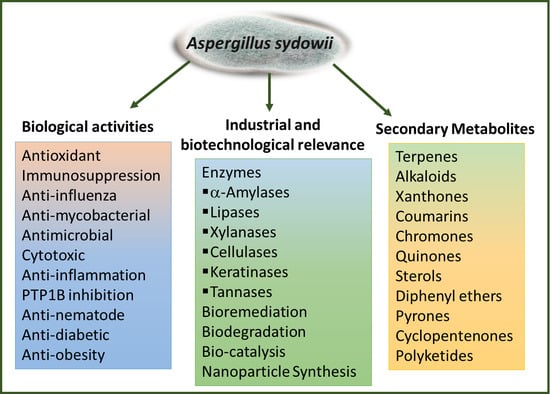
Secondary Metabolites, Biological Activities, and Industrial and Biotechnological Importance of Aspergillus sydowii
Abstract
:1. Introduction
2. Secondary Metabolites of Aspergillus sydowii
2.1. Sesquiterpenes
2.2. Mono- and Triterpenoids and Sterols
2.3. Xanthone and Anthraquinone Derivatives
2.4. Alkaloids
2.5. Phenyl Ether Derivatives
2.6. Chromane and Coumarin Derivatives
2.7. Pyrane, Cyclopentene, Cyclopropane, and Lactone Derivatives
2.8. Other Metabolites
3. Biological Activities of A. sydowii Extracts and Its Metabolites
3.1. Cytotoxic Activity
3.2. Antioxidant and Immunosuppression Activities
3.3. Anti-Mycobacterial, Anti-Microalgal, and Antimicrobial Activities
3.4. Anti-Influenza Virus Activity
3.5. Anti-Diabetic and Anti-Obesity Activities
3.6. Protein Tyrosine Phosphatase Inhibition
3.7. Anti-Inflammation Activity
3.8. Anti-Nematode Activity
4. Industrial and Biotechnological Applications
4.1. α-Amylase, Tannases, and Lipase Enzymes
4.2. Bioremediation and Biodegradation
4.2.1. Polycyclic Aromatic Hydrocarbons
4.2.2. Heavy Metals and Insecticides
4.2.3. Lignocellulosic Biomasses
4.2.4. Keratinous Wastes
4.3. Biocatalysis
5. Nanoparticle Synthesis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, S.R.; Mohamed, S.G.; Altyar, A.E.; Mohamed, G.A. Natural Products of the Fungal Genus Humicola: Diversity, Biological Activity, and Industrial Importance. Curr. Microbiol. 2021, 78, 2488–2509. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Altyar, A.E.; Mohamed, S.G.; Mohamed, G.A. Genus Thielavia: Phytochemicals, Industrial Importance and Biological Relevance. Nat. Prod. Res. 2022, 36, 5108–5123. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically Active Fungal Depsidones: Chemistry, Biosynthesis, Structural Characterization, and Bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A.; Khedr, A.I. γ-Butyrolactones from Aspergillus Species: Structures, Biosynthesis, and Biological Activities. Nat. Prod. Commun. 2017, 12, 791–800. [Google Scholar] [CrossRef]
- Lange, L. The Importance of Fungi and Mycology for Addressing Major Global Challenges. IMA Fungus 2014, 5, 463–471. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Abdallah, H.M.; Mohamed, G.A.; Deshmukh, S.K. Exploring Potential of Aspergillus Sclerotiorum: Secondary Metabolites and Biotechnological Relevance. Mycol. Prog. 2023, 22, 8. [Google Scholar]
- Ibrahim, S.R.M.; Fadil, S.A.; Fadil, H.A.; Eshmawi, B.A.; Mohamed, S.G.A.; Mohamed, G.A. Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential. Toxins 2022, 14, 154. [Google Scholar] [CrossRef]
- Ghazawi, K.F.; Fatani, S.A.; Mohamed, S.G.; Mohamed, G.A.; Ibrahim, S.R. Aspergillus nidulans—Natural Metabolites Powerhouse: Structures, Biosynthesis, Bioactivities, and Biotechnological Potential. Fermentation 2023, 9, 325. [Google Scholar] [CrossRef]
- Wang, Y.; Mou, Y.; Dong, Y.; Wu, Y.; Liu, B.; Bai, J.; Yan, D.; Zhang, L.; Feng, D.; Pei, Y. Diphenyl Ethers from a Marine-Derived Aspergillus sydowii. Mar. Drugs 2018, 16, 451. [Google Scholar]
- Hamasaki, T.; Nakajima, H.; Yokota, T.; Kimura, Y. A New Metabolite, 3-Carboxy-2, 4-Diphenyl-but-2-Enoic Anhydride, Produced by Aspergillus nidulans. Agric. Biol. Chem. 1983, 47, 891–892. [Google Scholar] [CrossRef]
- Hamasaki, T.; Nagayama, K.; Hatsuda, Y. Two New Metabolites, Sydonic Acid and Hydroxysydonic Acid, from Aspergillus Sydowi. Agric. Biol. Chem. 1978, 42, 37–40. [Google Scholar]
- Ishida, M.; Hamasaki, T.; Hatsuda, Y. The Structure of Two New Metabolites, Emerin and Emericellin, from Aspergillus nidulans. Agric. Biol. Chem. 1975, 39, 2181–2184. [Google Scholar] [CrossRef]
- Jiménez-Gómez, I.; Valdés-Muñoz, G.; Moreno-Ulloa, A.; Pérez-Llano, Y.; Moreno-Perlín, T.; Silva-Jiménez, H.; Barreto-Curiel, F.; Sanchez-Carbente, M.d.R.; Folch-Mallol, J.L.; Gunde-Cimerman, N. Surviving in the Brine: A Multi-Omics Approach for Understanding the Physiology of the Halophile Fungus Aspergillus sydowii at Saturated NaCl Concentration. Front. Microbiol. 2022, 13, 1520. [Google Scholar]
- Alker, A.P.; Smith, G.W.; Kim, K. Characterization of Aspergillus sydowii (Thom Et Church), a Fungal Pathogen of Caribbean Sea Fan Corals. Hydrobiologia 2001, 460, 105–111. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, S.; Yin, L.; Yu, Y.; Chen, Z.; Shen, H.; Zhou, L. A New Sydonic Acid Derivative from a Marine Derived-Fungus Aspergillus sydowii. Chem. Nat. Compd. 2017, 53, 1056–1058. [Google Scholar] [CrossRef]
- Toledo-Hernández, C.; Zuluaga-Montero, A.; Bones-González, A.; Rodríguez, J.A.; Sabat, A.M.; Bayman, P. Fungi in Healthy and Diseased Sea Fans (Gorgonia Ventalina): Is Aspergillus sydowii always the Pathogen? Coral Reefs 2008, 27, 707–714. [Google Scholar] [CrossRef]
- Takahata, Y.; Hiruma, M.; Sugita, T.; Muto, M. A Case of Onychomycosis due to Aspergillus sydowii Diagnosed using DNA Sequence Analysis. Mycoses 2008, 51, 170–173. [Google Scholar] [CrossRef]
- Hayashi, A.; Crombie, A.; Lacey, E.; Richardson, A.J.; Vuong, D.; Piggott, A.M.; Hallegraeff, G. Aspergillus sydowii Marine Fungal Bloom in Australian Coastal Waters, its Metabolites and Potential Impact on Symbiodinium Dinoflagellates. Mar. Drugs 2016, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Adegoke, S.A.; Odibo, F. Production, Purification and Characterization of A-Amylase of Aspergillus sydowii IMI 502692. Plant Cell Biotechnol. Mol. Biol. 2019, 20, 1050–1058. [Google Scholar]
- Albuquerque, K.K.; Albuquerque, W.W.; Costa, R.M.; Batista, J.M.S.; Marques, D.A.; Bezerra, R.P.; Herculano, P.N.; Porto, A.L. Biotechnological Potential of a Novel Tannase-Acyl Hydrolase from Aspergillus sydowii using Waste Coir Residue: Aqueous Two-Phase System and Chromatographic Techniques. Biocatal. Agric. Biotechnol. 2020, 23, 101453. [Google Scholar] [CrossRef]
- Alwakeel, S.S.; Ameen, F.; Al Gwaiz, H.; Sonbol, H.; Alghamdi, S.; Moharram, A.M.; Al-Bedak, O.A. Keratinases Produced by Aspergillus Stelliformis, Aspergillus sydowii, and Fusarium Brachygibbosum Isolated from Human Hair: Yield and Activity. J. Fungi 2021, 7, 471. [Google Scholar]
- Elwan, S.H.; Ammar, M.S.; Mohawed, S.M. Lipases from Aspergillus Sydowi. Zent. Für Mikrobiol. 1986, 141, 233–239. [Google Scholar]
- Ghosh, M.; Nanda, G. Purification and some Properties of a Xylanase from Aspergillus sydowii MG49. Appl. Environ. Microbiol. 1994, 60, 4620–4623. [Google Scholar] [CrossRef]
- Amin, M.; Liang, X.; Ma, X.; Dong, J.; Qi, S. New Pyrone and Cyclopentenone Derivatives from Marine-Derived Fungus Aspergillus sydowii SCSIO 00305. Nat. Prod. Res. 2021, 35, 318–326. [Google Scholar]
- Bu, C.; Zhang, Q.; Zeng, J.; Cao, X.; Hao, Z.; Qiao, D.; Cao, Y.; Xu, H. Identification of a Novel Anthocyanin Synthesis Pathway in the Fungus Aspergillus sydowii H-1. BMC Genom. 2020, 21, 29. [Google Scholar]
- Chen, K.; Sun, S.; Cao, H.; Yi, C.; Yang, C.; Liu, Y. Two Sydowic Acid Derivatives and a Sulfonyl Metabolite from the Endophytic Fungus Aspergillus sydowii. J. Asian Nat. Prod. Res. 2022, 24, 1128–1133. [Google Scholar]
- Fukuyama, K.; Tsukihara, T.; Katsube, Y.; Hamasaki, T.; Hatsuda, Y. Structural Analysis of Sydowic Acid by X-Ray Diffraction. Agric. Biol. Chem. 1976, 40, 1053–1054. [Google Scholar]
- Gao, T.; Cao, F.; Yu, H.; Zhu, H. Secondary Metabolites from the Marine Fungus Aspergillus sydowii. Chem. Nat. Compd. 2017, 53, 1204–1207. [Google Scholar]
- Hamasaki, T.; Sato, Y.; Hatsuda, Y. Isolation of New Metabolites from Aspergillus Sydowi and Structure of Sydowic Acid. Agric. Biol. Chem. 1975, 39, 2337–2340. [Google Scholar] [CrossRef] [Green Version]
- Hamasaki, T. Sydowic Acid, a New Metabolite from Aspergillus Sydowi. Tetrahedron Lett. 1975, 16, 659–660. [Google Scholar] [CrossRef]
- He, F.; Sun, Y.; Liu, K.; Zhang, X.; Qian, P.; Wang, Y.; Qi, S. Indole Alkaloids from Marine-Derived Fungus Aspergillus sydowii SCSIO 00305. J. Antibiot. 2012, 65, 109–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Li, X.; Meng, L.; Wang, B. Antioxidant Bisabolane-Type Sesquiterpenoids from Algal-Derived Fungus Aspergillus sydowii EN-434. J. Oceanol. Limnol. 2020, 38, 1532–1536. [Google Scholar]
- Kaur, A.; Raja, H.A.; Darveaux, B.A.; Chen, W.; Swanson, S.M.; Pearce, C.J.; Oberlies, N.H. New Diketopiperazine Dimer from a Filamentous Fungal Isolate of Aspergillus sydowii. Magn. Reson. Chem. MRC 2015, 53, 616. [Google Scholar]
- Kim, H.S.; Park, I.Y.; Park, Y.J.; Lee, J.H.; Hong, Y.S.; Lee, J.J. A Novel Dihydroxanthenone, AGI-B4 with Inhibition of VEGF-Induced Endothelial Cell Growth. J. Antibiot. 2002, 55, 669–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Peng, S.; Yang, J.; Cong, Z.; Lin, X.; Liao, S.; Yang, B.; Zhou, X.; Zhou, X.; Liu, Y. Structurally Diverse Sesquiterpenoids and Polyketides from a Sponge-Associated Fungus Aspergillus sydowii SCSIO41301. Fitoterapia 2019, 135, 27–32. [Google Scholar]
- Liu, S.; Wang, H.; Su, M.; Hwang, G.J.; Hong, J.; Jung, J.H. New Metabolites from the Sponge-Derived Fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res. 2017, 31, 1682–1686. [Google Scholar] [CrossRef]
