2.1. Timing of MC-LR Accumulation in Yellow Perch
The preliminary experiment that was used to select the appropriate time points for sampling fish after MC-LR exposure showed that after a dose of 5 µg·fish−1, MC concentration in both the liver and muscle peaked at 8–10 h and returned to concentrations below 4 ng g−1 MC within 24 h, remaining low through the end of the experiment at 240 h (unpublished data). This preliminary experiment suggested that a more detailed analysis of time points up through 24 h should be the focus of the dosing experiments, which resulted in an increased number of time points sampled between 0–24 h and no further sampling past 24 h in the remainder to the experiments, described below.
Environmentally relevant doses were chosen for the experiments. Yellow perch less than 150 mm in size generally consume 1.31 g dry food per 100 g fish (wet weight) each day [
31] and it is known from stomach content analysis by Wilson
et al. [
28] that early in the summer, juvenile yellow perch in western Lake Erie are eating predominantly benthic invertebrates (76%), followed by fish (18%) and zooplankton (7%). The total weight of food consumed was scaled to the average fish size in this experiment and partitioned based on this expected diet. Literature sources were used to identify some minimum and maximum concentrations of microcystins commonly found in those groups via natural routes of exposure. Concentrations of microcystins ranged 1–30 µg g
−1 in benthic macroinvertebrates in Michigan lakes with
Microcystis, 0.017–1.19 µg g
−1 in the muscle and liver of Lake Erie juvenile yellow perch and 0.2–1352 µg g
−1 in zooplankton (mostly
Daphnia) [
17,
28]. Although these are approximations and the exact concentration of microcystins in each component of the diet is not known, particularly for chironomids which are a significant part of the diet at this life stage, daily exposure to microcystins for this size fish via an early summer diet during a toxic
Microcystis bloom is estimated to be in the range of 1–25 µg for yellow perch.
The dose of MC-LR given on each food pellet was confirmed by extraction and quantification by ELISA from spare pellets. The 5 µg MC-LR dose was actually 6.38 ± 0.58 µg and the 20 µg dose was 19.70 ± 2.20 µg. The analytical error was determined by quantifying the recovery of a known concentration of MC-LR spiked into control tissue samples before extraction. The recovery of spiked MC-LR into muscle tissue was 89.3 ± 9.3% (n = 11) and from liver tissue was 77.0 ± 10.6% (n = 4). The liver and muscle MC data presented below have not been corrected for these recovery rates.
Yellow perch of similar size were chosen for the experiments, but there was still variability in fish weight within and between experiments. Fish in the 5 µg experiment were 12.61 ± 4.80 g wet weight (range of 5 to 24.5 g) and in the 20 µg experiment were 17.62 ± 5.65 g wet weight (range of 9.6 to 29.3 g) even though the lengths only varied by a factor of 2.1 for the 5 µg dose and 1.4 for the 20 µg dose. Thus, the condition of the fish may have been different even though they were from the same source. Fish of smaller size are known to have a higher metabolism and potentially higher concentrations of contaminant relative to similarly exposed fish of a larger size [
32,
33]; therefore the lack of uniform fish size may have increased the variability of MC concentrations between replicate fish. To control for this variability and better assess the impacts of time on MC accumulation in fish tissues, the lowest 5% of all fish in each experiment by weight were not included in the data analysis to determine peak periods of MC concentrations in the tissues. This was a total of three fish per experiment. In addition, one fish per experiment was rejected as an outlier based on both tissue and liver concentrations (
Q-test, 95% confidence interval) [
34].
