH on Cytokine mRNA Expression of Mouse Dorsal Skin
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Materials
4.2. Experimental Animals
4.3. Hair Regrowth Score and Relative Area for Each Score
4.4. Hair Diameter, Length, and Weight [85]
4.5. Hair Density per Area [86]
4.6. Scanning Electron Microscope (SEM) [87]
4.7. Quantitative Reverse Transcriptase PCR for Cytokine Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Saewan, N. Effect of coffee berry extract on anti-aging for skin and hair-in vitro approach. Cosmetics 2022, 9, 66. [Google Scholar] [CrossRef]
- Yenin, J.; Serarslan, G.; Yönden, Z.; Ulutaş, K. Investigation of oxidative stress in patients with alopecia areata and its relationship with disease severity, duration, recurrence and pattern. Clin. Exp. Dermatol. 2015, 40, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, L.-H.; Guo, L.-L.; Wang, G.-Q.; Zhou, X.-P.; Jiang, Y.; Shang, J.; Murao, K.; Chen, J.-W.; Fu, W.-Q. Chronic restraint stress inhibits hair growth via substance P mediated by reactive oxygen species in mice. PLoS ONE 2013, 8, e61574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Hsieh, J.-H.; Wang, I.-T.; Jheng, P.-R.; Yeh, Y.-Y.; Lee, J.-W.; Bolouki, N.; Chuang, E.-Y. Transferred cold atmospheric plasma treatment on melanoma skin cancer cells with/without catalase enzyme in vitro. Appl. Sci. 2021, 11, 6181. [Google Scholar] [CrossRef]
- Abdel Fattah, N.; Ebrahim, A.; El Okda, E. Lipid peroxidation/antioxidant activity in patients with alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 403–408. [Google Scholar] [CrossRef]
- Patro, G.; Bhattamisra, S.K.; Mohanty, B.K.; Sahoo, H.B. In vitro and in vivo antioxidant evaluation and estimation of total phenolic, flavonoidal content of Mimosa pudica L. Pharmacogn. Res. 2016, 8, 22. [Google Scholar] [CrossRef]
- Turkoglu, M.; Pekmezci, E.; Kilic, S.; Dundar, C.; Sevinc, H. Effect of Ficus carica leaf extract on the gene expression of selected factors in HaCaT cells. J. Cosmet. Dermatol. 2017, 16, e54–e58. [Google Scholar] [CrossRef]
- Kasumagić-Halilovic, E.; Ovcina-Kurtovic, N. Serum levels of interleukin-1 (IL-1α, IL-1β) in patients with alopecia areata. Age 2012, 36, 32–36. [Google Scholar] [CrossRef]
- Sadick, N.S.; Callender, V.D.; Kircik, L.H.; Kogan, S. New insight into the pathophysiology of hair loss trigger a paradigm shift in the treatment approach. J. Drugs Dermatol. 2017, 16, s135–s140. [Google Scholar]
- Dai, B.; Sha, R.N.; Yuan, J.L.; Liu, D.J. Multiple potential roles of thymosin β4 in the growth and development of hair follicles. J. Cell Mol. Med. 2021, 25, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, M.; Martins, A.M.; Sousa-Oliveira, I.; Ribeiro, H.M.; Veiga, F.; Marto, J.; Paiva-Santos, A.C. Nanomaterials in hair care and treatment. Acta Biomater. 2022, 142, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Powar, M.A.D.; Nitave, S.A. A review: Polyherbal antidandruff hair oil. World J. Pharm. Res. 2020, 10, 440–457. [Google Scholar]
- Lin, H.-Y.; Yang, L.-T. Differential response of epithelial stem cell populations in hair follicles to TGF-β signaling. Dev. Biol. 2013, 373, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, H. Fundamental hair follicle biology and fine fibre production in animals. Animal 2010, 4, 1490–1509. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Kishimoto, J. Molecular control of epithelial–mesenchymal interactions during hair follicle cycling. J. Investig. Dermatol. Symp. Proc. 2003, 8, 46–55. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, Z.; Gu, L.; Wang, Y.; Sung, C. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-β1 in C57BL/6 mice in vivo. Growth Horm. IGF Res. 2014, 24, 89–94. [Google Scholar] [CrossRef]
