Mycosporine-like Amino Acids in Palmaria palmata (Rhodophyta): Specific Implication of Usujirene in Photoprotection
Abstract
:1. Introduction
2. Results
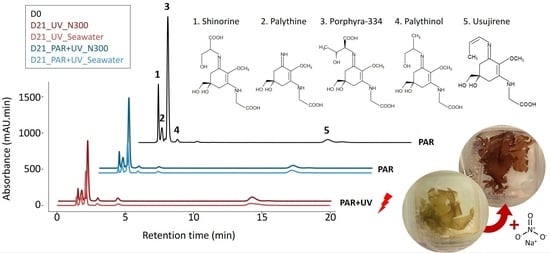
2.1. MAA Composition
2.2. Pigment Composition
3. Discussion
4. Materials and Methods
4.1. Algal Collection and Cultivation Conditions
4.2. Extraction and Identification of Mycosporine-like Amino Acids
4.3. Extraction and Quantification of Pigments
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-like Amino Acids (MAAs): Biology, chemistry and identification features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Naveira, R.I.; Granone, L.I.; Massa, A.E.; Churio, M.S. Argentine squid (Illex argentinus): A source of mycosporine-like amino acids with antioxidant properties. Food Chem. 2024, 438, 137955. [Google Scholar] [CrossRef]
- Bedoux, G.; Pliego-Cortés, H.; Dufau, C.; Hardouin, K.; Boulho, R.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N. Production and properties of mycosporine-like amino acids isolated from seaweeds. Adv. Bot. Res. 2020, 75, 213–245. [Google Scholar] [CrossRef]
- Peng, J.; Guo, F.; Liu, S.; Fang, H.; Xu, Z.; Wang, T. Recent advances and future prospects of mycosporine-like amino acids. Molecules 2023, 28, 5588. [Google Scholar] [CrossRef]
- Peinado, N.K.; Abdala Díaz, R.T.; Figueroa, F.L.; Helbling, E.W. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J. Phycol. 2004, 40, 248–259. [Google Scholar] [CrossRef]
- Torres, P.B.; Chow, F.; Santos, D.Y.A.C. Growth and photosynthetic pigments of Gracilariopsis tenuifrons (Rhodophyta, Gracilariaceae) under high light in vitro culture. J. Appl. Phycol. 2015, 27, 1243–1251. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Effects of UV radiation on photosynthesis, antioxidant capacity and the accumulation of bioactive compounds in Gracilariopsis longissima, Hydropuntia cornea and Halopithys incurva (Rhodophyta). J. Appl. Phycol. 2019, 55, 1258–1273. [Google Scholar] [CrossRef]
- Karsten, U.; Franklin, L.A.; Lüning, K.; Wiencke, C. Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta 1998, 205, 257–262. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stiger-Pouvreau, V.; Connan, S. Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. J. Appl. Phycol. 2020, 32, 2641–2656. [Google Scholar] [CrossRef]
- La Barre, S.; Roullier, C.; Boustie, J. Mycosporine-like Amino Acids (MAAs) in Biological Photosystems. In Outstanding Marine Molecules; La Barre, S., Kornprobst, J.M., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014; pp. 333–360. [Google Scholar] [CrossRef]
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmet. 2006, 9, 1–4. [Google Scholar]
- Candelo, V.; Llewellyn, C.A. Separating and Purifying Mycosporine-like Amino Acids from Cyanobacteria for Application in Commercial Sunscreen Formulations. BioTech 2023, 12, 16. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Figueroa, F.L.; Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-Inflammation Activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Hartmann, A.; Gostner, J.; Fuchs, J.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Med. 2015, 81, 813–820. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Ying, R.; Zhang, Z.; Song, W.; Li, B.; Hou, H. Protective effect of MAAs extracted from Porphyra tenera against UV irradiation-induced photoaging in mouse skin. J. Photochem. Photobiol. B Biol. 2019, 192, 26–33. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Gao, Q.; Garcia-Pichel, F. An ATP-grasp ligase involved in the last biosynthetic step of the iminomycosporine shinorine in Nostoc punctiforme ATCC 29133. J. Bacteriol. 2011, 193, 5923–5928. [Google Scholar] [CrossRef]