- Liu, X.; Song, F.; Ma, L.; Chen, C.; Xiao, X.; Ren, B.; Liu, X.; Dai, H.; Piggott, A.M.; Av-Gay, Y. Sydowiols A–C: Mycobacterium Tuberculosis Protein Tyrosine Phosphatase Inhibitors from an East China Sea Marine-Derived Fungus, Aspergillus sydowii. Tetrahedron Lett. 2013, 54, 6081–6083. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Wang, B.; Ji, N. Phenol Derivatives from the Cold-Seep Fungus Aspergillus sydowii 10–31. Phytochem. Lett. 2022, 52, 63–66. [Google Scholar] [CrossRef]
- Handayani, D.; Dwinatrana, K.; Rustini, R. Antibacterial Compound from Marine Sponge Derived Fungus Aspergillus sydowii DC08. Rasayan J. Chem. 2022, 15, 2485–2492. [Google Scholar] [CrossRef]
- Li, W.; Luo, D.; Huang, J.; Wang, L.; Zhang, F.; Xi, T.; Liao, J.; Lu, Y. Antibacterial Constituents from Antarctic Fungus, Aspergillus sydowii SP-1. Nat. Prod. Res. 2018, 32, 662–667. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, C.; Mu, X.; Liu, X.; Wang, L.; Zhao, Y.; Zhang, P.; Li, X.; Zhang, X. Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9. Molecules 2019, 24, 4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.; Huang, S.; Hong, B.; Huang, Q.; Liu, X.; Shao, Z.; Zhang, G. Antiviral Cyclopropane Acids from Deep-Sea-Derived Fungus Aspergillus sydowii. Mar. Drugs 2022, 20, 410. [Google Scholar] [PubMed]
- Ren, H.; Liu, R.; Chen, L.; Zhu, T.; Zhu, W.M.; Gu, Q.Q. Two New Hetero-Spirocyclic Γ-Lactam Derivatives from Marine Sediment-Derived Fungus Aspergillus Sydowi D2–6. Arch. Pharm. Res. 2010, 33, 499–502. [Google Scholar] [CrossRef]
- Tian, L.; Cai, S.; Li, D.; Lin, Z.; Zhu, T.; Fang, Y.; Liu, P.; Gu, Q.; Zhu, W. Two New Metabolites with Cytotoxicities from Deep-Sea Fungus, Aspergillus Sydowi YH11-2. Arch. Pharm. Res. 2007, 30, 1051–1054. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lin, X.; Qin, C.; Liao, S.; Wan, J.; Zhang, T.; Liu, J.; Fredimoses, M.; Chen, H.; Yang, B. Antimicrobial and Antiviral Sesquiterpenoids from Sponge-Associated Fungus, Aspergillus sydowii ZSDS1-F6. J. Antibiot. 2014, 67, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Wiese, J.; Aldemir, H.; Schmaljohann, R.; Gulder, T.A.; Imhoff, J.F. Asperentin B, a New Inhibitor of the Protein Tyrosine Phosphatase 1B. Mar. Drugs 2017, 15, 191. [Google Scholar]
- Yang, X.; Yu, H.; Ren, J.; Cai, L.; Xu, L.; Liu, L. Sulfoxide-Containing Bisabolane Sesquiterpenoids with Antimicrobial and Nematicidal Activities from the Marine-Derived Fungus Aspergillus sydowii LW09. J. Fungi 2023, 9, 347. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Cytotoxic Alkaloids and Antibiotic Nordammarane Triterpenoids from the Marine-Derived Fungus Aspergillus Sydowi. J. Nat. Prod. 2008, 71, 985–989. [Google Scholar]
- Zhang, C.; Chen, Z.; Tao, Y.; Ke, T.; Li, S.; Wang, P.; Chen, L. Enhanced Removal of Trichlorfon and Cd (II) from Aqueous Solution by Magnetically Separable Chitosan Beads Immobilized Aspergillus sydowii. Int. J. Biol. Macromol. 2020, 148, 457–465. [Google Scholar] [PubMed]
- Nayak, B.K.; Anitha, K. Amplified Antibiotic Potency of Two Different Drugs Combined with Biosynthesized AgNPs from Aspergillus sydowii Isolated from Sand Dunes. Int. J. Pharm. Tech. Res. 2014, 6, 1751–1755. [Google Scholar]
- Zhang, C.; Liu, S.; Li, S.; Tao, Y.; Wang, P.; Ma, X.; Chen, L. Enahanced Biosorption of Cu (II) by Magnetic Chitosan Microspheres Immobilized Aspergillus sydowii (MCMAs) from Aqueous Solution. Colloids Surf. Physicochem. Eng. Asp. 2019, 581, 123813. [Google Scholar] [CrossRef]
- Vala, A.K. Exploration on Green Synthesis of Gold Nanoparticles by a Marine-derived Fungus Aspergillus sydowii. Environ. Prog. Sustain. Energy 2015, 34, 194–197. [Google Scholar] [CrossRef]
- Teuscher, F.; Lin, W.; Wray, V.; Edrada, R.; Padmakumar, K.; Proksch, P.; Ebel, R. Two New Cyclopentanoids from the Endophytic Fungus Aspergillus sydowii Associated with the Marine Alga Acanthophora Spicifera. Nat. Prod. Commun. 2006, 1, 927–933. [Google Scholar]
- Chung, Y.; Wei, C.; Chuang, D.; El-Shazly, M.; Hsieh, C.; Asai, T.; Oshima, Y.; Hsieh, T.; Hwang, T.; Wu, Y. An Epigenetic Modifier Enhances the Production of Anti-Diabetic and Anti-Inflammatory Sesquiterpenoids from Aspergillus sydowii. Bioorg. Med. Chem. 2013, 21, 3866–3872. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, M.; Luo, Z.; Liao, Y.; Zhang, B.; Ke, W.; Shao, Z.; Li, F.; Chen, J. Secondary Metabolites Isolated from the Deep Sea-Derived Fungus Aspergillus sydowii C1-S01-A7. Nat. Prod. Res. 2019, 33, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and Xanthone Derivatives from the Sea Fan-Derived Fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2011, 74, 1663–1667. [Google Scholar] [CrossRef]
- Niu, S.; Yang, L.; Zhang, G.; Chen, T.; Hong, B.; Pei, S.; Shao, Z. Phenolic Bisabolane and Cuparene Sesquiterpenoids with Anti-Inflammatory Activities from the Deep-Sea-Derived Aspergillus sydowii MCCC 3A00324 Fungus. Bioorg. Chem. 2020, 105, 104420. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.; Shi, X.; Zheng, H.; Zheng, Z.; Lu, X.; Xing, Y.; Ji, K.; Liu, M.; Dong, Y. Inducing Secondary Metabolite Production of Aspergillus sydowii through Microbial Co-Culture with Bacillus Subtilis. Microb. Cell Factories 2021, 20, 42. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.; He, L.; Xing, Y.; Guo, Q.; Xiu, Z.; Dong, Y. Biosynthetic Profile in the Co-Culture of Aspergillus sydowii and Bacillus Subtilis to Produce Novel Benzoic Derivatives. Microb. Ecol. 2022, 85, 1288–1299. [Google Scholar] [CrossRef]
- Niu, S.; Yang, L.; Chen, T.; Hong, B.; Pei, S.; Shao, Z.; Zhang, G. New Monoterpenoids and Polyketides from the Deep-Sea Sediment-Derived Fungus Aspergillus sydowii MCCC 3A00324. Mar. Drugs 2020, 18, 561. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Wu, H.; Yuan, M.; Zheng, C.; Xu, H. Xanthone Glucosides: Isolation, Bioactivity and Synthesis. Molecules 2021, 26, 5575. [Google Scholar] [CrossRef]
- Badiali, C.; Petruccelli, V.; Brasili, E.; Pasqua, G. Xanthones: Biosynthesis and Trafficking in Plants, Fungi and Lichens. Plants 2023, 12, 694. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; Han, Q.; Li, X.; Li, G.; Li, R.; Jiao, Y.; Zhou, J.; Lou, H. Xanthone Derivatives from Aspergillus sydowii, an Endophytic Fungus from the Liverwort Scapania Ciliata S. Lac and their Immunosuppressive Activities. Phytochem. Lett. 2013, 6, 318–321. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Z.; Zhao, F.; Zheng, J.; Zheng, X.; Zhang, K.; Huang, H. Two New Pyrone Derivatives from the Mangrove-Derived Endophytic Fungus Aspergillus sydowii# 2B. Nat. Prod. Res. 2022, 36, 3872–3878. [Google Scholar]
- Niu, S.; Chen, Z.; Pei, S.; Shao, Z.; Zhang, G.; Hong, B. Acremolin D, a New Acremolin Alkaloid from the Deep-Sea Sediment Derived Aspergillus sydowii Fungus. Nat. Prod. Res. 2022, 36, 4936–4942. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.; Che, Y. N-Hydroxypyridones, Phenylhydrazones, and a Quinazolinone from Isaria Farinosa. J. Nat. Prod. 2011, 74, 32–37. [Google Scholar] [CrossRef]
- Tian, Y.; Qin, X.; Lin, X.; Kaliyaperumal, K.; Zhou, X.; Liu, J.; Ju, Z.; Tu, Z.; Liu, Y. Sydoxanthone C and Acremolin B Produced by Deep-Sea-Derived Fungus Aspergillus Sp. SCSIO Ind09F01. J. Antibiot. 2015, 68, 703–706. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.C. Chapter 55-Aflatoxins, Ochratoxins and Citrinin. In Reproductive and Developmental Toxicology; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Li, X.; Li, H.; Meng, L.; Wang, B. New Citrinin Analogues Produced by Coculture of the Marine Algal-Derived Endophytic Fungal Strains Aspergillus sydowii EN-534 and Penicillium Citrinum EN-535. Phytochem. Lett. 2018, 25, 191–195. [Google Scholar] [CrossRef]
- Tang, Q.; Guo, K.; Li, X.; Zheng, X.; Kong, X.; Zheng, Z.; Xu, Q.; Deng, X. Three New Asperentin Derivatives from the Algicolous Fungus Aspergillus Sp. F00785. Mar. Drugs 2014, 12, 5993–6002. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Gao, X.; Yue, J. Attractive Natural Products with Strained Cyclopropane and/Or Cyclobutane Ring Systems. Sci. China Chem. 2016, 59, 1126–1141. [Google Scholar] [CrossRef]
- Burmudžija, A.Z.; Muškinja, J.M.; Kosanić, M.M.; Ranković, B.R.; Novaković, S.B.; Đorđević, S.B.; Stanojković, T.P.; Baskić, D.D.; Ratković, Z.R. Cytotoxic and Antimicrobial Activity of Dehydrozingerone based Cyclopropyl Derivatives. Chem. Biodiver. 2017, 14. [Google Scholar] [CrossRef]
- Ma, S.; Mandalapu, D.; Wang, S.; Zhang, Q. Biosynthesis of Cyclopropane in Natural Products. Nat. Prod. Rep. 2022, 39, 926–945. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yoon, Y.; Yoon, H.; Park, H.; Song, S.; Yeum, K. Dietary Anthocyanins Against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef] [PubMed]
- Coque, T.M.; Cantón, R.; Pérez-Cobas, A.E.; Fernández-de-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Sandrawati, N.; Hati, S.P.; Yunita, F.; Putra, A.E.; Ismed, F.; Tallei, T.E.; Hertiani, T.; Handayani, D. Antimicrobial and Cytotoxic Activities of Marine Sponge-Derived Fungal Extracts Isolated from Dactylospongia sp. J. Appl. Pharm. Sci. 2020, 10, 28. [Google Scholar]
- Sarker, A.; Gu, Z.; Mao, L.; Ge, Y.; Hou, D.; Fang, J.; Wei, Z.; Wang, Z. Influenza-Existing Drugs and Treatment Prospects. Eur. J. Med. Chem. 2022, 232, 114189. [Google Scholar] [CrossRef]