- Wu, X.-J.; Jing, J.; Lu, Z.-F.; Zheng, M. VEGFR-2 is in a state of activation in hair follicles, sebaceous glands, eccrine sweat glands, and epidermis from human scalp: An in situ immunohistochemistry study of phosphorylated VEGFR-2. Med. Sci. Monit. Basic Res. 2019, 25, 107. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dickkopf 1 promotes regression of hair follicles. J. Investig. Dermatol. 2012, 132, 1554–1560. [Google Scholar] [CrossRef]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Inui, S.; Itami, S. Androgen actions on the human hair follicle: Perspectives. Exp. Dermatol. 2013, 22, 168–171. [Google Scholar] [CrossRef]
- Inui, S.; Fukuzato, Y.; Nakajima, T.; Yoshikawa, K.; Itami, S. Identification of androgen-inducible TGF-β1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J. Investig. Dermatol. Symp. Proc. 2003, 8, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-Q.; Wu, Z.-B.; Chu, X.-Y.; Bi, Z.-G.; Fan, W.-X. An investigation of crosstalk between Wnt/β-catenin and transforming growth factor-β signaling in androgenetic alopecia. Medicine 2016, 95, e4297. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Pawlus, A.D.; Thornton, M.J. Getting under the skin of hair aging: The impact of the hair follicle environment. Exp. Dermatol. 2020, 29, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Mancini, E.; Xu, L.; Moore, A.; Jahanbani, F.; Hebestreit, K.; Srinivasan, R.; Li, X.; Devarajan, K.; Prélot, L. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 2019, 574, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Desprez, P.Y.; Campisi, J.; Velarde, M.C. Cell autonomous and non-autonomous effects of senescent cells in the skin. J. Investig. Dermatol. 2015, 135, 1722–1726. [Google Scholar] [CrossRef]
- Shin, W.; Rosin, N.L.; Sparks, H.; Sinha, S.; Rahmani, W.; Sharma, N.; Workentine, M.; Abbasi, S.; Labit, E.; Stratton, J.A. Dysfunction of hair follicle mesenchymal progenitors contributes to age-associated hair loss. Dev. Cell 2020, 53, 185–198.e187. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef]
- Rompolas, P.; Deschene, E.R.; Zito, G.; Gonzalez, D.G.; Saotome, I.; Haberman, A.M.; Greco, V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 2012, 487, 496–499. [Google Scholar] [CrossRef]
- Le Vu, P.; Takatori, R.; Iwamoto, T.; Akagi, Y.; Satsu, H.; Totsuka, M.; Chida, K.; Sato, K.; Shimizu, M. Effects of food-derived collagen peptides on the expression of keratin and keratin-associated protein genes in the mouse skin. Skin Pharmacol. Physiol. 2015, 28, 227–235. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels-a randomized, double-blind, placebo-controlled trial. Mar. Drugs 2018, 16, 482. [Google Scholar] [CrossRef] [PubMed]
- Chiriacò, G.; Cauci, S.; Mazzon, G.; Trombetta, C. An observational retrospective evaluation of 79 young men with long-term adverse effects after use of finasteride against androgenetic alopecia. Andrology 2016, 4, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Randolph, M.; Tosti, A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Dermatol. 2021, 84, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2014, 27, 47–55. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Jiménez-Alvarado, R.; Aguirre-Álvarez, G. Collagen hydrolysates for skin protection: Oral administration and topical formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef]