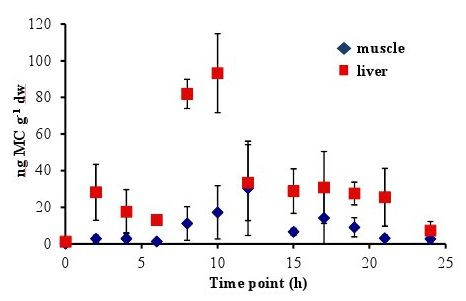
In all experiments, the concentration of MC in the fish liver was elevated by 4–6 h and peaked at 8–10 h after oral dosing. In the 5 µg experiment, there was a sharp increase at 8 h to 86.58 ± 20.01 ng MC g
−1 dw (dry weight), followed by a significant decrease to less than 38 ng MC g
−1 dw by 12 h (
Figure 1). In the 20 µg experiment, the MC concentration in the liver remained elevated at 8–10 h, with a peak concentration of 93.19 ± 21.56 ng MC g
−1 dw, and then decreased to less than 35 ng MC g
−1 dw by 12 h post-dosing (
Figure 2). In both the 5 µg and 20 µg dose experiments, the concentration in the liver remained between 20–30 ng MC g
−1 dw through 21 h post-dosing. By 24 h, the liver MC concentration remained at 24.78 ± 22.04 ng MC g
−1 dw in the 5 µg experiment, but in the 20 µg dose decreased further to 7.27 ± 4.96 ng MC g
−1 dw. At 24 h, MC concentrations in the liver of control fish
not dosed with MC was 9.03 ± 4.14 ng MC g
−1 dw for the 5 µg experiment and 0.72 ± 0.54 ng MC g
−1 dw for the 20 µg experiment.
Concentrations of MC in the fish muscle tissue peaked at 12 h after the fish was dosed in both the 5 µg and 20 µg experiment. Concentrations were lower in the muscle than in the liver, with maximum concentrations of 41.38 ± 51.49 ng MC g
−1 dw in the 5 µg experiment (
Figure 2) and 30.35 ± 25.75 ng MC g
−1 dw in the 20 µg experiment (
Figure 2). By 24 h post-dosing, the average MC concentrations in the 5 µg experiment (
Figure 1) was 8.54 ± 2.58 ng MC g
−1 dw and 2.74 ± 2.14 ng MC g
−1 dw for the 20 µg experiment (
Figure 2). The muscle tissue of control fish at the 24 h time point contained 0.08 ± 0.06 ng MC g
−1 dw for the 5 µg experiment (
Figure 1) and 1.81 ± 1.03 ng MC g
−1 dw in the 20 µg experiment (
Figure 2).
Figure 1.
Concentrations of microcystin (ng microcystin per g dw fish tissue) in yellow perch liver and muscle tissue for time points 0–24 h after given a single oral dose of 5 µg MC-LR. Error is expressed as standard deviation of four replicate fish.
Figure 1.
Concentrations of microcystin (ng microcystin per g dw fish tissue) in yellow perch liver and muscle tissue for time points 0–24 h after given a single oral dose of 5 µg MC-LR. Error is expressed as standard deviation of four replicate fish.
Figure 2.
Concentrations of microcystin (ng microcystin per g dw fish tissue) in yellow perch liver and muscle tissue for time points 0–24 h after given a single oral dose of 20 µg MC-LR. Error is expressed as standard deviation of four replicate fish.
Figure 2.
Concentrations of microcystin (ng microcystin per g dw fish tissue) in yellow perch liver and muscle tissue for time points 0–24 h after given a single oral dose of 20 µg MC-LR. Error is expressed as standard deviation of four replicate fish.