- Zhang, Z.; Morris-Natschke, S.L.; Cheng, Y.; Lee, K.; Li, R. Development of Anti-influenza Agents from Natural Products. Med. Res. Rev. 2020, 40, 2290–2338. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Ducluzeau, P.H.; Fletcher, L.M.; Vidal, H.; Laville, M.; Tavare, J.M. Molecular Mechanisms of Insulin-Stimulated Glucose Uptake in Adipocytes. Diabetes Metab. 2002, 28, 85–92. [Google Scholar]
- Ruberg, F.L. Myocardial Lipid Accumulation in the Diabetic Heart. Circulation 2007, 116, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- Krecek, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, RC Anderson: Book Review. J. S. Afr. Vet. Assoc. 2000, 71, 239. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An Appraisal of Natural Products Active Against Parasitic Nematodes of Animals. Parasites Vectors 2019, 12, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, P.M.d.; Magalhães, P.d.O. Application of Microbial A-Amylase in Industry-A Review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef]
- Castro, A.M.d.; Carvalho, D.F.; Freire, D.M.G.; Castilho, L.d.R. Economic Analysis of the Production of Amylases and Other Hydrolases by Aspergillus Awamori in Solid-State Fermentation of Babassu Cake. Enzym. Res. 2010, 2010, 576872. [Google Scholar] [CrossRef]
- Hareeri, R.H.; Aldurdunji, M.M.; Abdallah, H.M.; Alqarni, A.A.; Mohamed, S.G.; Mohamed, G.A.; Ibrahim, S.R. Aspergillus Ochraceus: Metabolites, Bioactivities, Biosynthesis, and Biotechnological Potential. Molecules 2022, 27, 6759. [Google Scholar] [CrossRef]
- Fagunwa, O.E.; Olanbiwoninu, A.A. Accelerating the Sustainable Development Goals through Microbiology: Some Efforts and Opportunities. Access Microbiol. 2020, 2, acmi000112. [Google Scholar]
- Toop, T.A.; Ward, S.; Oldfield, T.; Hull, M.; Kirby, M.E.; Theodorou, M.K. AgroCycle–developing a Circular Economy in Agriculture. Energy Procedia 2017, 123, 76–80. [Google Scholar] [CrossRef]
- Sallach, J.B.; Thirkell, T.J.; Field, K.J.; Carter, L.J. The Emerging Threat of Human-use Antifungals in Sustainable and Circular Agriculture Schemes. Plants People Planet 2021, 3, 685–693. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Brar, S.K.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Fungal Enzymes: A Review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef]
- Haroune, L.; Saibi, S.; Bellenger, J.; Cabana, H. Evaluation of the Efficiency of Trametes Hirsuta for the Removal of Multiple Pharmaceutical Compounds Under Low Concentrations Relevant to the Environment. Bioresour. Technol. 2014, 171, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Overview on the Biochemical Potential of Filamentous Fungi to Degrade Pharmaceutical Compounds. Front. Microbiol. 2017, 8, 1792. [Google Scholar] [CrossRef] [PubMed]
- González-Abradelo, D.; Pérez-Llano, Y.; Peidro-Guzmán, H.; del Rayo Sánchez-Carbente, M.; Folch-Mallol, J.L.; Aranda, E.; Vaidyanathan, V.K.; Cabana, H.; Gunde-Cimerman, N.; Batista-García, R.A. First Demonstration that Ascomycetous Halophilic Fungi (Aspergillus sydowii and Aspergillus destruens) are Useful in Xenobiotic Mycoremediation Under High Salinity Conditions. Bioresour. Technol. 2019, 279, 287–296. [Google Scholar] [CrossRef]
- Ganesh Kumar, A.; Manisha, D.; Sujitha, K.; Magesh Peter, D.; Kirubagaran, R.; Dharani, G. Genome Sequence Analysis of Deep Sea Aspergillus sydowii BOBA1 and Effect of High Pressure on Biodegradation of Spent Engine Oil. Sci. Rep. 2021, 11, 9347. [Google Scholar] [CrossRef]
- Birolli, W.G.; Santos, D.d.A.; Alvarenga, N.; Garcia, A.C.; Romão, L.P.; Porto, A.L. Biodegradation of Anthracene and several PAHs by the Marine-Derived Fungus Cladosporium Sp. CBMAI 1237. Mar. Pollut. Bull. 2018, 129, 525–533. [Google Scholar] [CrossRef]
- Birolli, W.G.; Yamamoto, K.Y.; de Oliveira, J.R.; Nitschke, M.; Seleghim, M.H.; Porto, A.L. Biotransformation of Dieldrin by the Marine Fungus Penicillium Miczynskii CBMAI 930. Biocatal. Agric. Biotechnol. 2015, 4, 39–43. [Google Scholar] [CrossRef]
- Zhang, C.; Tao, Y.; Li, S.; Ke, T.; Wang, P.; Wei, S.; Chen, L. Bioremediation of Cadmium-Trichlorfon Co-Contaminated Soil by Indian Mustard (Brassica Juncea) Associated with the Trichlorfon-Degrading Microbe Aspergillus sydowii: Related Physiological Responses and Soil Enzyme Activities. Ecotoxicol. Environ. Saf. 2020, 188, 109756. [Google Scholar] [CrossRef]
- Olszowski, T.; Baranowska-Bosiacka, I.; Rębacz-Maron, E.; Gutowska, I.; Jamioł, D.; Prokopowicz, A.; Goschorska, M.; Chlubek, D. Cadmium Concentration in Mother’s Blood, Milk, and Newborn’s Blood and its Correlation with Fatty Acids, Anthropometric Characteristics, and Mother’s Smoking Status. Biol. Trace Elem. Res. 2016, 174, 8–20. [Google Scholar] [CrossRef]
- Tian, J.; Dong, Q.; Yu, C.; Zhao, R.; Wang, J.; Chen, L. Biodegradation of the Organophosphate Trichlorfon and its Major Degradation Products by a Novel Aspergillus sydowii PA F-2. J. Agric. Food Chem. 2016, 64, 4280–4287. [Google Scholar] [CrossRef] [PubMed]
- Birolli, W.G.; Alvarenga, N.; Vacondio, B.; Seleghim, M.H.R.; Porto, A.L.M. Growth Assessment of Marine-Derived Fungi in the Presence of Esfenvalerate and its Main Metabolites. J. Microb. Biochem. Technol. 2014, 6, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, N.; Birolli, W.G.; Nitschke, M.; Rezende, M.O.; Seleghim, M.H.; Porto, A.L. Biodegradation of Chlorpyrifos by Whole Cells of Marine-Derived Fungi Aspergillus sydowii and Trichoderma sp. J. Microb. Biochem. Technol. 2015, 7, 133–139. [Google Scholar]
- Soares, P.R.S.; Birolli, W.G.; Ferreira, I.M.; Porto, A.L.M. Biodegradation Pathway of the Organophosphate Pesticides Chlorpyrifos, Methyl Parathion and Profenofos by the Marine-Derived Fungus Aspergillus sydowii CBMAI 935 and its Potential for Methylation Reactions of Phenolic Compounds. Mar. Pollut. Bull. 2021, 166, 112185. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, N.; Birolli, W.G.; Seleghim, M.H.; Porto, A.L. Biodegradation of Methyl Parathion by Whole Cells of Marine-Derived Fungi Aspergillus sydowii and Penicillium Decaturense. Chemosphere 2014, 117, 47–52. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M. Untapped Potential of Marine-Associated Cladosporium Species: An Overview on Secondary Metabolites, Biotechnological Relevance, and Biological Activities. Mar Drugs 2021, 19, 645. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, S.G.; Sindi, I.A.; Mohamed, G.A. Biologically Active Secondary Metabolites and Biotechnological Applications of Species of the Family Chaetomiaceae (Sordariales): An Updated Review from 2016 to 2021. Mycol. Prog. 2021, 20, 595–639. [Google Scholar] [CrossRef]
- Cong, B.; Wang, N.; Liu, S.; Liu, F.; Yin, X.; Shen, J. Isolation, Characterization and Transcriptome Analysis of a Novel Antarctic Aspergillus sydowii Strain MS-19 as a Potential Lignocellulosic Enzyme Source. BMC Microbiol. 2017, 17, 129. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.; Choudhry, H.; Asseri, A.H.; Elfaky, M.A.; Mohamed, S.G.; Mohamed, G.A. Stachybotrys Chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance. J. Fungi 2022, 8, 504. [Google Scholar] [CrossRef]
- Brandt, S.C.; Ellinger, B.; Van Nguyen, T.; Harder, S.; Schlüter, H.; Hahnke, R.L.; Rühl, M.; Schäfer, W.; Gand, M. Aspergillus sydowii: Genome Analysis and Characterization of Two Heterologous Expressed, Non-Redundant Xylanases. Front. Microbiol. 2020, 11, 2154. [Google Scholar] [CrossRef]
- Nair, S.G.; Sindhu, R.; Shashidhar, S. Purification and Biochemical Characterization of Two Xylanases from Aspergillus sydowii SBS 45. Appl. Biochem. Biotechnol. 2008, 149, 229–243. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current Perspective on Production and Applications of Microbial Cellulases: A Review. Bioresour. Bioprocess. 2021, 8, 95. [Google Scholar] [CrossRef]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From Bioactivity to a Variety of Industrial Applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef]
- Matkar, K.; Chapla, D.; Divecha, J.; Nighojkar, A.; Madamwar, D. Production of Cellulase by a Newly Isolated Strain of Aspergillus sydowii and its Optimization Under Submerged Fermentation. Int. Biodeterior. Biodegrad. 2013, 78, 24–33. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Bright Side of Fusarium Oxysporum: Secondary Metabolites Bioactivities and Industrial Relevance in Biotechnology and Nanotechnology. J. Fungi 2021, 7, 943. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Abol-Fotouh, D.; Omer, A.M.; Tamer, T.M.; Abbas, E. Comprehensive Insights into Microbial Keratinases and their Implication in various Biotechnological and Industrial Sectors: A Review. Int. J. Biol. Macromol. 2020, 154, 567–583. [Google Scholar] [CrossRef]