- Oesser, S. The oral intake of specific bioactive collagen peptides has a positive effect on hair thickness. Int. J. Nutraceuticals Funct. Foods Novel Foods 2020, 2, 134–138. [Google Scholar]
- Yang, T.; Zhang, K.; Li, B.; Hou, H. Effects of oral administration of peptides with low molecular weight from Alaska Pollock (Theragra chalcogramma) on cutaneous wound healing. J. Funct. Foods 2018, 48, 682–691. [Google Scholar] [CrossRef]
- Tanaka, M.; Koyama, Y.-I.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kwon, C.-J.; Inoue, N.; Koizumi, S.; Hwang, J.S. The effect of collagen peptide intake on UVB-induced skin damage in hairless mice. J. Korea Acad.-Ind. Coop. Soc. 2016, 17, 611–621. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.a.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Rahman, A.; Silva, T.H. Collagens from marine organisms towards biomedical applications. Mar. Drugs 2022, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Matsumoto, H.; Ito, K.; Iwai, K.; Sato, K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J. Agric. Food Chem. 2007, 55, 1532–1535. [Google Scholar] [CrossRef]
- Tak, Y.J.; Shin, D.K.; Kim, A.H.; Kim, J.I.; Lee, Y.L.; Ko, H.-C.; Kim, Y.-W.; Lee, S.Y. Effect of collagen tripeptide and adjusting for climate change on skin hydration in middle-aged women: A randomized, double-blind, placebo-controlled trial. Front. Med. 2021, 7, 608903. [Google Scholar] [CrossRef] [PubMed]
- Sethuramalingam, S.; Ravi, R.L.; Rajiah, J.R. Anti-oxidant, anti-inflammatory and anti-atherosclerotic activity of bioactive peptide HPAEDR isolated from Catla catal muscle on LPS induced inflammation on 246.7 raw macrophage cells and HCF induced hyperlipidemic Zebrafish Larvae. Biol. Med. Nat. Prod. Chem. 2022, 11, 151–160. [Google Scholar] [CrossRef]
- Sivaraman, K.; Shanthi, C. Purified fish skin collagen hydrolysate attenuates TNF-α induced barrier dysfunction in-vitro and DSS induced colitis in-vivo model. Int. J. Biol. Macromol. 2022, 222, 448–461. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Sivaraman, K.; Shanthi, C. Role of fish collagen hydrolysate in attenuating inflammation—An in vitro study. J. Food Biochem. 2021, 45, e13876. [Google Scholar] [CrossRef]
- Qian, B.; Zhao, X.; Yang, Y.; Tian, C. Antioxidant and ant-inflammatory peptide fraction from oyster soft tissue by enzymatic hydrolysis. Food Sci. Nutr. 2020, 8, 3947–3956. [Google Scholar] [CrossRef]
- Taheri, A.; Farvin, K.S.; Jacobsen, C.; Baron, C.P. Antioxidant activities and functional properties of protein and peptide fractions isolated from salted herring brine. Food Chem. 2014, 142, 318–326. [Google Scholar] [CrossRef]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem. 2010, 58, 835–841. [Google Scholar] [CrossRef]
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M.-a.; Ohno, S.; Yamaguchi, K. Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin. J. Agric. Food Chem. 2017, 65, 2315–2322. [Google Scholar] [CrossRef]
- Yamamoto, S.; Deguchi, K.; Onuma, M.; Numata, N.; Sakai, Y. Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans. Biol. Pharm. Bull. 2016, 39, 428–434. [Google Scholar] [CrossRef]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: A single-blind case-control clinical study. Oxidative Med. Cell Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef]