Initial concentrations of MC were also higher in fish from the 5 µg experiment in comparison to the 20 µg experiment. At the beginning of the experiment, before oral dosing, MC concentrations in the liver and muscle were 10.36 ± 11.75 and 0.38 ± 0.22 ng MC g
−1 dw, respectively, in the 5 µg experiment and 1.25 ± 0.76 and 0.21 ± 0.10 ng MC g
−1 dw in the 20 µg experiment. As a hepatotoxin targeting the liver, at least some portion of any measurable accumulation of MC in the fish organs would be expected in the liver tissue. In this study, an initial peak in MC concentrations was measured in the liver at 8–10 h followed by maximum MC accumulation in the muscle tissue at 12 h and a significant decrease in the concentration of unbound MC in both tissues by 24 h after exposure. The measurement of maximum MC concentrations in the liver that were as much as three times higher than those in the muscle also further supports the preferential accumulation of this toxin in the liver. Multiple field studies have documented the uptake of microcystins into the liver and muscle of planktivorous [
22,
23], omnivorous [
18,
19] and piscivorous fish [
19] and lab studies using fish dosed through oral gavage or intraperitoneal (IP) injection [
29,
35,
36,
37] have confirmed the accumulation of microcystins in these organs. However, despite the useful data these studies have contributed, there still remains an incomplete understanding of the capacity of fish to accumulate and eliminate microcystins and the timing over which this occurs. This is in part due to the difficulty in determining the actual levels of exposure in field-caught fish and the lack of environmentally relevant modes of exposure in laboratory studies. While it is acknowledged that the current study also lacks some environmental relevance due to the use of purified toxin instead of microcystins delivered in the form of
Microcystis cells, or other food source which has accumulated toxins by feeding or filtering, the advantage here is that a known dose of MC-LR is provided, eliminating variability associated with cell size, mucilage content, internal microcystin content or other matrix effects of
Microcystis cells.
The current study assessed the effect of a single dose, in contrast to many of the previous feeding studies in which fish were exposed to MC-LR over a multiple day period [
38,
39]. A single dose was chosen in order to better determine the timing of uptake into tissues. However, despite the differences in feeding frequency and experiment duration between this and other studies, the pattern of accumulation of microcystins in the liver first followed by the muscle is relatively consistent. In a 15 day study in which juvenile tilapia were fed
Microcystis cells daily containing concentrations of microcystins in a similar range to our 20 µg MC-LR dose, maximum liver concentrations of microcystins were observed on day 6 and maximum muscle concentrations on day 9 [
38]. In a similar experiment, Smith and Haney [
39] measured the accumulation and elimination of microcystins in pumpkinseed sunfish using a 1000 fold lower dose of microcystins given over 9 days via a zooplankton food source. Their first trial showed a similar pattern of accumulation with the maximum concentration of microcystins found in the liver at 2–4 days and in the muscle by day 4. However, the liver and muscle concentrations of microcystins did not show this clear pattern in their second trial, likely due to the high degree of variability measured in samples [
39], which also highlights the heterogeneity in response between individual fish. Such heterogeneity among fish may be due to size, as observed in this study.
Two different doses of MC-LR were administered in this study, but there was not a clear dose dependent accumulation detected. Maximum MC concentrations in both the liver and muscle tissues were similar in the 5 µg and 20 µg doses, as was the timing of accumulation and elimination of the toxin. While this could be due to the maximum capacity of the tissues to absorb the compound, there were also a number of other confounding factors that may have impacted the measured MC concentrations. The presence of microcystins in the fish livers of the fish at the 0 h time point (before dosing) in the 5 µg experiment was evidence that the fish had previous exposure to microcystins in the fish farm from which they originated. Although the fish were held in microcystin-free water for 24–28 h before the start of the experiment, low levels of microcystins in the liver persisted, likely resulting in higher maximum MC concentrations than would have otherwise been measured. Additionally, the fish used in the 5 µg experiment were smaller on average than those used in the 20 µg experiment. A previous study [
40] also observed that smaller fish have higher concentrations of microcystins than larger fish of the same species from the same lake. This difference in mass between dosing groups is potentially a main driving force in tissue concentrations of microcystins. A fish with more mass will require a greater accumulation of toxin to achieve a higher tissue concentration.
