Potential Human Health Risk by Metal(loid)s, 234,238U and 210Po due to Consumption of Fish from the “Luis L. Leon” Reservoir (Northern México)
Abstract
:1. Introduction

2. Materials and Methods
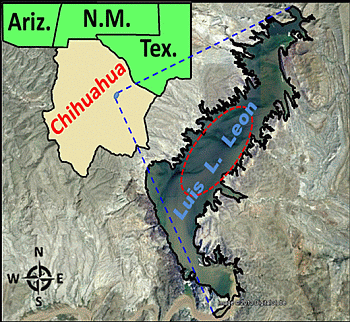
2.1. Study Area
2.2. Sampling
Sample Preparation
2.3. Analytical Methods and Measurement Techniques
2.3.1. Radiochemical Method
2.3.2. Metal(loid)s Analysis
| Element | DOLT-4 concentration (mg∙kg−1 dw ± SD) | Recovery (%) | |
|---|---|---|---|
| Certified | Measured | ||
| As | 9.66 ± 0.62 | 8.7 ± 0.6 | 90 |
| Cu | 31.2 ± 1.1 | 28.4 ± 0.6 | 91 |
| Fe | 1833 ± 75 | 1686 ± 40 | 92 |
| Pb | 0.16 ± 0.04 | 0.15 ± 0.03 | 93 |
| Hg | 2.58 ± 0.22 | 2.76 ± 0.13 | 107 |
| Zn | 116 ± 6 | 113 ± 8 | 97 |
2.4. Bioacumulation Factor

2.5. Estimates of Theoretical Intake and Effective Dose for Metal(loid)s in Fish
2.5.1. Intake Estimation

2.5.2. Effective Dose Estimation

2.6. Statistical Analysis
3. Results and Discussion
3.1. General Information of Analyzed Species
3.2. 234,238U and 210Po Activity Concentration
 . From results of Table 3, uranium activity concentrations present in fish fillet are low, in comparison with both the reference values established by UNSCEAR 2008 [57], and the values of 0.14–2.6 and 0.6–4.5 Bq∙kg−1 (dry weight) for 238U and 234U, respectively, reported in samples of species Cichlasoma labridens in San Marcos Dam (Chihuahua) [33]. The uranium concentrations found in this study are also lower than those presented in other publications related with uranium mine activities [58,59]. The activity ratios 234U/238U were 1.3–1.5 in fillet samples and 1.1–2.7 in water. It is noteworthy that this slight disequilibrium was expected, because it is known that 234U is more soluble in water than 238U and it is well known that isotope disequilibria of 234U/238U in natural waters can occur due to alpha recoil effects [22]. Studies developed in San Marcos Dam have reported a greater radioactive disequilibrium between 234U and 238U than the ones reported in the present work. Renteria-Villalobos et al. [33] reported significantly higher radioactive disequilibrium in fillet of species Cichlasoma labridens, ranging from 0.9–4.5. Also Burillo-Montufar et al. [60] found activity ratios in water samples ranging from 1.1 to 1.9. However, the differences in isotopic activity concentration may be attributed to the geological environment where San Marcos Dam is placed.
. From results of Table 3, uranium activity concentrations present in fish fillet are low, in comparison with both the reference values established by UNSCEAR 2008 [57], and the values of 0.14–2.6 and 0.6–4.5 Bq∙kg−1 (dry weight) for 238U and 234U, respectively, reported in samples of species Cichlasoma labridens in San Marcos Dam (Chihuahua) [33]. The uranium concentrations found in this study are also lower than those presented in other publications related with uranium mine activities [58,59]. The activity ratios 234U/238U were 1.3–1.5 in fillet samples and 1.1–2.7 in water. It is noteworthy that this slight disequilibrium was expected, because it is known that 234U is more soluble in water than 238U and it is well known that isotope disequilibria of 234U/238U in natural waters can occur due to alpha recoil effects [22]. Studies developed in San Marcos Dam have reported a greater radioactive disequilibrium between 234U and 238U than the ones reported in the present work. Renteria-Villalobos et al. [33] reported significantly higher radioactive disequilibrium in fillet of species Cichlasoma labridens, ranging from 0.9–4.5. Also Burillo-Montufar et al. [60] found activity ratios in water samples ranging from 1.1 to 1.9. However, the differences in isotopic activity concentration may be attributed to the geological environment where San Marcos Dam is placed.| Scientific Name | Common Name | Diet | Habitat | n | L. Range | W. Range | Equation | R2 Value |
|---|---|---|---|---|---|---|---|---|
| Cyprinus carpio | Common carp | Insects, Plant, Algae | Cloudy or stagnant waterin areas with less than 10 m deep | 24 | 30–45 (36 ± 3.9) | 0.10–0.30 (0.16 ± 0.04) | L = 22.29 + 83.44W | 0.90 |
| Lepomis cyanellus | Bluegill | Insects, Small fish, Shellfish | Shallow areas with sand, gravel and vegetation | 24 | 31–48 (36 ± 4.5) | 0.11–0.23 (0.16 ± 0.03) | L = 16.63 + 124.1W | 0.92 |