- Verma, A.; Singh, H.; Anwar, S.; Chattopadhyay, A.; Tiwari, K.K.; Kaur, S.; Dhilon, G.S. Microbial Keratinases: Industrial Enzymes with Waste Management Potential. Crit. Rev. Biotechnol. 2017, 37, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, A. Sustainable Management of Keratin Waste Biomass: Applications and Future Perspectives. Braz. Arch. Biol. Technol. 2016, 59, e16150684. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.P.; Mouad, A.M.; Boschini, L.; Regali Seleghim, M.H.; Sette, L.D.; Meleiro Porto, A.L. Marine Fungi Aspergillus sydowii and Trichoderma Sp. Catalyze the Hydrolysis of Benzyl Glycidyl Ether. Mar. Biotechnol. 2011, 13, 314–320. [Google Scholar] [CrossRef]
- Martins, M.P.; Ouazzani, J.; Arcile, G.; Jeller, A.H.; de Lima, J.P.; Seleghim, M.H.; Oliveira, A.L.L.; Debonsi, H.M.; Venâncio, T.; Yokoya, N.S. Biohydroxylation of (-)-ambrox®, (-)-sclareol, and (+)-sclareolide by whole cells of Brazilian marine-derived fungi. Mar. Biotechnol. 2015, 17, 211–218. [Google Scholar] [CrossRef]
- de Paula, S.F.C.; Porto, A.L.M. Cascate Reactions of Progesterone by Mycelia and Culture Broth from Marine-Derived Fungus Aspergillus sydowii CBMAI 935. Biocatal. Agric. Biotechnol. 2020, 25, 101546. [Google Scholar] [CrossRef]
- de Matos, I.L.; Nitschke, M.; Porto, A.L.M. Regioselective and Chemoselective Biotransformation of 2′-Hydroxychalcone Derivatives by Marine-Derived Fungi. Biocatal. Biotransform. 2023, 41, 46–56. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Seleghim, M.H.R.; Porto, A.L.M. Biotransformation of Methylphenylacetonitriles by Brazilian Marine Fungal Strain Aspergillus sydowii CBMAI 934: Eco-Friendly Reactions. Mar. Biotechnol. 2014, 16, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, C.; Zheng, C.; Xia, T.; Ma, B.; Liu, X. 3-Methylxanthine Production through Biodegradation of Theobromine by Aspergillus sydowii PT-2. BMC Microbiol. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, D.E.; Barreiro, J.C.; dos Santos, F.M., Jr.; de Vasconcellos, S.P.; Porto, A.L.; Batista, J.M., Jr. Enantioselective Ene-reduction of E-2-cyano-3-(Furan-2-yl) Acrylamide by Marine and Terrestrial Fungi and Absolute Configuration of (R)-2-cyano-3-(Furan-2-yl) Propanamide Determined by Calculations of Electronic Circular Dichroism (ECD) Spectra. Chirality 2019, 31, 534–542. [Google Scholar] [CrossRef]
- Morais, A.T.d.B.; Ferreira, I.M.; Jimenez, D.E.; Porto, A.L. Synthesis of A-Chloroacetophenones with NH4Cl/Oxone® in Situ Followed by Bioreduction with Whole Cells of Marine-Derived Fungi. Biocatal. Agric. Biotechnol. 2018, 16, 314–319. [Google Scholar] [CrossRef]
- Rocha, L.C.; Ferreira, H.V.; Pimenta, E.F.; Berlinck, R.G.S.; Rezende, M.O.O.; Landgraf, M.D.; Seleghim, M.H.R.; Sette, L.D.; Porto, A.L.M. Biotransformation of A-Bromoacetophenones by the Marine Fungus Aspergillus sydowii. Mar. Biotechnol. 2010, 12, 552–557. [Google Scholar] [CrossRef]
- Alvarenga, N.; Porto, A.L. Stereoselective Reduction of 2-Azido-1-Phenylethanone Derivatives by Whole Cells of Marine-Derived Fungi Applied to Synthesis of Enantioenriched Β-Hydroxy-1, 2, 3-Triazoles. Biocatal. Biotransform. 2017, 35, 388–396. [Google Scholar] [CrossRef]
- Rocha, L.C.; Ferreira, H.V.; Luiz, R.F.; Sette, L.D.; Porto, A.L. Stereoselective Bioreduction of 1-(4-Methoxyphenyl) Ethanone by Whole Cells of Marine-Derived Fungi. Mar. Biotechnol. 2012, 14, 358–362. [Google Scholar] [CrossRef]
- Prasad, R.; Pandey, R.; Barman, I. Engineering Tailored Nanoparticles with Microbes: Quo Vadis? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 316–330. [Google Scholar] [CrossRef]
- Fariq, A.; Khan, T.; Yasmin, A. Microbial Synthesis of Nanoparticles and their Potential Applications in Biomedicine. J. Appl. Biomed. 2017, 15, 241–248. [Google Scholar] [CrossRef]
- Singh, C.R.; Kathiresan, K.; Anandhan, S. A Review on Marine Based Nanoparticles and their Potential Applications. Afr. J. Biotechnol. 2015, 14, 1525–1532. [Google Scholar]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of Nanoparticles using Microbes—A Review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-Mediated Green Synthesis of Nano-Silver using Aspergillus sydowii and its Antifungal/Antiproliferative Activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef] [PubMed]





















| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, Location | Ref. |
|---|---|---|---|---|
| (+)-(7S)-Sydonic acid (1) | 266 | C15H22O4 | Cultured, IFO 7531, Japan | [11] |
| - | - | Acanthophora spicifera (red alga), Rameswaram, India | [53] | |
| Marine sediment, Hsinchu, Taiwan | [54] | |||
| - | - | CUGB-F126, seawater, Bohai Sea, Tianjin | [15] | |
| - | - | C1-S01-A7, seawater sample, West Pacific Ocean | [55] | |
| - | - | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] | |
| - | - | MSX19583, spruce litter, Colorado, USA | [33] | |
| - | - | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] | |
| - | - | C1-S01-A7, seawater sample, West Pacific Ocean | [55] | |
| - | - | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] | |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] | |
| - | - | Deep-sea mud, Dalian, China | [58] | |
| - | - | CPCC 401353, cultured, China | [59] | |
| - | - | LW09, deep-sea sediment, Southwest Indian Ridge | [47] | |
| (7S)-(+)-Hydroxysydonic acid = Aspergoterpenin C (2) | 282 | C15H22O5 | Cultured, IFO 7531, Japan | [11] |
| - | - | Acanthophora spicifera (red alga), Rameswaram, India | [53] | |
| - | - | SP-1, marine sediment sample, Antarctic Great Wall Station | [40] | |
| - | - | EN-434, Symphyocladia latiuscula (red alga), Qingdao coastline, China | [32] | |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] | |
| - | - | Piece of deep-sea mud, Dalian, China | [58] | |
| - | - | CPCC 401353, cultured, China | [59] | |
| - | - | LW09, deep-sea sediment, Southwest Indian Ridge | [47] | |
| (7S)-(−)-10-Hydroxysydonic acid (3) | 282 | C15H22O5 | Piece of deep-sea mud, Dalian, China | [58] |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] | |
| - | - | CPCC 401353, cultured, China | [59] | |
| (+)-(7S)-7-O-Methylsydonic acid (4) | 280 | C16H24O4 | PSU-F154, genus Annella sp. (marine gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| (7S,11S)-(+)-12-Hydroxysydonic acid (5) | 282 | C15H22O5 | Marine sediment, Hsinchu, Taiwan | [54] |
| - | - | SP-1, marine sediment, Antarctic Great Wall Station | [40] | |
| - | - | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] | |
| - | - | LW09, deep-sea sediment, Southwest Indian Ridge | [47] | |
| (7S,11S)-(+)-12-Acetoxysydonic acid (6) | 324 | C17H24O6 | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] |
| (S)-(+)-Dehydrosydonic acid (7) | 264 | C15H20O4 | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] |
| 7-Deoxy-7,14-didehydrosydonic acid (8) | 248 | C15H20O3 | CUGB-F126, seawater, Bohai Sea, Tianjin | [15] |
| - | - | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] | |
| (E)-7-deoxy-7,8-didehydrosydonic acid (9) | 248 | C15H20O3 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| (Z)-7-deoxy-7,8-didehydrosydonic acid (10) | 248 | C15H20O3 | SCSIO 41301, marine sponge Phakellia fusca, Xisha Islands, China | [35] |
| (−)-(R)-Cyclohydroxysydonic acid (11) | 280 | C15H20O5 | LW09, deep-sea sediment, Southwest Indian Ridge | [47] |
| Penicibisabolane G (12) | 264 | C15H20O4 | LW09, deep-sea sediment, Southwest Indian Ridge | [47] |
| 11,12-Dihydroxysydonic acid (13) | 298 | C15H22O6 | LW09, deep-sea sediment, Southwest Indian Ridge | [47] |
| Expansol G (14) | 324 | C17H24O6 | LW09, deep-sea sediment, Southwest Indian Ridge | [47] |
| Aspergillusene C (15) | 264 | C15H20O4 | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] |
| Aspergillusene D (16) | 250 | C15H22O3 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| Methyl (S)-(3-Hydroxy-4-(2-hydroxy-6-methylheptan-2-yl)benzoyl)glycinate = (+)-(7S)-Sydonic acid glycinate (17) | 337 | C18H27NO5 | CUGB-F126, seawater, Bohai Sea, Tianjin | [15] |
| Serine sydonate (18) | 353 | C18H27NO6 | Deep-sea mud, Dalian, China | [58] |
| - | - | Cultured, CPCC 401353, China | [59] | |
| 4′-Alkenyl serine sydonate (19) | 351 | C18H25NO6 | Deep-sea mud, Dalian, China | [58] |
| 4′-Hydroxyl serine sydonate (20) | 369 | C18H27NO7 | Deep-sea mud, Dalian, China | [58] |
| 5′-Hydroxyl serine sydonate (21) | 369 | C18H27NO7 | Deep-sea mud, Dalian, China | [58] |
| cyclo-12-Hydroxysydonic acid (22) | 264 | C15H20O4 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| Sydowic acid (23) | 264 | C15H20O4 | Cultured, Japan | [27,29,30] |
| - | - | IFO 4284, cultured, Japan | [29,30] | |
| - | - | Acanthophora spicifera (red alga), Rameswaram, India | [53] | |
| - | - | CUGB-F126, seawater, Bohai Sea, Tianjin | [15] | |
| - | - | C1-S01-A7, seawater sample, West Pacific Ocean | [55] | |
| - | - | EN-434, Symphyocladia latiuscula (red alga), Qingdao coastline, China | [32] | |
| - | - | Rhododendron mole (leaves), Xing’an, Guangxi, China | [26] | |
| (7S,8S)-8-Hydroxysydowic acid (24) | 280 | C15H20O5 | EN-434, Symphyocladia latiuscula (red alga), Qingdao coastline, China | [32] |
| (±)-(7R*,10R*)-10-Hydroxysydowic acid (25) | 280 | C15H20O5 | EN-434, Symphyocladia latiuscula (red alga), Qingdao coastline, China | [32] |
| (−)-(7R,10S)-10-Hydroxysydowic acid (26) | 280 | C15H20O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| - | - | Rhododendron mole (leaves), Xing’an, Guangxi, China | [26] | |