As pointed out by Smith and Haney [
39], the amount of microcystins that accumulate in fish may be determined by the route by which the fish is exposed. By 24 h of exposure, 80% of microcystins administered to pumpkinseed sunfish through a zooplankton diet was accumulated in a non-covalently bound form in the liver [
39], while there was only approximately 1.7–10% absorption in the liver of MC-LR delivered to rainbow trout via gavage of toxic
Microcystis cells [
20,
35], 5% absorption of MC-LR in the livers of yellow perch via an oral dose of MC-LR (this study) and 0.3% uptake in the liver when rainbow trout were orally gavaged with purified MC [
36]. While the purified MC-LR would be expected to be more available for uptake than intracellular microcystin, it is also possible that much of the extracellular microcystin passes directly through the gut and is excreted without being taken up into the organ tissues. The peptide bonds linking D-amino acids in microcystins are not susceptible to normal hydrolytic enzymes, making these toxins resistant to digestion in the gastrointestinal tract [
41,
42]. The microcystin congener also impacts the degree to which microcystins are taken up by the fish. MC-LR alone was used as the microcystin dose in this study and there is an indication that MC-LR may be taken up to a much lower degree than microcystin-RR, another of the 80+ variants of microcystin. Xie
et al. [
22] measured microcystins in silver carp (
Hypophthalmichthys molitrix) fed toxic
Microcystis viridis cells containing both MC-LR and -RR and, despite a high concentration of MC-RR in blood, liver, muscle and intestines, MC-LR was only found in the intestines of these fish. Comparing the ratio of MC-LR:RR in the seston
vs. fish gut and feces suggested that the transport of MC-LR across the intestines is selectively inhibited, but MC-RR is able to cross through the intestines and into muscle tissues [
22,
43]. MC-RR was also measured in trace amounts in the brain of fish (
Jenynsia multidentata) exposed to dissolved MC-RR in the laboratory, in addition to accumulating in the muscle at the end of the 24 h exposure [
18].
Microcystis blooms generally contain multiple microcystin congeners so determining which are present may be important to assess potential toxicity and accumulation in fish. Therefore, despite the lower toxicity of MC-RR compared to many other microcystin variants, including MC-LR [
44], its increased bioavailability may result in increased transport through trophic levels [
22]. Though there is no evidence for biomagnification of microcystins in the food web, this toxin is vectorially transported to higher trophic levels [
23]. Thus, the trophic level at which fish feed is also an important indicator of their potential tissue toxin concentration. There is no consensus in the literature about whether phytoplanktivorous or piscivorous fish generally have the highest levels of microcystins [
19,
23,
45] and, even within these groups, there are likely significant species specific differences in metabolism of microcystins [
30,
46].
2.2. Microcystin Elimination from Yellow Perch and Mass Balance
Dissolved MC concentrations in the tank water increased with time. Microcystins were measurable in the tank water by 4 h after the fish were dosed in both the experiments and then steadily increased over the course of the experiment, with the highest concentrations measured at 24 h. In the 5 µg dose experiment, the average MC concentration in the tank water was 0.50 ± 0.37 µg at 12 h and 3.5 ± 1.2 µg by 24 h (
Figure 3). Overall, concentrations were higher in the 20 µg dose experiment, with an average dissolved MC concentration in the tank water of 1.01 ± 0.11 µg at 12 h and 11.71 ± 1.2 µg by 24 h (
Figure 3).
Figure 3.
Average microcystin concentration (µg MC) in fish tanks for time points 0–24 h after fish given a single oral dose of MC-LR (either 5 µg or 20 µg dose). This concentration represents the MC excreted by the fish, including both dissolved MC and any feces present. Error is expressed as standard deviation of four replicate fish tanks.
Figure 3.
Average microcystin concentration (µg MC) in fish tanks for time points 0–24 h after fish given a single oral dose of MC-LR (either 5 µg or 20 µg dose). This concentration represents the MC excreted by the fish, including both dissolved MC and any feces present. Error is expressed as standard deviation of four replicate fish tanks.
In order to evaluate how the MC is partitioned, a rough mass balance was calculated by summing the total amount of MC in the fish muscle, liver, and tank water for each time point. Total MC measured in the fish and tank water is almost 3% of the initial 5 µg dose at 4 h, 10.5% by 12 h and a maximum of 70.3% accounted for by 24 h. In the 20 µg experiment, the total MC in fish liver, muscle and tank water combined was less than 1% of the initial dose at 4 h, less than 10% through 16 h and was 58.6% of the initial dose by 24 h (
Table 1). Microcystins in the liver and muscle tissues never exceeded 0.3% of the total initial dose.