| Ictalurus furcatus | Catfish | Fish, Insect larvae and mollusks Detritus | Environments with braking currents on sand or rock bottom | 24 | 30–49 (39 ± 5.5 ) | 0.11–0.31 (0.20 ± 0.06) | L = 21.48 + 89.07W | 0.92 |
| Sample | n | Tissue | Activity concentrations | ||||
|---|---|---|---|---|---|---|---|
| 238U | 234U | U Total | 234U/238U | 210Po | |||
| Cyprinus carpio | 24 | Fillet | 0.008 (0.003–0.025) | 0.012 (0.004–0.042) | 0.020 (0.007–0.067) | 1.5 (1.1–1.6) | -- |
| Ictalurus furcatus | 24 | Fillet | 0.011 (0.003–0.034) | 0.016 (0.006–0.048) | 0.028 (0.01–0.076) | 1.5 (1.1–1.6) | -- |
| Lepomis cyanellus | 24 | Fillet | 0.012 (0.004–0.034) | 0.017 (0.005–0.041) | 0.029 (0.008–0.075) | 1.4 (1.1–1.5) | -- |
| Cyprinus carpio | 18 | Liver | -- | -- | -- | -- | 0.85 (0.7–1.13) |
| Ictalurus furcatus | 18 | Liver | -- | -- | -- | -- | 1.13 (0.93–1.37) |
| Lepomis cyanellus | 18 | Liver | -- | -- | -- | -- | 1.73 (1.16–3.26) |
| Water * | 8 | 0.008 (0.004–0.012) | 0.011 (0.006–0.018) | 0.02 (0.01–0.03) | 1.8 (1.1–2.7) | -- | |
| BAF ** | 24 | 0.94 1 | 1.0 1 | 1.0 1 | -- | -- | |
| 24 | 1.3 2 | 1.4 2 | 1.4 2 | -- | -- | ||
| 24 | 1.5 3 | 1.5 3 | 1.5 3 | -- | -- | ||
3.3. Metal(loid)s
| Sample | n | Tissue | Metal(loid)s concentration | |||||
|---|---|---|---|---|---|---|---|---|
| As | Cu | Fe | Hg | Pb | Zn | |||
| Cyprinus carpio | 24 | Fillet | 0.15 (0.03–0.56) | 0.18 (0.02–0.46) | 4.2 (1–11) | 0.008 (0.0003–0.34) | 0.037 (0.0003–0.32) | 11.1 (4–19) |
| 24 | Gill | 0.12 (0.02–0.45) | 0.15 (0.06–0.26) | 11.0 (5–19) | 0.004 (0.0003–0.19) | 0.14 (0.018–0.48) | 13.5 (15–85) | |
| Ictalurus furcatus | 24 | Fillet | 0.15 (0.04–0.28) | 0.14 (0.01–0.36) | 4.4 (0.3–12) | 0.079 (0.0005–0.88) | 0.065 (0.002–1.48) | 8.9 (2–16) |
| 24 | Gill | 0.17 (0.01–0.52) | 0.2 (0.08–0.4) | 10.3 (6–18) | 0.025 (0.0003–0.22) | 0.22 (0.01–0.53) | 14.5 (14–91) | |
| Lepomis cyanellus | 24 | Fillet | 0.29 (0.10–0.65) | 0.22 (0.06–0.62) | 3.2 (1–12) | 0.069 (0.0003–1.02) | 0.023 (0.0003–0.28) | 11.4 (4–18) |
| 24 | Gill | 0.31 (0.1–0.7) | 0.23 (0.01–0.58) | 8.3 (5–19) | 0.014 (0.0003–0.25) | 0.185 (0.011–0.38) | 13.1 (14–81) | |
| Water * | 8 | -- | 0.008 (0.003–0.01) | 0.014 (0.01–0.06) | 0.044 (0.007–0.25) | 0.002 (0.001–0.009) | 0.009 (0.002–0.1) | 0.016 (0.003–0.28) |
| BAF ** | 24 | Fillet | 19 1 | 13 1 | 96 1 | 4 1 | 3.7 1 | 594 1 |
| 24 | Gill | 15 1 | 11 1 | 249 1 | 2 1 | 14 1 | 720 1 | |
| 24 | Fillet | 19 2 | 10 2 | 100 2 | 40 2 | 6.5 2 | 469 2 | |
| 24 | Gill | 22 2 | 14 2 | 233 2 | 13 2 | 22 2 | 776 2 | |
| 24 | Fillet | 37 3 | 16 3 | 73 3 | 35 3 | 2.3 3 | 554 3 | |
| 24 | Gill | 40 3 | 17 3 | 188 3 | 7 3 | 19 3 | 702 3 | |
3.4. Human Health Effects by Intake of Metal(loid)s and Uranium Due to Fish Fillets Consumption

| Species | n | Metal(loid)s Concentration * | EDI a (µg∙day−1) | PTDI b (µg∙day−1) | EWI c (µg∙week−1) | PTWI d (µg∙week−1) | H d (µSv∙year−1) |
|---|---|---|---|---|---|---|---|
| Cyprinus carpio | 24 | As: 0.15 (0.56) | -- | -- | 21 (78) | 350 | -- -- -- -- -- -- 0.01 (0.03) |
| Cu: 0.18 (0.46) | 36 ** (92) | 35,000 | -- | -- | |||
| Fe: 4.24 (11) | 85 (220) | 56,000 | -- | -- | |||
| Hg: 0.008 (0.34) | -- | -- | 1.1 (48) | 350 | |||
| Pb: 0.037 (0.32) | -- | -- | 5.2 (45) | 1750 | |||
| Zn: 11 (19) | 222 (380) | 71,429 | -- | -- | |||
| 238U: 0.008 (0.025) | -- | -- | -- | -- | |||
| 234U: 0.012 (0.067) | -- | -- | -- | -- | |||
| Lepomis cyanellus | 24 | As: 0.29 (0.65) | -- | -- | 40 (91) | 350 | -- |
| Cu: 0.22 (62) | 4.3 (1240) | 35,000 | -- | -- | -- | ||
| Fe: 3.24 (12) | 65 (240) | 56,000 | -- | -- | -- | ||
| Hg: 0.07 (1.02) | -- | -- | 9.7 (143) | 350 | -- | ||
| Pb: 0.02 (0.28) | -- | -- | 3.2 (39) | 1750 | -- | ||
| Zn: 11 (18) | 228 (360) | 71,429 | -- | -- | -- | ||
| 238U: 0.012 (0.032) | -- | -- | -- | -- | -- | ||
| 234U: 0.017 (0.041) | -- | -- | -- | -- | 0.01 (0.02) | ||
| Ictalurus furcatus | 24 | As: 0.15 (0.65) | -- | -- | 21 (91) | 350 | -- |
| Cu: 0.14 (36) | 2.8 (7.2) | 35,000 | -- | -- | -- | ||
| Fe: 4.43 (12) | 89 (240) | 56,000 | -- | -- | -- | ||
| Hg: 0.079 (1.02) | -- | -- | 11 (143) | 350 | -- | ||
| Pb: 0.065 (0.84) | -- | -- | 9.1 (118) | 1750 | -- | ||
| Zn: 9 (18) | 178 (360) | 71,429 | -- | -- | -- | ||
| 238U: 0.011 (0.034) | -- | -- | -- | -- | -- | ||
| 234U: 0.016 (0.076) | -- | -- | -- | -- | 0.01 (0.04) |
 .