| (−)-(7R,10R)-iso-10-Hydroxysydowic acid (27) | 280 | C15H20O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane A (28) | 278 | C15H18O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane B (29) | 292 | C15H16O6 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane C (30) | 280 | C15H20O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane D (31) | 278 | C15H18O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane E (32) | 280 | C15H20O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane F (33) | 278 | C15H18O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane G (34) | 280 | C15H20O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane H (35) | 280 | C15H20O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane I (36) | 280 | C15H20O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane J (37) | 264 | C14H16O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane K (38) | 284 | C13H16O5S | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane L (39) | 206 | C12H14O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane M (40) | 280 | C15H20O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Asperbisabolane N (41) | 340 | C17H24O7 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Aspergillusene A = (E)-5-(Hydroxymethyl)-2-(6′-methylhept-2′-en-2′-yl)phenol (42) | 234 | C15H22O2 | PSU-F154, marine gorgonian sea fan of the genus Annella sp., coastal area, Surat Thani, Thailand | [56] |
| - | - | Marine sediment, Hsinchu, Taiwan | [54] | |
| - | - | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] | |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] | |
| - | - | LW09, deep-sea sediment, Southwest Indian Ridge | [47] | |
| Aspergillusene B (43) | 246 | C15H18O3 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| - | - | LW09, deep-sea sediment, Southwest Indian Ridge | [47] | |
| β-D-Glucopyranosyl aspergillusene A (44) | 396 | C21H32O7 | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] |
| (+)-(7S)-Sydonol (45) | 252 | C15H24O3 | MSX19583, spruce litter, Colorado, USA | [33] |
| - | - | Marine sediment, Hsinchu, Taiwan | [54] | |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] | |
| (+)-(7S)-7-O-Methylsydonol (46) | 266 | C16H26O3 | Marine sediment, Hsinchu, Taiwan | [54] |
| 7-Deoxy-7,14-didehydrosydonol (47) | 234 | C15H22O2 | Marine sediment, Hsinchu, Taiwan | [54] |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] | |
| (−)-5-(hydroxymethyl)-2-(2′,6′,6′-trimethyltetrahydro-2H-pyran-2-yl)phenol (48) | 250 | C15H22O3 | Rhododendron mole (leaves), Xing’an, Guangxi, China | [26] |
| Anhydrowaraterpol B (49) | 250 | C15H22O3 | Marine sediment, Hsinchu, Taiwan | [54] |
| - | - | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] | |
| (Z)-5-(Hydroxymenthyl)-2-(6′)-methylhept-2′-en-2′-yl)-phenol (50) | 234 | C15H22O2 | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] | |
| Methyl(R,E)-6-(2,3-dihydroxy-4-methylpenyl)-2-methylhept-5-enoate (51) | 278 | C16H22O4 | SW9, seawater sample, Yangma Island, Yantai, China | [41] |
| Cyclowaraterpol A (52) | 250 | C15H22O3 | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] |
| (7S)-Flavilane A (53) | 298 | C16H26O3S | 10–31, deep-sea sediments, cold seep off southwestern Taiwan | [38] |
| (7S)-4-Iodo-flavilane A (54) | 424 | C16H25IO3S | 10–31, deep-sea sediments, cold seep off southwestern Taiwan | [38] |
| Aspersydosulfoxide A (55) | 280 | C16H24O2S | LW09, deep-sea sediment, Southwest Indian Ridge | [47] |
| Aspercuparene A (56) | 262 | C15H18O4 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Aspercuparene B (57) | 264 | C15H20O4 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Aspercuparene C (58) | 260 | C15H16O4 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, Location | Ref. |
|---|---|---|---|---|
| Monoterpenoids | ||||
| Aspermonoterpenoid A (59) | 198 | C10H14O4 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] |
| Aspermonoterpenoid B (60) | 182 | C10H14O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] |
| Triterpenoids | ||||
| (4S,5S,6S,8S,9S,10R,13R,14S,16S,17Z)-6,16-Diacetoxy-25-hydroxy-3,7-dioxy-29-nordammara-1,17(20)-dien-21-oic acid (61) | 572 | C32H44O9 | PFW1-13, driftwood, beach of Baishamen, Hainan, China | [48] |
| Helvolic acid (62) | 554 | C32H42O8 | PFW1-13, driftwood, beach of Baishamen, Hainan, China | [48] |
| Sterols | ||||
| Ergosterol peroxide (63) | 430 | C28H46O3 | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| Ergosta-7,22-dien-3β-ol (64) | 398 | C28H46O | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| Ergosterol (65) | 396 | C28H44O | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| β-Sitosterol (66) | 414 | C29H50O | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| Cerevisterol (67) | 430 | C28H46O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44] |
| (17R)-17-Methylincistererol (68) | 346 | C22H34O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44] |
| Compound Name/Chemical Class | Mol. Wt. | Mol. Formula | Strain, Host, and Location | Ref. |
|---|---|---|---|---|
| Xanthones | ||||
| Sydowinin A (69) | 300 | C16H12O6 | Cultured, IFO 4284, Japan | [29] |
| - | - | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] | |
| 12-O-Acetyl-sydowinin A (70) | 342 | C18H14O7 | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| Sydowinin B (71) | 316 | C16H12O7 | Cultured, IFO 4284, Japan | [29] |
| - | - | Marine sediment, Hsinchu, Taiwan | [54] | |
| - | - | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] | |
| - | - | Marine sediment, Hsinchu, Taiwan | [54] | |
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55] | |
| 13-O-Acetylsydowinin B (72) | 358 | C18H14O8 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| - | - | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] | |
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55] | |
| Methyl 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (73) | 284 | C16H12O5 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| Pinselin (74) | 300 | C16H12O6 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| - | - | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] | |
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55] | |
| Methyl 1,6-dihydroxy-3-methyl-9-oxo-9H-xanthene-1-carboxylate (75) | 300 | C16H12O6 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [56] |
| Sydoxanthone A (76) | 388 | C19H16O7S | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| Sydoxanthone B (77) | 346 | C17H14O6S | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| 8-Hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ester (78) | 284 | C16H12O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| 2-Hydroxy-6-formyl-vertixanthone (79) | 314 | C16H10O7 | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| 2-Hydroxy-1-(hydroxymethyl)-8-methoxy-3-methyl-9H-xanthen-9-one (80) | 286 | C16H14O5 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| 2-Hydroxy-1-(hydroxymethyl)-7,8-dimethoxy-3-methyl-9H-xanthen-9-one (81) | 316 | C17H16O6 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| Austocystin A (82) | 372 | C19H13ClO6 | SCSIO 00305, Verrucella unbracculum (gorgonian), South China Sea, Sanya, Hainan, China | [24] |
| 6-Methoxyl austocystin A (83) | 402 | C20H15ClO7 | SCSIO 00305, Verrucella unbracculum (gorgonian), South China Sea, Sanya, Hainan, China | [24] |
| Sterigmatocystin (84) | 324 | C18H12O6 | DC08, Dachtylospongia sp. (marine sponge), South Coast, West Sumatra, Indonesia | [39] |
| Sydowinol (85) | 318 | C16H14O7 | IFO 4284, Cultured, Japan | [29] |
| Aspergillusone A (86) | 304 | C16H16O6 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55] | |
| Aspergillusone B (87) | 338 | C16H18O8 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| (7R,8R)-AGI-B4 (88) | 320 | C16H16O7 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| - | - | Marine sediment, Hsinchu, Taiwan | [54] | |
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55] | |
| 12-O-Acetyl (7R,8R)-AGI-B4 (89) | 362 | C18H18O8 | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| (7R,8R)-α-Diversonolic ester (90) | 322 | C16H18O7 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| Quinones | ||||
| Emodin (91) | 270 | C15H10O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55] | |
| Emodic acid (92) | 300 | C15H8O7 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| Parietinic acid (93) | 314 | C16H10O7 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| Questin (94) | 284 | C16H12O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55] | |
| - | - | SCSIO 41301, marine sponge Phakellia fusca, Xisha Islands, China | [35] | |
| 1,6,8-Trihydroxy-3-methylanthraquinone (95) | 270 | C15H10O5 | SCSIO 41301, marine sponge Phakellia fusca, Xisha Islands, China | [35] |
| Yicathin C (96) | 312 | C17H12O6 | C1-S01-A7, seawater sample, West Pacific Ocean | [55] |
| 1-Hydroxy-6,8-dimethoxy-3-methylanthraquinone (97) | 298 | C17H14O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| (+)-3,3′,7,7′,8,8′-hexahydroxy-5,5′-dimethyl-bianthra-quinone (98) | 538 | C30H18O10 | #2B, leaves, Aricennia marina, Yangjiang, Guangdong, China | [64] |