- Hu, C.; Völler, G.; Süßmuth, R.; Dittmann, E.; Kehr, J.C. Functional assessment of mycosporine-like amino acids in Microcystis aeruginosa strain PCC 7806. Environ. Microbiol. 2015, 17, 1548–1559. [Google Scholar] [CrossRef]
- Spence, E.; Dunlap, W.C.; Shick, J.M.; Long, P.F. Redundant pathways of sunscreen biosynthesis in a cyanobacterium. ChemBioChem 2012, 13, 531–533. [Google Scholar] [CrossRef]
- Belcour, A.; Girard, J.; Aite, M.; Delage, L.; Trottier, C.; Marteau, C.; Leroux, C.; Dittami, S.M.; Sauleau, P.; Corre, E.; et al. Inferring biochemical reactions and metabolite structures to understand metabolic pathway drift. iScience 2020, 23, 100849. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N.P. Seasonal variation of mycosporine-like amino acids in three subantarctic red seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Korbee, N.; Huovinen, P.; Figueroa, F.L.; Aguilera, J.; Karsten, U. Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales, Rhodophyta). Mar. Biol. 2005, 146, 645–654. [Google Scholar] [CrossRef]
- Huovinen, P.; Matos, J.; Pinto, I.S.; Figueroa, F.L. The role of ammonium in photoprotection against high irradiance in the red alga Grateloupia lanceola. Aquat. Bot. 2006, 84, 308–316. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Bueno, A.; Korbee, N.; Santos, R.; Mata, L.; Schuenhoff, A. Accumulation of mycosporine-like amino acids in Asparagopsis armata grown in tanks with fishpond effluents of Gilthead sea bream, Sparus aurata. J. World Aquac. Soc. 2008, 39, 692–699. [Google Scholar] [CrossRef]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Borg, M.; Krueger-Hadfield, S.A.; Destombe, C.; Collén, J.; Lipinska, A.; Coelho, S.M. Red macroalgae in the genomic era. New Phytol. 2023, 240, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, K.; Karsten, U.; Sawall, T.; Wiencke, C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar. Ecol. Prog. Ser. 2001, 211, 117–129. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Álvarez-Gómez, F.; Bonomi-Barufi, J.; Vega, J.; Massocato, T.F.; Gómez-Pinchetti, J.L.; Korbee, N. Interactive effects of solar radiation and inorganic nutrients on biofiltration, biomass production, photosynthetic activity and the accumulation of bioactive compounds in Gracilaria cornea (Rhodophyta). Algal Res. 2022, 68, 102890. [Google Scholar] [CrossRef]
- Bonomi-Barufi, J.; Figueroa, F.L.; Korbee, N.; Momoli, M.M.; Martins, A.P.; Colepicolo, P.; Van Sluys, M.-A.; Oliveira, M.C. How macroalgae can deal with radiation variability and photoacclimation capacity: The example of Gracilaria tenuistipitata (Rhodophyta) in laboratory. Algal Res. 2020, 50, 102007. [Google Scholar] [CrossRef]
- Karsten, U.; Wiencke, C. Factors controlling the formation of UV-absorbing mycosporine-like amino acids in the marine red alga Palmaria palmata from Spitsbergen (Norway). J. Plant Physiol. 1999, 155, 407–415. [Google Scholar] [CrossRef]