.3.5. Discussion
| Specie | Tissue | Correlation | n | r | p-value |
|---|---|---|---|---|---|
| Cyprinus carpio | Fillet | As-Wet | 24 | 0.500 | 0.013 |
| As-Length | 24 | 0.534 | 0.007 | ||
| Cu-Fe | 24 | 0.482 | 0.017 | ||
| Cu-Zn | 24 | 0.546 | 0.006 | ||
| Gills | As-Wet | 24 | 0.525 | 0.008 | |
| Lepomis cyanellus | Fillet | Hg-Wet | 24 | 0.563 | 0.004 |
| Cu-Fe | 24 | 0.523 | 0.009 | ||
| Fe-Zn | 24 | 0.555 | 0.005 | ||
| Gills | Hg-Fe | 24 | 0.402 | 0.052 | |
| Ictalurus furcatus | Gills | Fe-Pb | 24 | 0.490 | 0.015 |
| Fe-Zn | 24 | 0.473 | 0.020 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jabeen, F.; Chaudhry, A. Environmental impacts of anthropogenic activities on the mineral uptake in Oreochromis mossambicus from indus river in Pakistan. Environ. Monit. Assess. 2009, 166, 641–651. [Google Scholar] [CrossRef]
- Quintero-Alvarez, J.M.; Soto-Jiménez, M.F.; Amezcua, F.; Voltolina, D.; Frías-Espericueta, M. Cadmium and lead concentrations in the fish tissues of a coastal lagoon system of the Gulf of California. Bull. Environ. Contam. Toxicol. 2012, 89, 820–823. [Google Scholar] [CrossRef]
- Ouédraogo, O.; Amyot, M. Mercury, arsenic and selenium concentrations in water and fish from sub-Saharan semi-arid freshwater reservoirs (Burkina Faso). Sci. Total Environ. 2012, 444, 243–254. [Google Scholar] [CrossRef]
- Türkmen, M.; Türkmen, A.; Tepe, Y.; Ates, A.; Gökkus, K. Determination of metal contaminations in sea foods from Marmara, Aegean and Mediterranean seas: Twelve fish species. Food Chem. 2008, 108, 794–800. [Google Scholar] [CrossRef]
- Korkmaz Görür, F.; Keser, R.; Akçay, N.; Dizman, S. Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea region of Turkey. Chemosfere 2012, 87, 356–361. [Google Scholar] [CrossRef]
- Labrot, F.; Narbonne, J.F.; Ville, P.; Saint Denis, M.; Ribera, D. Acute toxicity, toxicokinetics, and tissue target of lead and uranium in the clam Corbicula fluminea and the worm Eisenia fetida: Comparison with the fish Brachydanio rerio. Arch. Environ. Contam. Toxicol. 1999, 36, 167–178. [Google Scholar] [CrossRef]
- Schenone, N.F.; Avigliano, E.; Goessler, W.; Fernández Cirelli, A. Toxic metals, trace and major elements determined by ICPMS in tissues of Parapimelodus valenciennis and Prochilodus lineatus from Chascomus lake, Argentina. Microchem. J. 2014, 112, 127–131. [Google Scholar] [CrossRef]
- WHO, World Health Organization. Arsenic, Fact Sheet No. 372, (2012). Available online: http://www.who.int/mediacentre/factsheets/fs372/en/#.09/27/2013 (accessed on 16 June 2014).
- WHO, World Health Organization. Lead Poisoning and Health, Fact Sheet No. 379, (2013). Available online: http://www.who.int/mediacentre/factsheets/fs379/en/ (accessed on 16 June 2014).