| Xanthoradone A (99) | 490 | C27H22O9 | #2B, leaves, Aricennia marina, Yangjiang, Guangdong, China | [64] |
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, and Location | Ref. |
|---|---|---|---|---|
| Cyclotryprostatin B (100) | 425 | C23H27N3O5 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| Cyclotryprostatin E (101) | 443 | C23H29N3O6 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| Fumitremorgin B (102) | 479 | C27H33N3O5 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| 6-Methoxyspirotryprostatin B (103) | 393 | C22H23N3O4 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| 18-Oxotryprostatin A (104) | 395 | C22H25N3O4 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| 14-Hydroxyterezine D (105) | 341 | C19H23N3O3 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Spirotryprostatin A (106) | 365 | C21H23N3O2 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Terezine D (107) | 325 | C19H23N3O2 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Fumitremorgin C (108) | 379 | C22H25N3O3 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| 12,13-Dihydroxyfumitremorgin C (109) | 411 | C22H25N3O5 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| (11S,14S)-Cyclo-(L-Trp-L-Phe) (110) | 333 | C20H19N3O2 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| - | - | MSX19583, spruce litter, Colorado, USA | [33] | |
| - | - | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] | |
| - | - | ZSDS1-F6, unidentified marine sponge, Xisha Islands, China | [45] | |
| - | - | SP-1, marine sediment, Antarctic Great Wall Station | [40] | |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65] | |
| Didehydrobisdethiobis(methylthio)gliotoxin (111) | 356 | C15H20N2O4S2 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Verruculogen (112) | 354 | C15H18N2O4S2 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Cyclo-(S-Pro-S-Ile) (114) | 210 | C11H18N2O2 | Cultured, China | [28] |
| Cyclo-(S-Pro-R-Leu) (113) | 210 | C11H18N2O2 | Cultured, China | [28] |
| WIN 64821 (115) | 664 | C40H36N6O4 | MSX19583, spruce litter, Colorado, USA | [33] |
| - | - | C1-S01-A7, seawater, West Pacific Ocean | [55] | |
| Bisdethiobis(methylthio)-acetylaranotin (116) | 534 | C24H26N2O8S2 | Cultured, China | [28] |
| [4-(2-Methoxyphenyl)-1-piperazinyl][(1-methyl-1H-indol-3-yl)]-methanone (117) | 349 | C21H23N3O2 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| Fumiquinazoline A (118) | 445 | C24H23N5O4 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| Fumiquinazoline B (119) | 445 | C24H23N5O4 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| Fumiquinazoline C (120) | 443 | C24H21N5O4 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| Fumiquinazoline D (121) | 443 | C24H21N5O4 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| Fumiquinazoline F (122) | 358 | C21H18N4O2 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| Fumiquinazoline G (123) | 358 | C21H18N4O2 | SCSIO 00305, Verrucella umbraculum (gorgonian), Sanya, Hainan, China | [31] |
| 2-(4-Hydroxybenzyl)-4-(3-acetyl)quinazolin-one (124) | 294 | C17H14N2O3 | SW9, seawater, Yangma Island, Yantai, China | [41] |
| 2-(4-Hydroxybenzoyl)-4(3H)-quinazolinone (125) | 252 | C15H12N2O2 | SW9, seawater, Yangma Island, Yantai, China | [41] |
| 2-(4-Oxo-3,4-dihydroquinazolin-2-yl)benzoic acid (126) | 266 | C15H10N2O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65] |
| Acremolin (127) | 231 | C11H13N5O | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65] |
| Acremolin C (128) | 245 | C12H15N5O | SP-1, marine sediment, Antarctic Great Wall Station | [40] |
| Acremolin D (129) | 289 | C13H15N5O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65] |
| Pseustin A (130) | 431 | C22H25NO8 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| 14-Norpseurotin A (131) | 417 | C21H23NO8 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Azaspirofurans A (132) | 411 | C21H19NO7 | D2-6, Marine sediment, Jiaozhou Bay, China | [43] |
| Azaspirofurans B (133) | C22H21NO7 | D2-6, Marine sediment, Jiaozhou Bay, China | [43] | |
| Chrysotriazole A (134) | 311 | C17H17N3O3 | SW9, seawater, Yangma Island, Yantai, China | [41] |
| Indoleacetic acid (135) | 175 | C10H9NO2 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65] |
| Pyrrole-2-carboxylic acid (136) | 111 | C5H5NO2 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [65] |
| 2-Acetylaminobenzamide (137) | 178 | C9H10N2O2 | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| 1,4-Dioxa-9,12-diazacyclohexadecane-5,8,13,16-tetraone (138) | 286 | C12H18N2O6 | Cultured, China | [28] |
| N-Acetyltyramine (139) | 179 | C10H13NO2 | Cultured, China | [28] |
| Fumigaclavine B (140) | 366 | C23H30N2O2 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Fumigaclavine C (141) | 298 | C18H22N2O2 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Pyripyropene A (142) | 525 | C29H35NO8 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Pyripyropene E (143) | 569 | C30H35NO10 | PFW1-13, driftwood, Baishamen beach, Hainan, China | [48] |
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, Location | Ref. |
|---|---|---|---|---|
| Violaceol I (144) | 262 | C14H14O5 | MF357, sea sediment, East China Sea, China | [37] |
| - | - | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] | |
| Violaceol II (145) | 248 | C13H12O5 | MF357, sea sediment, East China Sea, China | [37] |
| - | - | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] | |
| Diorcinol (146) | 230 | C14H14O3 | Marine sediment, Hsinchu, Taiwan | [54] |
| - | - | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] | |
| - | - | FNA026, seawater, Xiamen, China | [9] | |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] | |
| 4-Carboxydiorcinal (147) | 274 | C15H14O5 | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] |
| FNA026, seawater, Xiamen, China | [9] | |||
| Diorcinolic acid (148) | 318 | C16H14O7 | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] |
| Glyceryl diorcinolic acid (149) | 392 | C19H20O9 | FNA026, seawater, Xiamen, China | [9] |
| 4-Methoxycarbonyl diorcinol (150) | 288 | C16H16O5 | FNA026, seawater, Xiamen, China | [9] |
| 10-Deoxygerfelin (151) | 274 | C15H14O5 | CPCC 401353, cultured, China | [59] |
| Cordyol C (152) | 246 | C14H14O4 | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] |
| - | - | FNA026, seawater, Xiamen, China | [9] | |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] | |
| Cordyol E (153) | 244 | C15H16O3 | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] |
| Cordyol F (154) | 276 | C15H16O5 | FNA026, seawater, Xiamen, China | [9] |
| Cordyol C-3-O-α-D-ribofuranoside (155) | 378 | C19H22O8 | FNA026, seawater, Xiamen, China | [9] |
| Diorcinol-3-O-α-D-ribofuranoside (156) | 362 | C19H22O7 | FNA026, seawater, Xiamen, China | [9] |
| 4-Methoxycarbonyl diorcinol-3-O-α-D-glucoside (157) | 450 | C22H26O10 | FNA026, seawater, Xiamen, China | [9] |
| Disydonol B (158) | 486 | C30H46O5 | FNA026, seawater, Xiamen, China | [55] |
| 2-(Ethoxycarbonyl)-4′-carboxydiorcinal (159) | 348 | C17H16O8 | FNA026, seawater, Xiamen, China | [9] |
| 7-Ethyldiorcinol (160) | 244 | C15H16O3 | FNA026, seawater, Xiamen, China | [9] |
| 3-Hydroxydiorcinol (161) | 246 | C14H14O4 | FNA026, seawater, Xiamen, China | [9] |
| Aspergilol E (162) | 304 | C16H16O6 | FNA026, seawater, Xiamen, China | [9] |
| 4-Hydroxy-2-(3′-hydroxy-4-methoxycarbonyl-5′-methylphenoxy)-6-methylbenzoic acid (163) | 332 | C17H16O7 | FNA026, seawater, Xiamen, China | [9] |
| Aspermutarubrol (164) | 262 | C14H14O5 | FNA026, seawater, Xiamen, China | [9] |
| Bisviolaceol II (165) | 506 | C28H26O9 | 10–31, sediments, deep-sea, cold seep off southwestern Taiwan | [38] |
| Sydowiol A (166) | 370 | C20H18O7 | MF357, sea sediment, East China Sea, China | [37] |
| Sydowiol B (167) | 384 | C21H20O7 | MF357, sea sediment, East China Sea, China | [37] |
| Sydowiol C (168) | 384 | C21H20O7 | MF357, sea sediment, East China Sea, China | [37] |
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, Location | Ref. |
|---|---|---|---|---|
| Citrinin (169) | 250 | C13H14O5 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| Penicitrinol A (170) | 382 | C23H26O5 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [[6] |
| seco-Penicitrinol A (171) | 398 | C23H26O6 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| Penicitrinol L (172) | 266 | C14H18O5 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| Penicitrinone A (173) | 380 | C23H24O5 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| Penicitrinone F (174) | 394 | C24H26O5 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| Dihydrocitrinone (175) | 266 | C13H14O6 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| Decarboxydihydrocitrinone (176) | 222 | C12H14O4 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| (−)-Asperentin (177) | 292 | C16H20O5 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| LF660, sea sediment, Mediterranean Sea, Levantine Basin SE of Crete | [46] | |||
| Asperentin B (178) | 308 | C16H20O6 | LF660, sea sediment, Mediterranean Sea, Levantine Basin SE of Crete | [46] |