- Sontakke, S.B.; Jung, J.-h.; Piao, Z.; Chung, H.J. Orally available collagen tripeptide: Enzymatic stability, intestinal permeability, and absorption of Gly-Pro-Hyp and Pro-Hyp. J. Agric. Food Chem. 2016, 64, 7127–7133. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-U.; Chung, H.-C.; Choi, J.; Sakai, Y.; Lee, B.-Y. Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: A randomized, double-blind, placebo-controlled study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Park, J.; Lee, M.; Park, S.-H.; Jung, J.; Kim, J.; Eun, S.; Kim, J. Gly-Pro-Val-Gly-Pro-Ser peptide fish collagen improves skin moisture and wrinkles with ameliorated the oxidative stress and pro-inflammatory factors in skin photoaging mimic models. Prev. Nutr. Food Sci. 2023, 28, 50. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, Y.; Iwai, K.; Morimatsu, F.; Iwamoto, T.; Mori, T.; Oda, C.; Taira, T.; Park, E.Y.; Nakamura, Y.; Sato, K. Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J. Agric. Food Chem. 2009, 57, 444–449. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine collagen peptides from the skin of Nile Tilapia (Oreochromis niloticus): Characterization and wound healing evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.B.; Park, H.J.; Lee, B.-H. Hair-growth-promoting effects of the fish collagen peptide in human dermal papilla cells and C57BL/6 mice modulating Wnt/β-Catenin and BMP signaling pathways. Int. J. Mol. Sci. 2022, 23, 11904. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-I.; Choi, J.-Y.; Lee, J.-B.; Yun, S.-J.; Moon, B.-K.; Ahn, Y.-G.; Lee, S.-Y.; Lee, S.-C. Protective activity against oxidative stress in dermal papillae with extracted herbal essential oils. Appl. Sci. 2023, 13, 3985. [Google Scholar] [CrossRef]
- Yun, M.-S.; Kim, C.; Hwang, J.-K. Agastache rugosa Kuntze attenuates UVB-induced photoaging in hairless mice through the regulation of MAPK/AP-1 and TGF-β/Smad pathways. J. Microbiol. Biotechnol. 2019, 29, 1349–1360. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, M.; Yun, J.-M.; Kim, D.; Lee, J.; Lee, Y.-H. Zingiber mioga extract improves moisturization and depigmentation of skin and reduces wrinkle formation in uvb-irradiated hrm-2 hairless mice. Appl. Sci. 2021, 11, 976. [Google Scholar] [CrossRef]
- Thibaut, S.; De Becker, E.; Bernard, B.; Huart, M.; Fiat, F.; Baghdadli, N.; Luengo, G.; Leroy, F.; Angevin, P.; Kermoal, A. Chronological ageing of human hair keratin fibres. Int. J. Cosmet. Sci. 2010, 32, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Nanotechnology-based cosmetics for hair care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Lim, Y.S.; Harland, D.P.; Dawson, T.L., Jr. Wanted, dead and alive: Why a multidisciplinary approach is needed to unlock hair treatment potential. Exp. Dermatol. 2019, 28, 517–527. [Google Scholar] [CrossRef]
- Jeganathan, S. Formulation of Hair Straightening Cream from Keratin Protein; UMP: Gambang, Malaysia, 2015. [Google Scholar]
- Phillippi, D.T.; Daniel, S.; Nguyen, K.N.; Penaredondo, B.A.; Lund, A.K. Probiotics function as immunomodulators in the Intestine in C57Bl/6 male mice exposed to inhaled diesel exhaust particles on a high-fat diet. Cells 2022, 11, 1445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, J.; Chen, Q.; Yang, N.; Bao, Z.; Hu, S.; Chen, Y.; Wu, X. A Treatment combination of IGF and EGF promotes hair growth in the Angora Rabbit. Genes 2021, 12, 24. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Q.; Bai, Y.; Yang, K.; Ye, Y.; Wu, K.; Huang, J.; Zhang, Y.; Zhang, X.; Thianthanyakij, T. Autologous activated platelet-rich plasma in hair growth: A pilot study in male androgenetic alopecia with in vitro bioactivity investigation. J. Cosmet. Dermatol. 2021, 20, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Colombo, F.; GFM-O-Trial Investigators Group; Baraldo, C.; Barbareschi, M.; Chieco, P.; Colonna, L.; Desmond, M.V.; Fiorucci, M.C. Efficacy and tolerability of an oral supplement containing amino acids, iron, selenium, and marine hydrolyzed collagen in subjects with hair loss (androgenetic alopecia, AGA or FAGA or telogen effluvium). A prospective, randomized, 3-month, controlled, assessor-blinded study. Skin Res. Technol. 2023, 29, e13381. [Google Scholar]