- Domingo, J.L. Reproductive and developmental toxicity of natural and depleted uranium: A review. Reprod. Toxicol. 2001, 15, 603–609. [Google Scholar] [CrossRef]
- Al Sayegh Petkovsek, S.; Mazej Grudnik, Z.; Pokorny, B. Heavy metals and arsenic concentrations in ten fish species from the Salek lakes (Slovenia): Assessment of potential human health risk due to fish consumption. Environ. Monit. Assess. 2012, 184, 2647–2662. [Google Scholar]
- Burger, J.; Gaines, K.; Boring, C.S.; Stephens, W.L.; Snodgrass, J.; Dixon, C.; McMahon, M.; Shukla, S.; Shukla, T.; Gochfeld, M. Metal levels in fish from the Savannah river: Potential hazards to fish and other receptors. Environ. Res. 2002, 89, 85–97. [Google Scholar] [CrossRef]
- Nawaz, S.; Nagra, S.A.; Saleem, Y.; Priyadarshi, A. Determination of heavy metals in fresh water fish species of the River Ravi, Pakistan compared to farmed fish varieties. Environ. Monit. Assess. 2010, 167, 461–471. [Google Scholar] [CrossRef]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef]
- Franco, J.L.; Posser, T.; Mattos, J.J.; Sánchez-Chardi, A.; Trevisan, R.; Oliveira, C.S.; Carvalho, P.S.M.; Leal, R.B.; Marques, M.R.F.; Bainy, A.C.D.; et al. Biochemical alterations in juvenile carp (Cyprinus carpio) exposed to zinc: Glutathione reductase as a target. Mar. Environ. Res. 2008, 66, 88–89. [Google Scholar] [CrossRef]
- Luna Porres, M.Y.; Montero Cabrera, M.E.; Manjón Collado, G.; Díaz Frances, I.; Rentería Villalobos, M. Determination of uranium and polonium in Sparus aurata by alpha spectrometry. Revista Mexicana de Física 2012, 224–227. [Google Scholar]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef]
- Cyrille, Y.D.A.; Victor, K.; Sanogo, T.A.; Boukary, S.; Joseph, W. Cadmium accumulation in tissues of Sarotherodon melanotheron (Rüppel, 1852) from the Aby lagoon system in Côte D’ivoire. Int. J. Environ. Res. Public Health 2012, 9, 821–830. [Google Scholar]
- Buet, A.; Barillet, S.; Camilleri, V. Changes in oxidative stress parameters in fish as response to direct uranium exposure. Radioprotection 2005, 40, S151–S155. [Google Scholar] [CrossRef]
- Beatrice, G.; Isabelle, C.; Virginie, C.; Christelle, A.-G. Effects of depleted uranium on oxidative stress, detoxification, and defence parameters of Zebrafish Danio rerio. Arch. Environ. Contam. Toxicol. 2013, 64, 140–150. [Google Scholar] [CrossRef]
- Díaz-Francés, I.; Mantero, J.; Manjón, G.; Díaz, J.; García-Tenorio, R. 210po and 238u isotope concentrations in commercial bottled mineral water samples in Spain and their dose contribution. Radiat. Prot. Dosim. 2013, 156, 336–342. [Google Scholar] [CrossRef]
- Stromman, G.; Rosseland, B.O.; Skipperud, L.; Burkitbaev, L.M.; Uralbekov, B.; Heier, L.S.; Salbu, B. Uranium activity ratio in water and fish from Pit lakes in Kurday, Kazakhstan and Taboshar, Tajikistan. J. Environ. Radioact. 2013, 123, 71–81. [Google Scholar] [CrossRef]
- Skipperud, L.; Stromman, G.; Yunusov, M.; Stegnar, P.; Uralbekov, B.; Tilloboev, H.; Zjazjev, G.; Heier, L.S.; Rosseland, B.O.; Salbu, B. Environmental impact assessment of radionuclide and metal contamination at the former U sites Taboshar and Digmai, Tajikistan. J. Environ. Radioact. 2013, 123, 50–62. [Google Scholar] [CrossRef]
- Skipperud, L.; Jørgensen, A.G.; Heier, L.S.; Salbu, B.; Rosseland, B.O. Po-210 and Pb-210 in water and fish from Taboshar uranium mining Pit lake, Tajikistan. J. Environ. Radioact. 2013, 123, 82–89. [Google Scholar] [CrossRef]
- Yılmaz, F.; Özdemir, N.; Demirak, A.; Tuna, A.L. Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chem. 2007, 100, 830–835. [Google Scholar] [CrossRef]
- Alhas, E.; Oymak, S.A.; Karadede Akin, H. Heavy metal concentrations in two barb, Barbus xanthopterus and Barbus rajanorum mystaceus from Ataturk dam lake, Turkey. Environ. Monit. Assess. 2009, 148, 11–18. [Google Scholar] [CrossRef]
- Salbu, B.; Skipperud, L. Challenges in Radioecotoxicology. In Multiple Stressors: A Challenge for the Future; Springer: Dordrecht, The Netherlands, 2007; pp. 3–12. [Google Scholar]
- Salbu, B. Speciation of radionuclides–analytical challenges within environmental impact and risk assessments. J. Environ. Radioact. 2007, 96, 47–53. [Google Scholar] [CrossRef]
- Sandoval-Solis, S. Water Planning and Management for Large Scale River Basins: Case of Study of the Rio Grande/Rio Bravo Transboundary Basin; The University of Texas: Austin, TX, USA, 2011. [Google Scholar]
- Ferríz, H. Uranium mineralization in the san marcos volcanic center Chihuahua, Mexico. In Procedings of the Technical Committee Meeting, Uranium Deposits in Volcanic Rocks, El Paso, TX, USA; 1985; pp. 197–216. [Google Scholar]
- Colmenero Sujo, L.; Montero Cabrera, M.E.; Villalba, L.; Rentería Villalobos, M.; Torres Moye, E.; García León, M.; García-Tenorio, R.; Mireles García, F.; Herrera Peraza, E.F.; Sánchez Aroche, D. Uranium-238 and thorium-232 series concentrations in soil, radon-222 indoor and drinking water concentrations and dose assessment in the city of Aldama, Chihuahua, Mexico. J. Environ. Radioact. 2004, 77, 205–219. [Google Scholar] [CrossRef]
- Luna Porres, M.Y.; Alarcon Herrera, M.T.; Montero Cabrera, M.E.; Rodriguez Villa, M.A.; Villalobos, M.R.; Peraza, E.H. Baccharis salicifolia development in the presence of high concentrations of uranium in the arid environment of San Marcos, Chihuahua. Revista Mexicana de Física 2011, 57, 40–43. [Google Scholar]
- Renteria-Villalobos, M.; Cortes, M.; Mantero, J.; Manjon, G.; Garcia-Tenoria, R.; Herrera, E.; Montero-Cabrera, M. Uranium in the surrounding of San Marcos-Sacramento river environment (Chihuahua, Mexico). Sci. World J. 2012, 2012. [Google Scholar] [CrossRef]
- De la Mora-Covarrubias, A.; Quiñonez-Martinez, M.; Sosa-Cerecedo, M.; Soto-Cruz, R. Estudio de la calidad del agua del Río Bravo en el área de influencia de Cd. Juárez, Chihuahua-El Paso, Texas. In Proceeding of the VI Congreso Internacional y XII Nacional de Ciencias Ambientales, Chihuahua, Mexico, 6 August 2007.