Table 1.
Percent of total microcystin dose measured at selected time points.
Table 1.
Percent of total microcystin dose measured at selected time points.
| Time point (h) | 5 µg | 20 µg |
|---|
| Muscle | Liver | Tank | Muscle | Liver | Tank |
|---|
| 4 | 0.01 | 0.04 | 2.65 | 0.01 | 0.01 | 0.27 |
| 8 | 0.03 | 0.05 | 3.41 | 0.03 | 0.03 | 1.27 |
| 10 | 0.16 | 0.04 | 3.76 | 0.04 | 0.05 | 3.21 |
| 12 | 0.29 | 0.09 | 10.10 | 0.10 | 0.08 | 5.04 |
| 16 | 0.22 | 0.03 | 47.02 | 0.02 | 0.01 | 9.19 |
| 24 | 0.07 | 0.02 | 70.17 | 0.01 | 0.01 | 58.56 |
Microcystin concentrations in the digestive track were not measured in this study, so it is not known what percentage of the total MC-LR dose passed through the gut unabsorbed compared to the percentage taken up by the tissues and subsequently detoxified and excreted within 24 h. To find out how quickly unabsorbed MC-LR might be expected to pass through the fish gut, an evacuation rate model was used. This model predicts the digestion of food pellets under given temperatures and fish sizes [
31]. For the conditions of this experiment (20 °C, 15 g fish, 1 g food pellet), this model predicts that by 24 h at least 90% of the food pellet would have been excreted, thus suggesting that at least 90% of the MC-LR not taken up into fish tissue would have been passed through the gut without being absorbed by the end of the experiment. This rapid passage of food through the gut based on the digestion model suggests a relatively low accumulation efficiency for MC from food into the fish tissues. The peak concentrations observed with a single exposure were somewhat lower than the average liver concentration for field collected perch but much greater for muscle tissue than found in the field [
28]. However, the rapid elimination rate from the tissue is independent of the uptake once exposure has ceased (e.g., the end of the ingestion of contaminated prey).
Even given this rapid passage of food, and potentially associated MC, through the gut, there was still at least 30% of the initial MC-LR dose that was unaccounted for in the muscle, liver and tank water by the end of this experiment. A small percentage of this dose may be found in bile or other organs, including the heart, gonads, and stomach, but the literature suggests this is minimal [
17,
47,
48]. A more significant percentage of the unaccounted fraction of the MC-LR dose is likely bound covalently and irreversibly to protein phosphatase 1 and 2A. The solvent extraction used in this study will not reverse the covalent bonds that can be formed between the MC-LR and the protein phosphatase enzyme active site and thus is only a measure of the unbound MC fraction, which may underestimate the true toxin load to the tissues (John Berry, Florida International University, pers. comm.). Previous studies have shown that as little as 24% of the total microcystins could be extracted from Atlantic salmon liver [
49].