- Kräbs, G.; Bischof, K.; Hanelt, D.; Kartsen, U.; Wiencke, C. Wavelength-dependent induction of UV-absorbing mycosporine-like amino acids in the red alga Chondrus crispus under natural solar radiation. J. Exp. Mar. Biol. Ecol. 2002, 268, 69–82. [Google Scholar] [CrossRef]
- Chandra, R.; Pons-Faudoa, F.P.; Saldívar, R.P.; Rittmann, B.E. Effect of ultra-violet exposure on production of mycosporine-like amino acids and lipids by Lyngbya purpurem. Biomass Bioenerg. 2020, 134, 105475. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Escassi, L.; Perez-Rodrıguez, E.; Korbee, N.; Giles, A.D.; Johnsen, G. Effects of short-term irradiation on photoinhibition and accumulation of mycosporine-like amino acids in sun and shade species of the red algal genus Porphyra. J. Photochem. Photobiol. B Biol. 2003, 69, 21–30. [Google Scholar] [CrossRef]
- Bonomi-Barufi, J.; Mata, M.T.; Oliveira, M.C.; Figueroa, F.L. Nitrate reduces the negative effect of UV radiation on photosynthesis and pigmentation in Gracilaria tenuistipitata (Rhodophyta): The photoprotection role of mycosporine-like amino acids. Phycologia 2012, 51, 636–648. [Google Scholar] [CrossRef]
- Parjikolaei, B.R.; Kloster, L.; Bruhn, A.; Rasmussen, M.B.; Fretté, X.C.; Christensen, L.V. Effect of light quality and nitrogen availability on the biomass production and pigment content of Palmaria palmata (Rhodophyta). Chem. Eng. Trans. 2013, 32, 967–972. [Google Scholar] [CrossRef]
- Eggert, A. Seaweed responses to temperature. In Seaweed Biology. Ecological Studies (Analysis and Synthesis); Wiencke, C., Bischof, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 47–66. [Google Scholar]
- Nishida, Y.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly variation and ultraviolet stability of mycosporine-like amino acids from red alga dulse Palmaria palmata in Japan. Phycology 2021, 1, 119–128. [Google Scholar] [CrossRef]
- Dumay, J.; Morancais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Phycoerythrins: Valuable proteinic pigments in red seaweeds. Adv. Bot. Res. 2014, 71, 321–343. [Google Scholar] [CrossRef]
- Bonomi-Barufi, J.; Korbee, N.; Oliveira, M.C.; Figueroa, F.L. Effects of N supply on the accumulation of photosynthetic pigments and photoprotectors in Gracilaria tenuistipitata (Rhodophyta) cultured under UV radiation. J. Appl. Phycol. 2011, 23, 457–466. [Google Scholar] [CrossRef]
- Figueroa, F.; Bonomi-Barufi, J.; Malta, E.; Conde-Alvarez, R.; Nitschke, U.; Arenas, F.; Mata, M.; Connan, S.; Abreu, M.H.; Marquardt, R.; et al. Short-term effects of increasing CO2, nitrate and temperature on three Mediterranean macroalgae: Biochemical composition. Aquat. Biol. 2014, 22, 177–193. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Montoya, N.G. A high-resolution reverse-phase liquid chromatography method for the analysis of mycosporine-like amino acids (MAAs) in marine organisms. Mar. Biol. 2005, 146, 237–252. [Google Scholar] [CrossRef]
- Gröniger, A.; Hallier, C.; Häder, D.P. Influence of UV radiation and visible light on Porphyra umbilicalis: Photoinhibition and MAA concentration. J. Appl. Phycol. 1999, 11, 437–445. [Google Scholar] [CrossRef]