- Gutierrez, M.; Alarcón-Herrera, M.; Camacho, L. Geographical distribution of arsenic in sediments within the rio Conchos basin, Mexico. Environ. Geol. 2009, 57, 929–935. [Google Scholar] [CrossRef]
- Hernández-Garcia, Y.; Sosa-Cerecedo, M.; Moreno, M.; Alcalá, J.; Puga, S. Evaluación de la contaminación por metales pesados y arsénico en sedimento en embalses del estado de Chihuahua, México. Revista Latinoamericana de Recursos Naturales 2008, 4, 89–94. [Google Scholar]
- Moreno-López, M.V.; Sosa, M.; Patiño, R.; Benavides, A.; Miranda, S.V.; Rubio, A.D.; Leal, L. Accumulation of Arsenic and Mercury in Mojarra, Catfish and Carp Fish Species from Three Water Reservoirs in Chihuahua State. In Proceedings of the 10th International Conference on the Biogeochemistry of Trace Elements, Chihuahua, Mexico, 13–18 July 2009.
- INEGI. Estudio hidrológico del estado de Chihuahua; Instituto Nacional de Estadística Geografía e Informática: Aguas Calientes, México, 1999. Available online: http://www.e-local.gob.mx/work/templates/enciclo/EMM08chihuahua/mediofisico.html (accessed on 16 June 2014).
- L’Annunziata, M.F.; Kessler, M.J. Liquid Scintillation Analysis: Principles and Practice. In Handbook of Radioactivity Analysis, 2nd ed.; Elsevier Science: Oceanside, CA, USA, 2003; pp. 347–535. [Google Scholar]
- SCFI. NMX-AA-051-SCFI-2001, Water Analysis—Determination of Metals by Atomic Absorption in Natural, Drinking, Wastewaters and Treated Wastewaters—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, DF, Mexico, 2001. [Google Scholar]
- HACH. Water Analysis Handbook, 5th ed.; HACH Co.: Loveland, CO, USA, 2008. [Google Scholar]
- SCFI. NMX-AA-073-SCFI-2001, Water Analysis—Determination of Total Chlorine in Natural, Drinking, Wastewaters and Treated Wastewaters—Test Method; Secretaría de Comercio y Fomento Industrial: Mexico City, DF, Mexico, 2001. [Google Scholar]
- SCFI. NMX-AA-036-SCFI-2001, Water Analysis—Determination of Acidity and Total Alkalinity in Natural, Drinking, Wastewaters and Treated Wastewaters; Secretaría de Comercio y Fomento Industrial: Mexico City, DF, Mexico, 2001. [Google Scholar]
- Blanco Rodriguez, M.P.; Vera Tomé, F.; Lozano, J.C.; Gómez Escobar, V. Sequential method for the determination of uranium, thorium and 226Ra by liquid scintillation alpha spectrometry. Appl. Radiat. Isot. 2000, 52, 705–710. [Google Scholar] [CrossRef]
- Currie, L.A. Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]
- Jabeen, F.; Chaudhry, A. Monitoring trace metals in different tissues of Cyprinus carpio from the Indus river in Pakistan. Environ. Monitor. Assess. 2009, 170, 645–656. [Google Scholar] [CrossRef]
- Scerbo, R.; Ristori, T.; Stefanini, B.; de Ranieri, S.; Barghigiani, C. Mercury assessment and evaluation of its impact on fish in the Cecina river basin (Tuscany, Italy). Environ. Pollut. 2005, 135, 179–186. [Google Scholar] [CrossRef]
- Karadede, H.; Oymak, S.A.; Ünlü, E. Heavy metals in mullet, liza abu, and catfish, Silurus triostegus, from the Atatürk dam lake (Euphrates), Turkey. Environ. Int. 2004, 30, 183–188. [Google Scholar] [CrossRef]
- Lau, S.; Mohamed, M.; Yen, A.T.; Su’ut, S. Accumulation of heavy metals in freshwater molluscs. Sci. Total Environ. 1998, 214, 113–121. [Google Scholar] [CrossRef]
- Shah, A.Q.; Kazi, T.G.; Arain, M.B.; Jamali, M.K.; Afridi, H.I.; Jalbani, N.; Baig, J.A.; Kandhro, G.A. Accumulation of arsenic in different fresh water fish species—Potential contribution to high arsenic intakes. Food Chem. 2009, 112, 520–524. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, C.; Yang, L. Occurrence of arsenic in two large shallow freshwater lakes in China and a comparison to other lakes around the world. Microchem. J. 2013, 110, 169–177. [Google Scholar] [CrossRef]
- Weisbrod, A.V.; Burkhard, L.P.; Arnot, J.; Mekenyan, O.; Howard, P.H.; Russom, C.; Boethling, R.; Sakuratani, Y.; Traas, T.; Bridges, T.; et al. Workgroup report: Review of fish bioaccumulation databases used to identify persistent, bioaccumulative, toxic substances. Environ. Health Perspect. 2007, 115, 255–261. [Google Scholar]