The degree to which microcystins covalently bound to fish tissue is biologically available to higher trophic levels, including human consumers, is unknown [
23,
42]. However, it is likely that the unbound fraction is more readily available for trophic transfer. Of the total
recoverable (
i.e., unbound fraction) of MC measured in fish tissues and tank water in this experiment, the amount distributed in the muscle and liver tissues is not more than 4.1% at any given time point. A very high percentage of the recovered MC was in the tank water fraction, which also included any feces that might have been present. For both doses, from 15 h through the end of the experiment, the MC in the tank water comprised >99% of the total unbound MC. This large percentage of the unbound MC found in the tank water by 24 h after exposure to a single oral dose indicates that human exposure to microcystin via consumption of yellow perch is reduced as long as just the muscle tissue is being consumed. Since in many cases fish are only exposed to cyanotoxins intermittently while swimming through patchy blooms, there may be sufficient opportunity for elimination before being caught for human consumption. In addition to the decline in measurable microcystins in the liver and muscle by 24 h after exposure, the hepatic effects on fish are short term and serum biochemistry and hepatocyte morphology return to normal within 30 days of removal from exposure [
24,
37]. However, in small lakes with persistent toxic blooms, regular exposure may increase the probability of microcystins being present in the tissues of resident fish at the time they are caught for consumption. In a study on wild caught yellow perch in western Lake Erie, which experiences regular summertime blooms of
Microcystis, maximum concentrations of microcystins of 4.02 ng g dw
−1 in the muscle tissue were measured over the course of the bloom season [
28], which is 8.6 times lower than the maximum muscle MC concentrations measured in this study. The maximum concentrations of microcystins measured in the liver of these wild caught fish was 12.7 times higher than in this experiment (1182 ng·g·dw
−1), suggesting that the lower toxin load in the muscle tissue was not solely due to a lower absorption of microcystins. There are many other commercially and recreationally important fish in the Great Lakes and a recent study has measured higher concentrations of microcystins in the muscle of wild caught fish belonging to these groups, particularly walleye, white bass, and smallmouth bass from Lake Erie (maximum concentrations of 43.6 ng·g·wet weight
−1) and alewives and northern pike from the Bay of Quinte, Lake Ontario (maximum concentrations of 37.5 ng·g·wet weight
−1) [
40]. Naturally, the amount of microcystins that fish will be exposed to in their natural environment will vary greatly with bloom dynamics and toxicity, frequency and duration of exposure, fish size and diet, timing and frequency of feeding, food availability, life history,
etc. 2.3. Kinetics of MC Uptake
The data were difficult to kinetically model in the sense that they were extremely variable (
Figure 1 and
Figure 2). The variability could have been due to several sources of uncertainty. First, an examination of analytical variation yielded a relative percent difference for muscle tissue, measured in duplicate, of 41.1 ± 36.6% for the 5 µg dose and 60.5 ± 37.7% for the 20 µg dose. While this analytical variation appears to be large, it was not sufficient to account for most of the variation in the observed data, which were up to ten-fold or more different among tissue concentrations of different fish sampled after the same length of exposure (
Figure 1 and
Figure 2). This variation appears to in part be due to differences in fish size, with smaller fish exhibiting greater tissue concentrations after the same length of exposure.
As a result of the above variations in concentration, the fit to the data for the two tissues were not as robust as preferred but do provide some insight into the rate processes for microcystin in these fish. First, the elimination is relatively rapid with an apparent elimination half-life (elimination
t0.5) calculated from
ke in the range of 3.3 to 7.8 h for muscle (
Table 2). Given this, the fish would be expected to eliminate 95% of the accumulated MC from muscle in 14.5 to 33.5 h (elimination
t0.05). The elimination half-life for MC in the liver was calculated to be 10.5 h, with 95% elimination from the fish occurring within 46 h. The elimination modeled in this study does not specify mechanism but includes actual loss from the tissue as well as biotransformation to an unextractable or unmeasurable form.
The loss rates for the exposure dose are the rates at which exposure of the fish to the initial dose decreases as the MC is taken up into the fish tissue or eliminated without being absorbed and no longer can serve as a source of exposure. These apparent loss rates for the exposure dose are similar between dose levels but different between tissues. The apparent loss of exposure was modeled as a faster (larger dose loss rate, λ) for the liver than for the muscle, meaning the period of time in which the liver was exposed to MC was shorter than the period of time the muscle was exposed. Microcystin-LR exposure in the liver has a shorter half-life (exposure
t0.5) (2.3–4.5 h) compared to the muscle (14.8–16 h) due to the lower loss rate for exposure in the muscle. This suggests that 95% of the exposure to the initial dose (exposure
t0.05) is complete by 9.9–19.3 h in the liver but not until 63.7–69.2 h in the muscle (
Table 2). This supports our approach of determining the elimination rate constant as a simple first order model since uptake would have been low and likely insignificant by 10 to 12 h, considering the very small size of the uptake rate constants.
Table 2.