- Hu, C.; Ludsin, S.A.; Martin, J.F.; Dittmann, E.; Lee, J. Mycosporine-like amino acids (MAAs)—Producing Microcystis in Lake Erie: Development of a qPCR assay and insight into its ecology. Harmful Algae 2018, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Babele, P.K.; Singh, G.; Singh, A.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. UV-B radiation and temperature stress-induced alterations in metabolic events and defense mechanisms in a bloom-forming cyanobacterium Microcystis aeruginosa. Acta Physiol. Plant. 2017, 39, 248. [Google Scholar] [CrossRef]
- Conde, F.R.; Carignan, M.O.; Sandra Churio, M.; Carreto, J.I. In vitro cis-trans photoisomerization of palythene and usujirene. Implications on the in vivo transformation of mycosporine-like amino acids. Photochem. Photobiol. 2003, 77, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Whittock, A.L.; Woolley, J.M.; Auckloo, N.; Corre, C.; Stavros, V.G. Investigating the ultrafast dynamics and long-term photostability of an isomer pair, usujirene and palythene, from the mycosporine-like amino acid family. Molecules 2022, 27, 2272. [Google Scholar] [CrossRef] [PubMed]
- Navarro, N.P.; Huovinen, P.; Jofre, J.; Gómez, I. Ultraviolet radiation stress response of haploid and diploid spores of Mazzaella laminarioides: Do bio-optical traits matter? Algal Res. 2021, 54, 102230. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Tamura, Y.; Kikuzaki, H.; Nakatani, N. Antioxidant effect of the constituents of Susabinori (Porphyra yezoensis). J. Am. Oil Chem. Soc. 1999, 76, 649–653. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring mycosporine-like amino acids (MAAs) as safe and natural protective agents against UV-induced skin damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef]
- Stévant, P.; Schmedes, P.S.; Le Gall, L.; Wegeberg, S.; Dumay, J.; Rebours, C. Concise review of the red macroalga dulse, Palmaria palmata (L.) Weber & Mohr. J. Appl. Phycol. 2023, 35, 523–550. [Google Scholar] [CrossRef]
- Grote, B. Recent developments in aquaculture of Palmaria palmata (Linnaeus) (Weber & Mohr 1805): Cultivation and uses. Rev. Aquac. 2019, 11, 25–41. [Google Scholar] [CrossRef]
- Schmid, M.; Stengel, D.B. Intra-thallus differentiation of fatty acid and pigment profiles in some temperate Fucales and Laminariales. J. Phycol. 2015, 51, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, S.; Gong, X.; Zhao, M.; Fu, X.; Wang, L. Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expr. Purif. 2009, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Beer, S.; Eshel, A. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust. J. Mar. Freshw. Res. 1985, 36, 785–792. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 1 March 2024).
- R Studio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 1 March 2024).





| Days | Light | Nutrient | % Shinorine | % Palythine | % A330 | % P334 | % Palythinol | % Unknown | % Usujirene | % Palythene |
|---|---|---|---|---|---|---|---|---|---|---|
| D0 | PAR | Seawater | 15.73 + 1.53 | 10.34 + 1.81 | 1.41 + 0.27 | 57.38 + 3.57 | 1.95 + 0.31 | 1.63 + 0.89 | 9.29 + 1.59 | 2.28 + 0.86 |
| D7 | PAR | Seawater | 10.49 + 1.51 | 8.97 + 2.10 | 1.75 + 0.24 | 56.22 + 2.02 | 2.00 + 0.15 | 7.10 + 0.53 | 11.82 + 1.37 | 1.65 + 0.50 |