- Subotic, S.; Spasic, S.; Visnjic-Jeftic, Z.; Hegedis, A.; Krpo-Cetkovic, J.; Mickovic, B.; Skoric, S.; Lenhardt, M. Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube river (Serbia). Ecotoxicol. Environ. Saf. 2013, 98, 196–202. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Statistics Division, Food Security Statistics, Food Consumption; FAO: Rome, Italy, 2005. [Google Scholar]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of dabaoshan mine, south China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Recommendations of the International Commission on Radiological Protection; ICRP: Ottawa, ON, Canada, 1990; pp. 1–3. [Google Scholar]
- UNSCEAR, United Nation Scientific Committee on the Effects of Atomic Radiation. Exposures from natural radiation sources (Annex B); Report of the United Nation Scientific Committee on the Effects of Atomic Radiation to the General Assemby; UNSCEAR: New York, NY, USA, 2008. [Google Scholar]
- Carvalho, F.P.; Oliveira, J.M.; Lopes, I.; Batista, A. Radionuclides from past ur anium mining in rivers of Portugal. J. Environ. Radioact. 2007, 98, 298–314. [Google Scholar] [CrossRef]
- Kraemer, L.D.; Evans, D. Uranium bioaccumulation in a freshwater ecosystem: Impact of feeding ecology. Aquat. Toxicol. 2012, 124, 163–170. [Google Scholar] [CrossRef]
- Burillo Montúfar, J.C.; Reyes Cortés, M.; Reyes Cortés, I.A.; Espino Valdez, M.S.; Hinojosa de la Garza, O.R.; Nevárez Ronquillo, D.P.; Herrera Peraza, E.; Rentería Villalobos, M.; Montero Cabrera, M.E. Uranium-series isotopes transport in surface, vadose and ground waters at San Marcos uranium bearing basin, Chihuahua, Mexico. Appl. Geochem. 2012, 27, 1111–1122. [Google Scholar] [CrossRef]
- Reyes-Cortés, M.; Reyes-Cortés, I.A.; Espino Valdez, S.; Rentería-Villalobos, M.; Burillo Montúfar, J.C.; Montero-Cabrera, M.E. Origen y distribución de la radiactividad natural en la zona norte de la cuenca de Chihuahua, México. Revista Mexicana de Ciencias Geológicas 2012, 29, 659–675. [Google Scholar]
- Panorama Minero del Estado de Chihuahua. Available online: http://www.sgm.gob.mx/pdfs/CHIHUAHUA.pdf (accessed on 13 June 2014).
- De la Maza Benignos, M. Los peces del Río Conchos, 1a ed.; Alianza WWF - FGRA y Gobierno del Estado de Chihuahua: Chihuahua, Mexico, 2009. [Google Scholar]
- Al-Masri, M.S.; Mamish, S.; Budeir, Y.; Nashwati, A. 210po and 210pb concentrations in fish consumed in Syria. J. Environ. Radioact. 2000, 49, 345–352. [Google Scholar] [CrossRef]
- Bustamante, P.; Germain, P.; Leclerc, G.; Miramand, P. Concentration and distribution of 210po in the tissues of the scallop Chlamys varia and the mussel Mytilus edulis from the coasts of Charente-Maritime (France). Mar. Pollut. Bull. 2002, 44, 997–1002. [Google Scholar] [CrossRef]
- CNA, Comisión Nacional del Agua. Las represas del estado de Chihuahua; Instituto Politécnico Nacional: Chihuahua, Mexico, 2001. [Google Scholar]
- Gutiérrez, M.; Borrego, P. Water quality assessment of the rio Conchos, Chihuahua, Mexico. Environ. Int. 1999, 25, 573–583. [Google Scholar] [CrossRef]
- Turkmen, M.; Turkmen, A.; Tepe, Y. Comparison of metals in tissues of fish from Paradeniz lagoon in the coastal area of northern east Mediterranean. Bull. Environ. Contam. Toxicol. 2011, 87, 381–385. [Google Scholar] [CrossRef]
- Otter, R.R.; Bailey, F.C.; Fortner, A.M.; Adams, S.M. Trophic status and metal bioaccumulation differences in multiple fish species exposed to coal ash-associated metals. Ecotoxicol. Environ. Saf. 2012, 85, 30–36. [Google Scholar] [CrossRef]
- Portant Fixation de Teneurs Maximales Pour Certains Contaminants dans les Denrées Alimentaires. Available online: http://eur-lex.europa.eu/legal-content/FR/TXT/PDF/?uri=CELEX:32006R1881&from=FR (accessed on 19 June 2014).