Kinetic parameter estimates for MC in yellow perch for each tissue at each exposure dose. ke is the elimination rate constant (h−1), ku is the uptake rate constant (g−1·h−1), λ is the loss rate for the dose (h−1), elimination t0.5 is the half-life in each tissue, elimination t0.05 is the time for 95% elimination from the tissue (both elimination values calculated based on ke), exposure t0.5 is the half-life for exposure in each tissue, exposure t0.05 is the time for completion of 95% of the exposure (both exposure values calculated based on λ).
Table 2.
Kinetic parameter estimates for MC in yellow perch for each tissue at each exposure dose. ke is the elimination rate constant (h−1), ku is the uptake rate constant (g−1·h−1), λ is the loss rate for the dose (h−1), elimination t0.5 is the half-life in each tissue, elimination t0.05 is the time for 95% elimination from the tissue (both elimination values calculated based on ke), exposure t0.5 is the half-life for exposure in each tissue, exposure t0.05 is the time for completion of 95% of the exposure (both exposure values calculated based on λ).
| Tissue/Concentration | ke (h−1) | ke r2 | ku (g−1h−1) | λ (h−1) | ku, λ r2 | Elimination | Exposure |
|---|
| t0.5 (h) | t0.05 (h) | t0.5 (h) | t0.05 (h) |
|---|
| 5 µg–Muscle | 0.089 ± 0.058 | 0.55 | 0.00058 ± 0.00024 | 0.047 ± 0.051 | 0.41 | 7.8 | 33.5 | 14.8 | 63.7 |
| 20 µg–Muscle | 0.21 ± 0.07 | 0.62 | 0.00017 ± 0.000059 | 0.043 ± 0.035 | 0.44 | 3.3 | 14.5 | 16.0 | 69.2 |
| 5 µg–Liver | 0.066 ± 0.037 | 0.71 | 0.0019 ± 0.0006 | 0.155 ± 0.069 | 0.59 | 10.5 | 45.4 | 4.5 | 19.3 |
| 20 µg–Liver | 0.066 ± 0.029 | 0.81 | 0.0016 ± 0.0007 | 0.30 ± 0.15 | 0.55 | 10.5 | 45.8 | 2.3 | 9.9 |
The uptake rate constants (ku) calculated in this model are somewhat different from more familiar values that are often calculated, such as clearance coefficients. The uptake rate constants indicate the fraction of the dose accumulated by the respective fish tissue each hour. As with the exposure constants, the uptake rate constants are similar between doses but different between tissues. The uptake of the liver is relatively fast, about ten times faster than for the muscle. These differences reflect the much greater concentrations found in the liver compared to the muscle and further support the idea that uptake occurs first into the liver with some later redistribution to the muscle. Despite the much lower concentration in the muscle, the total amount of MC found in the muscle is often greater than that found in the liver because of the relative size of the two organs.
Higher concentrations of MC in the liver compared to the muscle tissue has been observed in many other studies as well, regardless of dosing mechanism or species [
29,
38,
45,
47,
50]. Selective uptake of microcystins into hepatocytes occurs via active transport, resulting in organ specificity of accumulation [
51]. Reasons for these differences in liver and muscle MC concentrations are demonstrated by the kinetics model developed in this study. The first order model used shows the rapid uptake and elimination of MC from the liver and much slower rates in the muscle. This is consistent with overall higher MC concentrations measured in the liver as well as the maximum liver concentrations proceeding maximum muscle concentrations in time. This leads to the hypothesis that the initial uptake of MC is into the liver and then partially redistributed into the muscle, prolonging the exposure period for the muscle.