| 5-O-Methyl-asperentin B = 5-Hydroxyl-6-O-methylasperentin (179) | 322 | C17H22O6 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| LF660, sea sediment, Mediterranean Sea, Levantine Basin SE of Crete | [46] | |||
| 6-O-α-D-Ribosylasperentin (180) | 424 | C21H28O9 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| 6-O-α-D-Ribosyl-8-O-methylasperentin (181) | 438 | C22H30O9 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| 5′-Hydroxyasperentin (182) | 308 | C16H20O6 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| 4′-Hydroxyasperentin (183) | 308 | C16H20O6 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| Asperentin-8-methyl ether (184) | 306 | C17H22O5 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| 5′-Hydroxyasperentin-8-methyl ether (185) | 322 | C17H22O6 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| 4′-Hydroxyasperentin-6-methyl ether (186) | 322 | C17H22O6 | F00785, Enteromorpha prolifera (green alga), Jinjiang Saltern, Fujian province, China | [70] |
| (3R,4S)-3,4,5-Trimethyl-isochroman-6,8-diol (187) | 208 | C12H16O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44] |
| (3R,4S)-6,8-dihydroxy-3,4,5-trimethylisochroman-1-one (188) | 222 | C12H14O4 | YH11-2, deep-sea fungus, Guam, South Japan | [44] |
| 2-(12S-Hydroxypropyl)-3-hydroxymethyl-6-hydroxy-7-methoxychromone (189) | 280 | C14H16O6 | #2B, Aricennia marina (leaves), Yangjiang, Guangdong, China | [64] |
| 7-Hydroxy-2-(2-hydroxypropyl)-5-methyl chromone (190) | 234 | C13H14O4 | J05B-7F-4, Stelletta sp. (marine sponge), South Korea | [36] |
| Aspercoumarine acid (191) | 206 | C10H6O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] |
| (2R)-2,3-Dihydro-7-hydroxy-6, 8-dimethyl-2-[(E)-prop-1-enyl]chromen-4-one (192) | 232 | C14H16O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44] |
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, Location | Ref. |
|---|---|---|---|---|
| 4-Hydroxy-3,6-dimethyl-2-pyrone (194) | 140 | C7H8O3 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| 4-Methyl-5,6-dihydropyren-2-one (193) | 112 | C6H8O2 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| Sydowione A (195) | 226 | C12H18O4 | SCSIO 00305, Verrucella unbracculum (gorgonian), South China Sea, Sanya, Hainan, China | [24] |
| Sydowione B (196) | 226 | C12H18O4 | SCSIO 00305, Verrucella unbracculum (gorgonian), South China Sea, Sanya, Hainan, China | [24] |
| Paecilpyrone A (197) | 238 | C13H18O4 | SCSIO 00305, Verrucella unbracculum (gorgonian), South China Sea, Sanya, Hainan, China | [24] |
| (±)-Pyrenocine S (198) | 226 | C11H14O5 | #2B, Aricennia marina (leaves), Yangjiang, Guangdong, China | [64] |
| Pyrenocine A (199) | 208 | C11H12O4 | #2B, Aricennia marina (leaves), Yangjiang, Guangdong, China | [64] |
| (±)-Pyrenocine E (200) | 240 | C12H16O5 | #2B, Aricennia marina (leaves), Yangjiang, Guangdong, China | [64] |
| Asperphenylpyrone (201) | 310 | C18H14O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] |
| Macrolactin U′ (202) | 480 | C31H44O4 | Deep-sea mud, Dalian, China | [58] |
| Sydocyclopropane A (203) | 270 | C14H22O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [42] |
| Sydocyclopropane B (204) | 182 | C11H18O2 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [42] |
| Sydocyclopropane C (205) | 184 | C10H16O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [42] |
| Sydocyclopropane D (206) | 184 | C10H16O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [42] |
| Hamavellone B (207) | 180 | C11H16O2 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [42] |
| Sydowione C (208) | 284 | C15H24O5 | SCSIO 00305, Verrucella unbracculum (gorgonian), South China Sea, Sanya, Hainan, China | [24] |
| Cycloerodiol (209) | 240 | C15H28O2 | Cultured, China | [28] |
| Sydowin A (210) | 412 | C18H14Cl2O7 | Acanthophora spicifera (red alga), Rameswaram, India | [53] |
| Sydowin B (211) | 396 | C18H14Cl2O6 | Acanthophora spicifera (red alga), Rameswaram, India | [53] |
| 3-(2-Hydroxypropyl)-4-(hexa-2E,4E-dien-6-yl)furan-2(5H)-one (212) | 222 | C13H18O3 | Cultured, China | [28] |
| Pestalotiolactone A (213) | 184 | C10H16O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] |
| 1-Hydroxyboivinianin A (214) | 206 | C12H14O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [57] |
| (±)-Sydowiccal (215) | 222 | C12H14O4 | Rhododendron mole (leaves), Xing’an, Guangxi, China | [26] |
| Butyrolactone-I (216) | 424 | C24H24O7 | #2B, Aricennia marina (leaves), Yangjiang, Guangdong, China | [64] |
| Compound Name | Mol. Wt. | Mol. Formula | Strain, Host, Location | Ref. |
|---|---|---|---|---|
| Sydowether (217) | 354 | C18H26O7 | SW9, seawater, Yangma Island, Yantai, China | [41] |
| 1,9-Dihydroxy-3-(hydroxymethyl)-10-methoxydibenzo[b,e]oxepine- 6,11-dione (218) | 316 | C16H12O7 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| 8-Demethoxy-10-methoxy-wentiquinone C (219) | 300 | C16H12O6 | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| Moniliphenone (220) | 286 | C16H14O5 | Scapania ciliata (Chinese liverwort), Maoer Mountain, Guangxi, China | [63] |
| Phenol A acid (221) | 240 | C12H16O5 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| Phenol A (222) | 196 | C11H16O3 | EN-534, Laurencia okamurai (red alga), Qingdao, China | [69] |
| 3-(2,5-Dimethylbenzo[d][1,3]dioxol-2-yl)propanoic acid (223) | 222 | C12H14O4 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| 2-(5-Hydroxy-4-methylpentyl)-2-methylbenzo[d][1,3]dioxole-5- carboxylic acid (224) | 280 | C15H20O5 | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] |
| 4-Hydroxyphenylacetic acid (225) | 152 | C8H8O3 | SP-1, marine sediment, Antarctic Great Wall Station | [40] |
| Orcinol (226) | 124 | C7H8O2 | PSU-F154, genus Annella sp. (gorgonian sea fan), coastal area, Surat Thani, Thailand | [56] |
| 3-Hydroxybenzoic acid (227) | 138 | C7H6O3 | CPCC 401353, cultured, China | [59] |
| 4-Hydroxybenzoic acid (228) | 138 | C7H6O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] |
| 3,4,5-Trimethoxybenzoic acid (229) | 212 | C10H12O5 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] |
| 4-(3′,4′-Dihydroxyphenyl)-2-butanone (230) | 180 | C10H12O3 | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] |
| 4-Hydroxybenzaldehyde (231) | 122 | C7H6O2 | C1-S01-A7, seawater, West Pacific Ocean | [55] |
| Benzoic acid (232) | 122 | C7H6O2 | CPCC 401353, cultured, China | [59] |
| Gibellulin B (233) | 260 | C14H12O5 | FNA026, seawater, Xiamen, China | [9] |
| 3,7-Dihydroxy-1,9-dimethyldibenzofuran (234) | 228 | C14H12O3 | FNA026, seawater, Xiamen, China | [9] |
| - | - | SCSIO 41301, Phakellia fusca (marine sponge), Xisha Islands, China | [35] | |
| - | - | MCCC 3A00324, deep-sea sediment, South Atlantic Ocean | [60] | |
| Orsellinic acid (235) | 168 | C8H8O4 | Deep-sea mud, Dalian, China | [58] |
| - | - | CPCC 401353, cultured, China | [59] | |
| 2-Methoxy-5-methyl-3-(methylsulfonyl)phenol (236) | 216 | C9H12O4S | Rhododendron mole (leaves), Xing’an, Guangxi, China | [26] |
| 2,3,5-Trimethyl-6-(3-oxobutan-2-yl)-4H-pyran-4-one (237) | 208 | C12H16O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44] |
| 5-[(2S,3R)-3-Hydroxybutan-2-yl]-4-methylbenzene-1,3-diol (238) | 196 | C11H16O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44] |
| 2,4-Dihydroxy-3,5,6-trimethylbenzaldehyde (239) | 180 | C10H12O3 | YH11-2, deep-sea fungus, Guam, South Japan | [44] |
| Macrolactin A (240) | 402 | C24H34O5 | Piece of deep-sea mud, Dalian, China | [58] |
| (Z)-6-Tridecenoic acid (241) | 212 | C13H24O2 | Cultured, China | [28] |
| Malvidin 3-O-glucoside (242) | 479 | C22H23O12+\ | H-1, bacterial wilt-affected ginger humus, Chengdu, China | [25] |
| Malvidin 3-O-galactoside (243) | 449 | C21H21O11+ | H-1, bacterial wilt-affected ginger humus, Chengdu, China | [25] |
| Cyanidin 3-O-glucoside (244) | 493 | C23H25O12+ | H-1, bacterial wilt-affected ginger humus, Chengdu, China | [25] |
| Peonidin O-malonylhexoside (245) | 549 | C25H25O14+ | H-1, bacterial wilt-affected ginger humus, Chengdu, China | [25] |
| Cyanidin (246) | 287 | C15H11O6+ | H-1, bacterial wilt-affected ginger humus, Chengdu, China | [25] |
| Compound Name | Assay/Cell Line | Biological Results (IC50) * | Ref. | |
|---|---|---|---|---|
| Compound | Positive Control | |||
| Cerevisterol (67) | P388/SRB | 0.12 μM | CDDP 0.039 μM | [44] |
| 6-Methoxyl austocystin A (83) | Artemia salina | 2.9 µM | Toosendanin 2.2 µM | [24] |
| [4-(2-Methoxyphenyl)-1-piperazinyl][(1-methyl-1H-indol-3-yl)]-methanone (117) | MTT/A375 | 5.7 μM | - | [31] |
| (3R,4S)-3,4,5-Trimethylisochroman-6,8-diol (187) | P388/SRB | 1.95 μM | CDDP 0.039 μM | [44] |
| (2R)-2,3-Dihydro-7-hydroxy-6,8-dimethyl-2-[(E)-prop-1-enyl] chromen-4-one (192) | P388/SRB | 0.14 μM | CDDP 0.039 μM | [44] |
| Sydowione A (195) | Artemia salina | 19.5 µM | Toosendanin 2.2 µM | [24] |
| Sydowione B (196) | Artemia salina | 14.3 µM | Toosendanin 2.2 µM | [24] |
| Sydowione C (208) | Artemia salina | 8.3 µM | Toosendanin 2.2 µM | [24] |
| 2,4-Dihydroxy-3,5,6-trimethylbenzaldehyde (239) | P388/SRB | 0.59 μM | CDDP 0.039 μM | [44] |
| Compound Name | Assay/Organism | Biological Results | Ref. | |
|---|---|---|---|---|