| N100 | 12.53 + 3.31 | 8.49 + 1.49 | 1.49 + 0.22 | 58.24 + 2.52 | 1.78 + 0.26 | 5.59 + 0.98 | 10.54 + 3.51 | 1.33 + 0.30 | ||
| N300 | 13.28 + 1.45 | 8.77 + 1.08 | 1.62 + 0.19 | 56.09 + 3.46 | 1.84 + 0.03 | 6.67 + 2.06 | 10.04 + 0.44 | 1.69 + 0.74 | ||
| PAR+UV | Seawater | 11.57 + 2.37 | 8.27 + 3.20 | 1.68 + 0.39 | 54.81 + 4.70 | 1.85 + 0.29 | 9.06 + 0.20 | 10.70 + 1.85 | 2.06 + 0.86 | |
| N100 | 8.14 + 1.58 | 7.62 + 1.75 | 1.79 + 0.33 | 50.03 + 4.90 | 1.74 + 0.29 | 13.31 + 4.12 | 15.65 + 3.91 | 1.72 + 1.02 | ||
| N300 | 13.2 + 1.80 | 10.11 + 4.82 | 1.78 + 0.42 | 54.06 + 2.45 | 1.81 + 0.47 | 5.38 + 4.97 | 12.73 + 2.76 | 0.94 + 0.25 | ||
| D15 | PAR | Seawater | 5.91 + 0.97 | 9.65 + 1.92 | 2.59 + 1.09 | 49.57 + 2.23 | 2.28 + 0.09 | 10.17 + 0.25 | 18.75 + 2.31 | 1.09 + 0.15 |
| N100 | 8.38 + 4.25 | 9.10 + 3.75 | 1.59 + 0.50 | 53.77 + 8.88 | 2.05 + 0.60 | 8.46 + 1.24 | 15.16 + 7.05 | 1.48 + 0.69 | ||
| N300 | 9.66 + 1.41 | 10.1 + 1.43 | 1.66 + 0.07 | 54.01 + 2.17 | 2.02 + 0.25 | 8.27 + 1.79 | 12.66 + 2.49 | 1.61 + 0.93 | ||
| PAR+UV | Seawater | 7.22 + 1.71 | 9.11 + 2.22 | 1.98 + 0.23 | 46.86 + 4.15 | 2.23 + 0.35 | 12.42 + 1.83 | 18.46 + 4.54 | 1.73 + 0.27 | |
| N100 | 4.43 + 2.23 | 11.16 + 2.63 | 1.65 + 0.69 | 37.89 + 8.68 | 2.87 + 0.75 | 12.62 + 2.66 | 27.30 + 9.24 | 2.08 + 1.16 | ||
| N300 | 6.65 + 2.24 | 11.56 + 6.34 | 2.43 + 0.82 | 41.81 + 11.10 | 2.73 + 0.84 | 11.63 + 4.53 | 21.54 + 9.71 | 1.65 + 0.59 | ||
| D21 | PAR | Seawater | 4.10 + 1.28 | 14.64 + 2.79 | 1.40 + 0.22 | 38.78 + 3.78 | 3.00 + 0.20 | 7.46 + 1.43 | 28.41 + 5.41 | 2.22 + 0.37 |
| N100 | 6.43 + 1.99 | 11.63 + 1.88 | 1.67 + 0.18 | 52.06 + 6.59 | 2.32 + 0.45 | 5.69 + 3.52 | 18.76 + 6.99 | 1.43 + 0.98 | ||
| N300 | 7.52 + 2.28 | 12.77 + 1.73 | 1.74 + 0.24 | 54.12 + 3.76 | 2.46 + 0.32 | 4.30 + 0.69 | 15.62 + 4.65 | 1.47 + 0.40 | ||
| PAR+UV | Seawater | 5.29 + 1.39 | 15.75 + 2.17 | 0.35 + 0.61 | 36.80 + 7.37 | 2.87 + 0.44 | 8.46 + 1.91 | 27.66 + 7.41 | 2.82 + 0.27 | |
| N100 | 4.43 + 2.31 | 13.13 + 4.37 | 1.84 + 0.50 | 37.02 + 16.95 | 3.44 + 0.98 | 8.55 + 2.17 | 29.40 + 12.81 | 2.21 + 1.48 | ||
| N300 | 5.05 + 1.26 | 12.73 + 1.71 | 2.16 + 0.32 | 43.22 + 7.02 | 2.89 + 0.56 | 8.96 + 1.16 | 23.47 + 4.06 | 1.52 + 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalegerie, F.; Stiger-Pouvreau, V.; Connan, S. Mycosporine-like Amino Acids in Palmaria palmata (Rhodophyta): Specific Implication of Usujirene in Photoprotection. Mar. Drugs 2024, 22, 121. https://doi.org/10.3390/md22030121
Lalegerie F, Stiger-Pouvreau V, Connan S. Mycosporine-like Amino Acids in Palmaria palmata (Rhodophyta): Specific Implication of Usujirene in Photoprotection. Marine Drugs. 2024; 22(3):121. https://doi.org/10.3390/md22030121
Chicago/Turabian StyleLalegerie, Fanny, Valérie Stiger-Pouvreau, and Solène Connan. 2024. "Mycosporine-like Amino Acids in Palmaria palmata (Rhodophyta): Specific Implication of Usujirene in Photoprotection" Marine Drugs 22, no. 3: 121. https://doi.org/10.3390/md22030121
APA StyleLalegerie, F., Stiger-Pouvreau, V., & Connan, S. (2024). Mycosporine-like Amino Acids in Palmaria palmata (Rhodophyta): Specific Implication of Usujirene in Photoprotection. Marine Drugs, 22(3), 121. https://doi.org/10.3390/md22030121