The kinetics of MC uptake and loss were similar regardless of dose, potentially driven by the ability of MC to cross membranes [
22]. Without an increase in MC uptake rate with increased dose, total concentrations of MC in fish with a larger tissue mass would be overall lower than in smaller fish. Another toxicokinetic model, which was developed for accumulation of microcystins in the nile tilapia
Oreochromis niloticus, showed that for lower doses of microcystins, there may be a greater dose dependent effect [
45]. In this model, at doses of microcystins below 0.15 µg·MC·fish
−1·day
−1, accumulation in the liver was not accompanied by elimination, resulting in liver concentrations as high as 51% of the dose. At intermediate concentrations (0.15–2.5 µg·MC·fish
−1·day
−1), increasing available microcystins did not result in increased liver concentrations, due to an increased capacity for elimination. This model predicts that at concentrations greater than 2.5 µg·MC·fish
−1·day
−1, a saturation point is reached for nile tilapia and there is again an increase in liver microcystin concentrations with increasing dose [
45]. Thus the lack of dose response for tissue concentrations in the current experiment may be an indication that doses of 5–20 µg MC fish
−1 may be in the intermediate phase of accumulation for yellow perch of this size and that higher or lower concentrations of MC are necessary to see a dose response.
2.4. Potential Impacts of Microcystins Human Health via Fish Consumption
The variability in exposure and the lack of data on the biological availability of the covalently bound microcystins in fish tissue makes it difficult to discern the potential risk of microcystins to human health through fish consumption [
23,
42]. There is circumstantial evidence of exposure to microcystins and toxicity to humans via consumption of contaminated fish [
6] and the WHO recommended total daily intake (TDI) of 0.04 μg per kg bodyweight per day can be applied to concentrations of microcystins in fish to determine acceptable fish tissue concentrations. Populations that are in the greatest danger of health risks are those for which fish comprise a high percentage of the diet. Average consumption of sport fish by anglers and their families in the Great Lakes basin is estimated at 40 g fish·day
−1, which is higher than the US national average fish consumption of 6.5 g day
−1 [
52,
53,
54]. Of greater concern are native tribal members for whom fish is much larger proportion of their diet, estimated to be an average of 190 g day
−1 and as much as 328 g day
−1 [
55]. Using the WHO recommended TDI for a 70 kg adult and these average consumption rates, the maximum recommended fish muscle concentration would be 70 ng MC g fish
−1 for the average Great Lakes angler, 14.7 ng MC g
−1 for tribal members and 8.5 ng MC g
−1 for very high fish consumers to prevent potential human illness. A recent draft document from EPA (NCEA-C-1765) used data from Heinze [
56] to suggest a lower recommended limit for chronic exposure of 0.003 μg kg
−1 per day, which would lower the maximum recommended fish levels of microcystins to 5.3, 1.1, and 0.6 ng MC g
−1 for Great Lake anglers, tribal members and very high fish consumers, respectively. These recommended microcystin concentration would be even lower for children, elderly, or sensitive individuals.
The uptake and elimination experiments described in this manuscript suggest that juvenile yellow perch exposed to environmentally relevant doses of microcystins have the capacity to eliminate the unbound fraction in the muscle tissue from a maximum of 18.7 ng g−1 ww down to 0.6 ng g−1 ww (expressed as wet weight to more accurately compare to the weight of a fish meal instead of dry weight as reported in the results) by 24 h after exposure. These data suggest that during periods of significant microcystin-producing blooms, consumers for whom fish is a substantial part of their diet could receive a dose that exceeds the WHO TDI. If the more conservative EPA guidelines were adopted, the recommended limits for chronic microcystins would be exceeded even by the average Great Lakes fish consumer during a toxic bloom. However, the evidence of rapid elimination presented here suggests that during non-bloom periods, muscle concentrations of unbound microcystins in yellow perch are likely lower than the recommended limit for chronic exposure.
Poste
et al. [
40] also concluded that despite the higher concentration of microcystins measured in the muscle tissue of some Great Lakes fish causing individual fish to be of potential human health concern, the shorter length of the bloom season, time for elimination and amount of fish consumed made it likely that most consumers of Great Lakes fish were not receiving chronic exposure to microcystins through fish in excess of the WHO TDI. In contrast, an important finding in a study of concentrations of microcystins in fish from lakes in Uganda is that in communities in tropical locations with persistent year-round cyanobacterial blooms, which rely on local fish for subsistence and frequently consume the entire fish, may be at risk for health risks through chronic microcystin exposure [
40].