- Ablon, G. A 6-month, randomized, double-blind, placebo-controlled study evaluating the ability of a marine complex supplement to promote hair growth in men with thinning hair. J. Cosmet. Dermatol. 2016, 15, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; McMahon, H. Effect of marine-derived saccharides on human skin fibroblasts and dermal papilla cells. Mar. Drugs 2023, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cescon, M.; Bonaldo, P. Lack of collagen VI promotes wound-induced hair growth. J. Investig. Dermatol. 2015, 135, 2358–2367. [Google Scholar] [CrossRef]
- Wei, G.; Martirosyan, D. Hair loss: A review of the role of food bioactive compounds. Bioact. Compd. Health Dis. 2019, 2, 94–125. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhu, Z.; Zheng, F.; Gao, R. Food-derived collagen peptides: Safety, metabolism, and anti-skin-aging effects. Curr. Opin. Food Sci. 2023, 51, 101012. [Google Scholar] [CrossRef]
- Ohara, H.; Ichikawa, S.; Matsumoto, H.; Akiyama, M.; Fujimoto, N.; Kobayashi, T.; Tajima, S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010, 37, 330–338. [Google Scholar] [CrossRef]
- Lai, C.-S.; Tu, C.-W.; Kuo, H.-C.; Sun, P.-P.; Tsai, M.-L. Type II collagen from cartilage of Acipenser baerii promotes wound healing in human dermal fibroblasts and in mouse skin. Mar. Drugs 2020, 18, 511. [Google Scholar] [CrossRef]
- Chung, M.S.; Bae, W.J.; Choi, S.W.; Lee, K.W.; Jeong, H.C.; Bashraheel, F.; Jeon, S.H.; Jung, J.W.; Yoon, B.I.; Kwon, E.B. An Asian traditional herbal complex containing Houttuynia cordata Thunb, Perilla frutescens Var. acuta and green tea stimulates hair growth in mice. BMC Complement. Altern. Med. 2017, 17, 515. [Google Scholar] [CrossRef]
- Pulat, S.; Subedi, L.; Pandey, P.; Bhosle, S.R.; Hur, J.-S.; Shim, J.-H.; Cho, S.-S.; Kim, K.-T.; Ha, H.-H.; Kim, H. Topical delivery of atraric acid derived from Stereocaulon japonicum with enhanced skin permeation and hair regrowth activity for androgenic alopecia. Pharmaceutics 2023, 15, 340. [Google Scholar] [CrossRef]
- Mylan Parmaceuticals ULC. Pr Mylan-Finasteride hg. Available online: https://pdf.hres.ca/dpdpm/00025985.PDF (accessed on 21 August 2023).
- Plikus, M.V. New activators and inhibitors in the hair cycle clock: Targeting stem cells’ state of competence. J. Investig. Dermatol. 2012, 132, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Ha, W.H.; Park, D.H. Effect of seaweed extract on hair growth promotion in experimental study of C57BL/6 mice. Arch. Craniofac. Surg. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Hong, J.; Song, M.-Y.; Choi, I.-H.; Sohn, N.-W.; Chung, S.-H. Effect of Yikgeebohyul-tang on hair regrowth and cytokine changes on hair-removed C57BL/6 mice. J. Korean Med. 2010, 31, 138–152. [Google Scholar]
- Song, P.H.; Park, G.-R.; Kim, Y.-H.; Jung, D.H.; Ku, S.-K.; Song, C.-H. Hair-growth-promoting effects of fermented red ginseng marc and traditional polyherb formula in C57BL/6 mice. Appl. Sci. 2021, 11, 1195. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, J.M.; Park, S.Y.; Yang, J.E.; Kim, J.H.; Yi, T.H. Hair growth activity of Crataegus pinnatifida on C57BL/6 mouse model. Phytother. Res. 2013, 27, 1352–1357. [Google Scholar] [CrossRef]