| Compound | Positive Control | |||
| Antibacterial (MIC) | ||||
| (7S)-(+)-hydroxysydonic acid (2) | Broth microdilution/S. aureus | 0.5 µg/mL | Tigecycline 0.06 µg/mL | [40] |
| Broth microdilution/ MRSA | 1 µg/mL | Tigecycline 0.25 µg/mL | [40] | |
| Broth microdilution/S. epidermidis | 0.25 µg/mL | Tigecycline 0.03 µg/mL | [40] | |
| Broth microdilution/ MRAE | 0.5 µg/mL | Tigecycline 0.12 µg/mL | [40] | |
| (7S,11S)-(+)-12-Hydroxysydonic acid (5) | Broth microdilution/S. aureus | 0.5 µg/mL | Tigecycline 0.06 µg/mL | [40] |
| Broth microdilution/ MRSA | 1 µg/mL | Tigecycline 0.25 µg/mL | [40] | |
| Broth microdilution/S. epidermidis | 0.25 µg/mL | Tigecycline 0.03 µg/mL | [40] | |
| Broth microdilution/ MRAE | 0.5 µg/mL | Tigecycline 0.12 µg/mL | [40] | |
| (11S,14S)-Cyclo-(L-Trp-L-Phe) (110) | Broth microdilution/S. aureus | 0.25 µg/mL | Tigecycline 0.06 µg/mL | [40] |
| Broth microdilution/ MRSA | 1 µg/mL | Tigecycline 0.25 µg/mL | [40] | |
| Broth microdilution/S. epidermidis | 0.12 µg/mL | Tigecycline 0.03 µg/mL | [40] | |
| Broth microdilution/ MRAE | 0.5 µg/mL | Tigecycline 0.12 µg/mL | [40] | |
| Citrinin (169) | Microplate assay/E. coli | 8 µg/mL | Chloramphenicol 1 µg/mL | [69] |
| Microplate assay/Micrococcus luteus | 16 µg/mL | Chloramphenicol 2 µg/mL | [69] | |
| Microplate assay/Vibrio parahaemolyticus | 8 µg/mL | Chloramphenicol 2 µg/mL | [69] | |
| Penicitrinol A (170) | Microplate assay/E. coli | 8 µg/mL | Chloramphenicol 1 µg/mL | [69] |
| Microplate assay/Micrococcus luteus | 4 µg/mL | Chloramphenicol 2 µg/mL | [69] | |
| Microplate assay/Vibrio parahaemolyticus | 8 µg/mL | Chloramphenicol 2 µg/mL | [69] | |
| Antituberculosis (IC50) | ||||
| Sydowiol A (166) | M. tuberculosis protein tyrosine phosphatase inhibitor | 14.0 μg/mL | - | [37] |
| Sydowiol B (167) | 24.0 μg/mL | - | [37] | |
| Anti-microalgae (IC50) | ||||
| (7S)-Flavilane A (53) | Broth microdilution/ Prorocentrum micans | 4.6 µg/mL | CuSO4 2.7 µg/mL | [38] |
| Broth microdilution/ Prorocentrum minimum | 2.4 µg/mL | CuSO4 2.2 µg/mL | [38] | |
| (7S)- 4-Iodo-flavilane A (54) | Broth microdilution/ Prorocentrum micans | 11.0 µg/mL | CuSO4 2.7 µg/mL | [38] |
| Broth microdilution/ Prorocentrum minimum | 1.3 µg/mL | CuSO4 2.2 µg/mL | [38] | |
| Bisviolaceol II (165) | Broth microdilution/ Prorocentrum minimum | 5.2 µg/mL | CuSO4 2.2 µg/mL | [38] |
| Compound Name | Virus/Assay | Biological Results (IC50) | Ref. | |
|---|---|---|---|---|
| Compound | Positive Control | |||
| 7-Deoxy-7,14-didehydrosydonic acid (8) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 7.07 µM | Ribavirin 2.53 µM | [35] |
| cyclo-12-Hydroxysydonic acid (22) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 8.89 µM | Ribavirin 2.53 µM | [35] |
| Aichi/2/68 (H3N2)/Pseudovirus neutralization and MTT | 36.41 µM | Ribavirin 6.23 µM | [35] | |
| FM-1/1/47(H1N1)/Pseudovirus neutralization and MTT | 24.46 µM | Ribavirin 3.97 µM | [35] | |
| 2-Hydroxy-1-(hydroxymethyl)-8-methoxy-3-methyl-9H-xanthen-9-one (80) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 4.70 µM | Ribavirin 2.53 µM | [35] |
| FM-1/1/47 (H1N1)/Pseudovirus neutralization and MTT | 4.04 µM | Ribavirin 3.97 µM | [35] | |
| 2-Hydroxy-1-(hydroxymethyl)-7,8-dimethoxy-3-methyl-9H-xanthen-9-one (81) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 2.17 µM | Ribavirin 2.53 µM | [35] |
| Emodic acid (92) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 2.00 µM | Ribavirin 2.53 µM | [35] |
| Aichi/2/68 (H3N2)/Pseudovirus neutralization and MTT | 17.53 µM | Ribavirin 6.23 µM | [35] | |
| FM-1/1/47(H1N1)/Pseudovirus neutralization and MTT | 5.37 µM | Ribavirin 3.97 µM | [35] | |
| Parietinic acid (93) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 7.88 µM | Ribavirin 2.53 µM | [35] |
| Aichi/2/68 (H3N2)/Pseudovirus neutralization and MTT | 30.09 µM | Ribavirin 6.23 µM | [35] | |
| FM-1/1/47(H1N1)/Pseudovirus neutralization and MTT | 39.60 µM | Ribavirin 3.97 µM | [35] | |
| Questin (94) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 1.92 µM | Ribavirin 2.53 µM | [35] |
| Aichi/2/68 (H3N2)/Pseudovirus neutralization and MTT | 9.62 µM | Ribavirin 6.23 µM | [35] | |
| FM-1/1/47(H1N1)/Pseudovirus neutralization and MTT | 11.1 µM | Ribavirin 3.97 µM | [35] | |
| 1,6,8-Trihydroxy-3-methylanthraquinone (95) | Aichi/2/68 (H3N2)/Pseudovirus neutralization and MTT | 9.72 µM | Ribavirin 6.23 µM | [35] |
| FM-1/1/47(H1N1)/Pseudovirus neutralization and MTT | 18.48 µM | Ribavirin 3.97 µM | [35] | |
| Bisdethiobis(methylthio)-acetylaranotin (116) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 34.60 µM | Ribavirin 2.53 µM | [35] |
| Aichi/2/68 (H3N2)/Pseudovirus neutralization and MTT | 24.56 µM | Ribavirin 6.23 µM | [35] | |
| FM-1/1/47(H1N1)/Pseudovirus neutralization and MTT | 44.08 µM | Ribavirin 3.97 µM | [35] | |
| Citrinin (169) | H5N1/Influenza neuraminidase inhibition screen kit | 45.6 nM | Oseltamivir 3.6 nM | [69] |
| Penicitrinol A (170) | H5N1/Influenza neuraminidase inhibition screen kit | 21.2 nM | Oseltamivir 3.6 nM | [69] |
| seco-Penicitrinol A (171) | H5N1/Influenza neuraminidase inhibition screen kit | 24.7 nM | Oseltamivir 3.6 nM | [69] |
| Penicitrinol L (172) | H5N1/Influenza neuraminidase inhibition screen kit | 41.5 nM | Oseltamivir 3.6 nM | [69] |
| Penicitrinone A (173) | H5N1/Influenza neuraminidase inhibition screen kit | 12.9 nM | Oseltamivir 3.6 nM | [69] |
| Penicitrinone F (174) | H5N1/Influenza neuraminidase inhibition screen kit | 18.5 nM | Oseltamivir 3.6 nM | [69] |
| Sydocyclopropane A (203) | WSN/33 (H1N1)/Cytopathic effect reduction/A/WSN/33 (H1N1) | 26.7 μM | Oseltamivir 18.1 μM | [42] |
| Sydocyclopropane B (204) | Cytopathic effect reduction/A/WSN/33 (H1N1) | 29.5 μM | Oseltamivir 18.1 μM | [42] |
| Hamavellone B (207) | Cytopathic effect reduction/A/WSN/33 (H1N1) | 35.8 μM | Oseltamivir 18.1 μM | [42] |
| 3,7-Dihydroxy-1,9-dimethyldibenzofuran (234) | Puerto Rico/8/34 (H1N1)/Pseudovirus neutralization and MTT | 1.31 µM | Ribavirin 2.53 µM | [35] |
| Aichi/2/68 (H3N2)/Pseudovirus neutralization and MTT | 1.24 µM | Ribavirin 6.23 µM | [35] | |
| FM-1/1/47(H1N1)/Pseudovirus neutralization and MTT | 2.84 µM | Ribavirin 3.97 µM | [35] | |
| Compound Name | Assay | Biological Results | Ref. | |
|---|---|---|---|---|
| Compound | Positive Control | |||
| (S)-(+)-Sydonic acid (1) | Inhibition of superoxide anion | 17.82 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] |
| (7S,11S)-(+)-12-Hydroxysydonic acid (5) | Inhibition of superoxide anion | 31.95 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] |
| Aspergillusene A (42) | Inhibition of superoxide anion | 6.11 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] |
| Inhibition of elastase release | 8.80 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] | |
| (+)-(7S)-Sydonol (45) | Inhibition of superoxide anion | 5.23 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] |
| Inhibition of elastase release | 16.39 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] | |
| (7S)-(+)-7-O-Methylsydonol (46) | Inhibition of superoxide anion | 13.80 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] |
| Anhydrowaraterpol B (49) | Inhibition of superoxide anion | 21.52 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] |
| Sydowinin B (71) | Inhibition of superoxide anion | 21.20 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] |
| Inhibition of elastase release | 12.62 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] | |
| (7R,8R)-AGI-B4 (88) | Inhibition of superoxide anion | 6.00 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] |
| Inhibition of elastase release | 6.60 μM (IC50) | Sorafenib 1.27 μM (IC50) | [54] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.R.M.; Mohamed, S.G.A.; Alsaadi, B.H.; Althubyani, M.M.; Awari, Z.I.; Hussein, H.G.A.; Aljohani, A.A.; Albasri, J.F.; Faraj, S.A.; Mohamed, G.A. Secondary Metabolites, Biological Activities, and Industrial and Biotechnological Importance of Aspergillus sydowii. Mar. Drugs 2023, 21, 441. https://doi.org/10.3390/md21080441
Ibrahim SRM, Mohamed SGA, Alsaadi BH, Althubyani MM, Awari ZI, Hussein HGA, Aljohani AA, Albasri JF, Faraj SA, Mohamed GA. Secondary Metabolites, Biological Activities, and Industrial and Biotechnological Importance of Aspergillus sydowii. Marine Drugs. 2023; 21(8):441. https://doi.org/10.3390/md21080441
Chicago/Turabian StyleIbrahim, Sabrin R. M., Shaimaa G. A. Mohamed, Baiaan H. Alsaadi, Maryam M. Althubyani, Zainab I. Awari, Hazem G. A. Hussein, Abrar A. Aljohani, Jumanah Faisal Albasri, Salha Atiah Faraj, and Gamal A. Mohamed. 2023. "Secondary Metabolites, Biological Activities, and Industrial and Biotechnological Importance of Aspergillus sydowii" Marine Drugs 21, no. 8: 441. https://doi.org/10.3390/md21080441
APA StyleIbrahim, S. R. M., Mohamed, S. G. A., Alsaadi, B. H., Althubyani, M. M., Awari, Z. I., Hussein, H. G. A., Aljohani, A. A., Albasri, J. F., Faraj, S. A., & Mohamed, G. A. (2023). Secondary Metabolites, Biological Activities, and Industrial and Biotechnological Importance of Aspergillus sydowii. Marine Drugs, 21(8), 441. https://doi.org/10.3390/md21080441