- WHO/FAO, World Health Organization, Food and Agriculture Organization of the United Nations. Evaluation of Certain Food Additives and the Contaminants Mercury, Lead and Cadmium; World Health Organization Technical Report Series No. 505; WHO: Geneva, Switzerland, 1989. [Google Scholar]
- Patra, A.K.; Wagh, S.S.; Jain, A.K.; Hegde, A.G. Assessment of daily intake of trace elements by Kakrapar adult population through ingestion pathway. Environ. Monit. Assess. 2010, 169, 267–272. [Google Scholar] [CrossRef]
- JECFA. Evaluation of Certain Food Additives and Contaminants: Seventy Second Report of the Joint FAO/WHO Expert Committe on Food Additives; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- FAO/WHO, Food and Agriculture Organization of the United Nations and World Health Organization. Summary of Evaluations Performed by the Joint FAO/WHO Expert Committe on Food Additives (JECFA 1956–2003); ILSI Press International Life Sciences Institute: Washington, DC, USA, 2004. [Google Scholar]
- Adams, D.H. Consistently low mercury concentrations in dolphinfish, Coryphaena hippurus, an oceanic pelagic predator. Environ. Res. 2009, 109, 697–701. [Google Scholar] [CrossRef]
- Kojadinovic, J.; Potier, M.; Le Corre, M.; Cosson, R.P.; Bustamante, P. Bioaccumulation of trace elements in pelagic fish from the western Indian ocean. Environ. Pollut. 2007, 146, 548–566. [Google Scholar] [CrossRef] [Green Version]
- Ling, M.-P.; Wu, C.-C.; Yang, K.-R.; Hsu, H.-T. Differential accumulation of trace elements in ventral and dorsal muscle tissues in tilapia and milkfish with different feeding habits from the same cultured fishery pond. Ecotoxicol. Environ. Saf. 2013, 89, 222–230. [Google Scholar] [CrossRef]
- Rice, K. Trace-element concentrations in streambed sediment across the conterminous United States. Environ. Sci. Technol. 1999, 33, 2499–2504. [Google Scholar] [CrossRef]
- Uysal, K.; Köse, E.; Bülbül, M.; Dönmez, M.; Erdoğan, Y.; Koyun, M.; Ömeroğlu, Ç.; Özmal, F. The comparison of heavy metal accumulation ratios of some fish species in Enne Dame lake (Kütahya/Turkey). Environ. Monitor. Assess. 2009, 157, 355–362. [Google Scholar] [CrossRef]
- Kanayochukwu, N.J.; Ebere, O.O.; Obi, O.I. Nigeria: Environmental health concerns. Encycl. Environ. Health 2010, 4, 125–130. [Google Scholar]
- Rejomon, G.; Nair, M.; Joseph, T. Trace metal dynamics in fishes from the southwest coast of India. Environ. Monit. Assess. 2010, 167, 243–255. [Google Scholar] [CrossRef]
- Campbell, L.; Verburg, P.; Dixon, D.G.; Hecky, R.E. Mercury biomagnification in the food web of lake Tanganyika (Tanzania, east Africa). Sci. Total Environ. 2008, 402, 184–191. [Google Scholar]
- Karadede-Akin, H.; Ünlü, E. Heavy metal concentrations in water, sediment, fish and some benthic organisms from Tigris River, Turkey. Environ. Monitor. Assess. 2007, 131, 323–337. [Google Scholar] [CrossRef]
- Baldantoni, D.; Maisto, G.; Bartoli, G.; Alfani, A. Analyses of three native aquatic plant species to assess spatial gradients of lake trace element contamination. Aquat. Bot. 2005, 83, 48–60. [Google Scholar]
Appendix
| Sample | n | Tissue | Activity concentrations | |||
|---|---|---|---|---|---|---|
| 238U | 234U | U Total | 234U/238U | |||
| Cyprinus carpio a | 12 | Fillet | 0.009 (0.003–0.025) | 0.014 (0.006–0.042) | 0.023 (0.009–0.067) | 1.5 (1.1–1.6) |
| Lepomis cyanellus a | 12 | Fillet | 0.014 (0.013–0.032) | 0.020 (0.009–0.039) | 0.034 (0.008–0.071) | 1.4 (1.1–1.5) |
| Ictalurus furcatus a | 12 | Fillet | 0.011 (0.005–0.033) | 0.017 (0.006–0.043) | 0.028 (0.011–0.076) | 1.4 (1.1–1.6) |
| Cyprinus carpio b | 12 | Fillet | 0.007 (0.003–0.017) | 0.012 (0.004–0.025) | 0.017 (0.007–0.024) | 1.4 (1.1–1.5) |
| Lepomis cyanellus b | 12 | Fillet | 0.011 (0.004–0.034) | 0.014 (0.005–0.041) | 0.025 (0.008–0.075) | 1.3 (1.1–1.4) |
| Ictalurus furcatus b | 12 | Fillet | 0.011 (0.003–0.034) | 0.016 (0.006–0.048) | 0.027 (0.01–0.073) | 1.5 (1.1–1.6) |
| Sample | n | Tissue | Metal(loid)s concentration | |||||
|---|---|---|---|---|---|---|---|---|
| As | Cu | Fe | Hg | Pb | Zn | |||
| Cyprinus carpio a | 12 | Fillet | 0.13 (0.03–0.56) | 0.22 (0.07–0.39) | 6 (2–11) | 0.005 (0.0003–0.05) | 0.025 (0.0003–0.32) | 11 (4–18) |
| 12 | Gill | 0.11 (0.03–0.3) | 0.17 (0.08–0.26) | 10 (5–18) | 0.002 (0.0004–0.04) | 0.132 (0.018–0.21) | 37 (15–74) | |