- Lee, S.; Choi, A.; Baek, J.; Kim, H.; Shin, M.; Koh, J. Twelve-point scale grading system of scanning electron microscopic examination to investigate subtle changes in damaged hair surface. Skin Res. Technol. 2016, 22, 406–411. [Google Scholar] [CrossRef] [PubMed]











| Hair 1 Length (mm) | Hair Diameter (mm) | Hair Weight (g) | Hair Density (Score) | |
|---|---|---|---|---|
| NC 2 | 15.95 ± 0.32 b 3 | 0.36 ± 0.01 b | 0.019 ± 0.001 d | 48 ± 4.9 b |
| PC | 18.93 ± 0.44 a | 0.42 ± 0.01 a | 0.023 ± 0.001 c | 59.8 ± 3.8 a |
| CH500 | 19.08 ± 0.41 a | 0.41 ± 0.01 a | 0.029 ± 0.001 b | 61.5 ± 3 a |
| CH1000 | 19.95 ± 0.49 a | 0.43 ± 0.01 a | 0.035 ± 0.000 a | 68.8 ± 1.7 a |
| Grade | Skin Color | Score |
|---|---|---|
| Grade 1 | Pink (no hair) | 0 |
| Grade 2 | Gray (initial hair growth) | 1 |
| Grade 3 | Dark gray (visible hair growth) | 2 |
| Grade 4 | Black (full-grown hair) | 3 |
| Grade (Point) | Criterion |
|---|---|
| Grade 1 (1) | Intact hair (undamaged hair) |
| Grade 2 (2) | Irregular overlay, visible and lifted |
| Grade 3 (3) | Severely lifted and peeled |
| Grade 4 (4) | Cortex partially exposed |
| Grade 5 (5) | Cortex exposed without cuticle layer |
| Target Genes | Primer | Sequence |
|---|---|---|
| IGF-1 | Forward | 5′-TGCTCTTCAGTTCGTGTG-3′ |
| Reverse | 5′-ACATCTCCAGTCTCCTCAG-3′ | |
| VEGF | Forward | 5′-TCTTCAAGCCATCCTGTGTG-3′ |
| Reverse | 5′-GCGAGTCTGTGTTTTTGCAG-3′ | |
| TGF-β1 | Forward | 5′-GGCGGTGCTCGCTTTGTAC-3′ |
| Reverse | 5′-TCCCGAATGTCTGACGTATTGA-3′ | |
| TNF-α | Forward | 5′-GCTGCACTTTGGAGTGATCG-3′ |
| Reverse | 5′-TCACTCGGGGTTCGAGAAGA-3′ | |
| IL-1β | Forward | 5′-CTTCTGGGAAACTCACGGCA-3′ |
| Reverse | 5′-GTGAGACTCCAGACCTACGC-3′ | |
| Elastin | Forward | 5′-TGGTGACATGATCCCTCTCTCTT-3′ |
| Reverse | 5′-CCAGGGTGTCCCAGATGTG-3′ | |
| HAS2 | Forward | 5′-TGGCTGTGTCCAGTGCATAAG-3′ |
| Reverse | 5′-CACAAATTCATGCAGCAAGGA-3′ | |
| GAPDH | Forward | 5′-GGGAAGCCCATCACCATCT-3′ |
| Reverse | 5′-CGGCCTCACCCCATTTG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.B.; Park, H.J.; Lee, B.-H. Collagen Hydrolysate from the Scales of Mozambique Tilapia (Oreochromis mossambicus) Improve Hair and Skin Health by Alleviating Oxidative Stress and Inflammation and Promoting Hair Growth and Extracellular Matrix Factors. Mar. Drugs 2023, 21, 475. https://doi.org/10.3390/md21090475
Hwang SB, Park HJ, Lee B-H. Collagen Hydrolysate from the Scales of Mozambique Tilapia (Oreochromis mossambicus) Improve Hair and Skin Health by Alleviating Oxidative Stress and Inflammation and Promoting Hair Growth and Extracellular Matrix Factors. Marine Drugs. 2023; 21(9):475. https://doi.org/10.3390/md21090475
Chicago/Turabian StyleHwang, Su Bin, Hyeon Ju Park, and Bog-Hieu Lee. 2023. "Collagen Hydrolysate from the Scales of Mozambique Tilapia (Oreochromis mossambicus) Improve Hair and Skin Health by Alleviating Oxidative Stress and Inflammation and Promoting Hair Growth and Extracellular Matrix Factors" Marine Drugs 21, no. 9: 475. https://doi.org/10.3390/md21090475
APA StyleHwang, S. B., Park, H. J., & Lee, B. -H. (2023). Collagen Hydrolysate from the Scales of Mozambique Tilapia (Oreochromis mossambicus) Improve Hair and Skin Health by Alleviating Oxidative Stress and Inflammation and Promoting Hair Growth and Extracellular Matrix Factors. Marine Drugs, 21(9), 475. https://doi.org/10.3390/md21090475