| Lepomis cyanellus a | 12 | Fillet | 0.21 (0.10–0.52) | 0.18 (0.06–0.39) | 2 (1–6) | 0.013 (0.0004–0.97) | 0.009 (0.0003–0.28) | 9 (5–17) |
| 12 | Gill | 0.23 (0.1–0.7) | 0.2 (0.01–0.3) | 8.6 (5–19) | 0.009 (0.0003–0.25) | 0.12 (0.011–0.38) | 31 (14–64) | |
| Ictalurus furcatus a | 12 | Fillet | 0.17 (0.09–0.26) | 0.15 (0.08–0.28) | 4 (0.3–12) | 0.033 (0.0007–0.64) | 0.05 (0.006–0.84) | 10 (7–16) |
| 12 | Gill | 0.2 (0.07–0.43) | 0.17 (0.08–0.25) | 10 (6–16) | 0.016 (0.0003–0.22) | 0.02 (0.19–0.39) | 28 (14–68) | |
| Cyprinus carpio b | 12 | Fillet | 0.16 (0.05–0.56) | 0.15 (0.02–0.46) | 3 (1–8) | 0.011 (0.0004–0.34) | 0.05 (0.004–0.30) | 11 (7–19) |
| 12 | Gill | 0.13 (0.02–0.45) | 0.14 (0.06–0.26) | 12 (8–19) | 0.008 (0.0003–0.19) | 0.15 (0.02–0.48) | 39 (17–85) | |
| Lepomis cyanellus b | 12 | Fillet | 0.39 (0.21–0.65) | 0.26 (0.08–0.62) | 4 (1–12) | 0.126 (0.0003–1.02) | 0.036 (0.0003–0.14) | 12 (4–18) |
| 12 | Gill | 0.43 (0.15–0.68) | 0.34 (0.19–0.58) | 8 (5–15) | 0.022 (0.0006–0.19) | 0.26 (0.16–0.34) | 29 (15–81) | |
| Ictalurus furcatus b | 12 | Fillet | 0.13 (0.04–0.28) | 0.13 (0.01–0.36) | 5 (1–12) | 0.12 (0.0005–0.88) | 0.08 (0.002–1.48) | 8 (2–16) |
| 12 | Gill | 0.15 (0.01–0.52) | 0.23 (0.14–0.40) | 11 (6–18) | 0.039 (0.002–0.17) | 0.234 (0.01–0.53) | 33 (16–91) | |
| U/Metal(loid)s | Factor/Interaction | |||||
|---|---|---|---|---|---|---|
| Sampling | Species | Tissue | Sampling*Species | Sampling*Tissue | Tissue*Species | |
| 238U | -- | C: 0.009 (b) I: 0.014 (ab) L: 0.015 (a) | -- | -- | -- | -- |
| As | W-S: 0.213 (b) Su-A: 0.270 (a) | C: 0.180 (b) I: 0.199 (b) L: 0.347 (a) | -- | Su-A*C: 0.184 (b) Su-A*I: 0.190 (b) Su-A*L: 0.437 (a) | -- | -- |
| Cu | -- | C: 0.196 (a) I : 0.187 (a) L: 0.285 (b) | -- | -- | -- | F*C: 0.255 (ab) F*I: 0.168 (b) F*L: 0.258 (a) G*C: 0.167 (b) G*I: 0.206 (b) G*L: 0.313 (a) |
| Fe | W-S: 6.13 (b) Su-A: 7.90 (a) | C: 8.50 (a) I: 8.31 (a) L: 4.22 (b) | F: 5.06 (b) G: 8.96 (a) | W-S*C: 8.83 (a) W-S*I: 8.07 (a) W-S*L: 1.48 (b) | Su-A*F: 4.96 (b) Su-A*G: 10.8 (a) | G*C: 11.7 (a) G*I: 10.9 (a) G*L: 4.38 (b) |
| Hg | -- | C: 0.035 (b) I: 0.197 (a) L: 0.146 (a) | F: 0.189 (a) G: 0.064 (b) | -- | -- | F*C: 0.046 (b) F*I: 0.305 (a) F*L: 0.214 (a) G*C: 0.025 (b) G*I: 0.088 (a) G*L: 0.078 (a) |
| Pb | W-S: 0.149 (b) Su-A: 0.225 (a) | C: 0.147 (b) I: 0.267 (a) L: 0.147 (b) | F: 0.143 (b) G: 0.231 (a) | -- | -- | -- |
| Zn | -- | -- | F: 11.3 (b) G: 38.7 (a) | -- | -- | -- |
| Metal(loid)s/Parameters | Factor/Interaction | |
|---|---|---|
| Sampling | Depth | |
| Fe | -- | 0.1 m: 0.019 (b) 10 m: 0.130 (a) |
| TDS | W-S: 229 (a) Su-A: 131 (b) | -- |
| T | W-S: 21 (a) Su-A: 18 (b) | -- |


© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luna-Porres, M.Y.; Rodríguez-Villa, M.A.; Herrera-Peraza, E.F.; Renteria-Villalobos, M.; Montero-Cabrera, M.E. Potential Human Health Risk by Metal(loid)s, 234,238U and 210Po due to Consumption of Fish from the “Luis L. Leon” Reservoir (Northern México). Int. J. Environ. Res. Public Health 2014, 11, 6612-6638. https://doi.org/10.3390/ijerph110706612
Luna-Porres MY, Rodríguez-Villa MA, Herrera-Peraza EF, Renteria-Villalobos M, Montero-Cabrera ME. Potential Human Health Risk by Metal(loid)s, 234,238U and 210Po due to Consumption of Fish from the “Luis L. Leon” Reservoir (Northern México). International Journal of Environmental Research and Public Health. 2014; 11(7):6612-6638. https://doi.org/10.3390/ijerph110706612
Chicago/Turabian StyleLuna-Porres, Mayra Y., Marco A. Rodríguez-Villa, Eduardo F. Herrera-Peraza, Marusia Renteria-Villalobos, and María E. Montero-Cabrera. 2014. "Potential Human Health Risk by Metal(loid)s, 234,238U and 210Po due to Consumption of Fish from the “Luis L. Leon” Reservoir (Northern México)" International Journal of Environmental Research and Public Health 11, no. 7: 6612-6638. https://doi.org/10.3390/ijerph110706612
APA StyleLuna-Porres, M. Y., Rodríguez-Villa, M. A., Herrera-Peraza, E. F., Renteria-Villalobos, M., & Montero-Cabrera, M. E. (2014). Potential Human Health Risk by Metal(loid)s, 234,238U and 210Po due to Consumption of Fish from the “Luis L. Leon” Reservoir (Northern México). International Journal of Environmental Research and Public Health, 11(7), 6612-6638. https://doi.org/10.3390/ijerph110706612